- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Aggregation efficiency of kaolinite suspension as function of pH at 1 200 r/min for 5 min
- Fig.2 SEM images of aggregation of kaolinite particles: (a) Edge-face self-aggregation in acidic medium; (b) Edge-face and edge-edge aggregation in neutral medium; (c) Dispersion in alkaline medium; (d) Edge-face hydrophobic aggregation in acidic DDA medium; (e) Face-face hydrophobic aggregation in neutral medium; (f) Dispersion of face-face aggregates in alkaline medium
- Fig.3 Zeta potential of kaolinite as function of pH
- Fig.4 Zeta potential of kaolinite as function of cationic surfactant concentration at pH=7
- Fig.5 Aggregation efficiency of kaolinite suspension with pH=7 as function of cationic surfactant concentration at 1 200 r/min for 5 min
- Fig.6 SEM images of kaolinite particles in suspension at with different concentrations surfactant: (a) Small and loose aggre- gation at low concentration, c(DDA)=0.15 mmol/L; (b) Big and tight aggregation at high concentration, c(DDA)=0.8 mmol/L
J. Cent. South Univ. Technol. (2008) 15: 368-372
DOI: 10.1007/s11771-008-0069-9
![]()
Hydrophobic aggregation of ultrafine kaolinite
ZHANG Xiao-ping(张晓萍), HU Yue-hua(胡岳华), LIU Run-qing(刘润清)
(School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract:
The hydrophobic aggregation of ultrafine kaolinite in cationic surfactant suspension was investigated by sedimentation test, zeta potential measurement and SEM observation. SEM images reveal that kaolinite particles show the self-aggregation of edge-face in acidic media, the aggregation of edge-face and edge-edge in neutral media, and the dispersion in alkaline media due to electrostatic repulsion. In the presence of the dodecylammonium acetate cationic surfactant and in neutral and alkaline suspension, the hydrophobic aggregation of face-face is demonstrated. The zeta potential of kaolinite increases with increasing the concentration of cationic surfactant. The small and loose aggregation at a low concentration but big and tight aggregation at a high concentration is presented. At pH=7 alkyl quarterly amine salt CTAB has the best hydrophobic aggregation among three cationic surfactants, namely, dodecylammonium acetate, alkyl quarterly amine salts 1227 and CTAB.
Key words:
ultrafine kaolinite; cationic surfactant; SEM observation; hydrophobic aggregation;
1 Introduction
Diasporic-bauxite in China is characterized by a high content of aluminum oxide and silica but a low mass ratio of Al2O3 to SiO2 (average 5-6). Bayer process is a typical method to produce aluminum oxide from bauxite[1-2]. But for the diasporic-bauxite, the Bayer process can economically produce aluminum oxide only when the mass ratio of Al2O3 to SiO2 is higher than 10. Therefore, it is very required to effectively remove aluminosilicate minerals such as kaolinite, pyrophyllite and illite from the bauxite to increase the mass ratio of Al2O3 to SiO2 in the bauxite.
Flotation provides a promising way to remove silicates. Direct flotation has been proven to be an efficient method for de-silication from diasporic-bauxite by using oleic acid as collector of diaspore in the slurry of pH 9-10[3-7]. But the cationic collector reverse flotation has shown some promising advantages for diasporic-bauxite de-silication over direct floatation, such as lower cost, easy dewatering and less effect on aluminum oxide metallurgy. There are many reports on the collectors and modifiers of reverse flotation of diasporic-bauxite[8-14]. In reverse flotation of diasporic- bauxite, the particulate state (aggregation/dispersion) of suspension has an evident influence on selective flotation of silicate minerals from diasporic-bauxite. Therefore, the understanding of the aggregation fundament and dispersion behavior of kaolinite is useful for the successful development of a satisfactory reverse flotation process to increase the mass ratio of Al2O3 to SiO2 in the bauxite.
According to DLVO theory, the stability of a colloidal suspension is determined by an electrostatic repulsive force that drives particles apart and by attractive van der Waals forces that promote aggregation. The effects of solution and charging properties on the dispersion and aggregation of kaolinite particles were reported, but few articles reported the hydrophobic aggregation of kaolinite with cationic surfactants[15-18].
In this work, the hydrophobic aggregation of kaolinite was investigated through zeta potential measurement, SEM observation and sedimentation test.
2 Experimental
2.1 Materials and reagents
Lumps of kaolinite were taken from Shanxi Deposits, China. The mineral lumps were crushed and ground in a laboratory porcelain mill. Those particles with an average diameter of 3.85 μm were collected and used in sedimentation tests. The chemical components are listed in Table 1.
Table 1 Chemical components of kaolinite (mass fraction, %)

Dodecylammonium acetate (DDA) and two alkyl quarterly amine salts (1227 and CTAB) were used as surfactants. Solutions of HCl and NaOH were used to adjust pH of the system. All reagents were analytical grade. Distilled water was used in all tests.
2.2 Sedimentation test
In sedimentation test, the kaolinite suspension was prepared by adding 3 g mineral sample to a beaker with 40 mL distilled water. The resultant suspension was dispersed by an electric stirrer for 15 min. After adding surfactant, the pH of mixture was adjusted by HCl or NaOH and agitated at 1 200 r/min for 5 min. The transmittance of the suspension was inverted for 10 times in 100 mL cylinders. After settling for 8 min, the top 50 mL suspension was taken out to measure pH. The dried mass of solids was obtained after the bottom 50 mL suspension was leached. The results of the sedimentation tests were reported in terms of aggregation efficiency (Ea), which is defined as
![]() (1)
(1)
where m0 represents the dried mass of solids in the bottom 50 mL suspension; m represents the total mass of the sample.
2.3 Zeta potential measurement
Zeta potentials were measured by a Brookheaven zeta plus zetameter (USA). The suspension was prepared by adding 20 mg mineral samples to 50 mL desired solutions containing 1 mmol/L KNO3 as supporting electrolytes. The resultant suspension was dispersed by a magnetic stirrer for 5 min. Suspension pH was measured after adding surfactants. Measurements were made in triplicate and the average zeta potential values were reported as results. The measurement error was found to be within 5 mV. All zeta potential measurements were carried out at 25 ℃.
2.4 SEM analysis
At the same pH, the sedimentation slurry was diluted for 50 times. After the suspension was agitated for 2 min, a suspension drop of 5 mm base diameter was created on a sheet glass by a micro dropper. The sample was then air-dried at an ambient temperature. The sample was examined under a scanning electron microscope of Noran Jeol model J SM25600LV with energy dispersive X-ray analyzer.
3 Results and discussion
3.1 Aggregation and dispersion of kaolinite suspen- sion
Fig.1 shows the aggregation efficiency of the kaolinite suspension as a function of pH. It can be seen that the aggregation efficiency of the kaolinite suspension is higher in acidic pH range, indicating the occurrence of self-aggregation of kaolinite particles. With increasing pH, the aggregation efficiency of the kaolinite suspension decreases, indicating that the stability of the suspension is enhanced and then the particles are dispersed. According to the DLVO theory, CUI et al[14] calculated the electrostatic interaction and van der Waals interaction between edge and edge, face and face, edge and face of kaolinite particles to explain the aggregation of edge and face in acidic media and the dispersion of face and face in alkaline media.
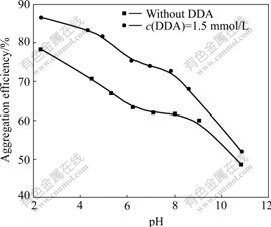
Fig.1 Aggregation efficiency of kaolinite suspension as function of pH at 1 200 r/min for 5 min
In an acidic pH range, the edges of kaolinite are positively charged and basal planes are small permanently negatively charged. The self-aggregation between edge and basal plane is possible through the electrostatic interactions. So the aggregation efficiency is higher in Fig.1. The SEM image of kaolinite suspension in acidic medium clearly shows the self-aggregation of edge-face(EF) (Fig.2(a)).
In neutral medium, the charge of edge of kaolinite is changed from positive to negative through a charge reversal around pH 7.3. Kaolinite particles may be aggregated by edge-face. The SEM image of kaolinite suspension in a neutral pH medium is shown in Fig.2(b). The aggregation of edge-face due to electrostatic attraction and edge-edge due to near point of zero charge (PZC) are observed in Fig.2(b). MAREK and HORN[18] reported that the long range edge-edge(EE) attractive forces were induced by the presence of nano-bubbles existing on the edges of clay crystals, which may cause clay particles to flocculate.
In an alkaline pH range, both edge and base are negatively charged, and the kaolinite suspension may be dispersed well due to the electrostatic repulsion among

Fig.2 SEM images of aggregation of kaolinite particles: (a) Edge-face self-aggregation in acidic medium; (b) Edge-face and edge-edge aggregation in neutral medium; (c) Dispersion in alkaline medium; (d) Edge-face hydrophobic aggregation in acidic DDA medium; (e) Face-face hydrophobic aggregation in neutral medium; (f) Dispersion of face-face aggregates in alkaline medium
various surfaces. Therefore, the aggregation efficiency is low in an alkaline medium. The dispersed kaolinite particles are demonstrated by the SEM image in Fig.2(c).
After adding DDA into the kaolinite suspension, the aggregation efficiency of the kaolinite suspension is obviously higher than that without DDA in all pH range, which indicates the occurrence of hydrophobic aggregation of kaolinite particles because of the adsorption of DDA cationic surfactant. In an acidic pH range and in the presence of DDA, the suspension of kaolinite particles still aggregates in form of edge-face (Fig.2(d)). In a neutral and alkaline pH range, the basal planes of dispersed kaolinite are negatively charged and adsorb DDA cationic surfactant. So the aggregation efficiency is higher than that without DDA. The SEM image of kaolinite suspension in a neutral medium clearly shows the hydrophobic aggregation of face-face (see Fig.2(e)). It can be found from Fig.2(f) that kaolinite particles are aggregated in an alkaline pH range and in the presence of DDA, which is different from that in Fig.2(c) mainly showing dispersion.
3.2 Zeta potential of kaolinite in the presence and absence of cationic surfactants
The zeta potentials of kaolinite as a function of pH in the absence and presence of the DDA cationic surfactant are shown in Fig.3. The isoelectric point of the kaolinite is around pH 3 and the zeta potential of kaolinite becomes more negative with increasing suspension pH. In a wide pH range, the adsorption of the DDA makes the kaolinite towards positively. The isoelectric point of kaolinite changes to 5.6 in the presence of DDA.
The zeta potentials of kaolinite as a function of the cationic surfactant concentration are shown in Fig.4. CTAB and 1227 have the same adsorption mechanism with DDA. The zeta potential of kaolinite markedly increases with the increase of cationic surfactant concentration, indicating a stronger adsorption of cationic surfactant on kaolinite and a stronger hydrophobicity. The zeta potential of kaolinite in surfactant solution increases in the order of CTAB>1227>DDA, indicating that the adsorption order of cationic surfactant is CTAB>1227>DDA, which is in accordance with the order of aggregation efficiency of kaolinite in Fig.5.
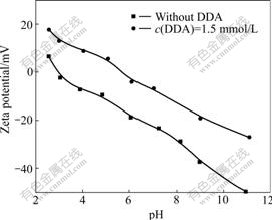
Fig.3 Zeta potential of kaolinite as function of pH
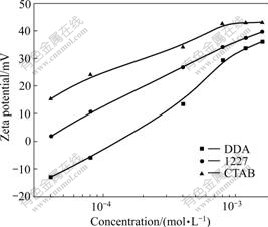
Fig.4 Zeta potential of kaolinite as function of cationic surfactant concentration at pH=7
The effect of cationic surfactant concentration on the aggregation efficiency of kaolinite suspension is presented in Fig.5. It follows that the aggregation efficiency increases with the increase of surfactant concentration, which may be attributed to the increase of adsorption of cationic surfactants and hence hydrophobicity.
At pH 7, kaolinite suspension shows small and loose aggregation at low DDA concentration while big and tight aggregation at high DDA concentration (Fig.6).
In addition, it can also be noted that the aggregation efficiencies of kaolinite are different in three different cationic surfactant suspensions. The aggregation efficiency of kaolinite suspension is in the order of CTAB>1227>DDA.

Fig.5 Aggregation efficiency of kaolinite suspension with pH=7 as function of cationic surfactant concentration at 1 200 r/min for 5 min
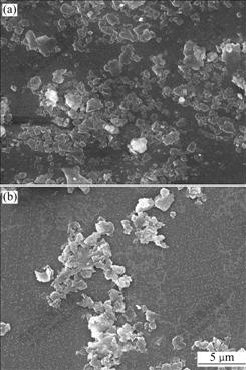
Fig.6 SEM images of kaolinite particles in suspension at with different concentrations surfactant: (a) Small and loose aggre- gation at low concentration, c(DDA)=0.15 mmol/L; (b) Big and tight aggregation at high concentration, c(DDA)=0.8 mmol/L
4 Conclusions
1) Kaolinite shows self-aggregation of edge-face in an acidic suspension, edge-face and edge-edge aggregation in a neutral suspension, and dispersion in an alkaline suspension. In the presence of the DDA cationic surfactant and in a neutral and alkaline suspension, the hydrophobic aggregation of face-face is demonstrated too.
2) The aggregation efficiency of kaolinite is progressively higher with the increase of cationic surfactant concentration. Accordingly, the zeta potential of kaolinite increases with increasing the cationic surfactants concentration. The aggregation is small and loose at low concentration but big and tight at high concentration.
3) CTAB has the best hydrophobic aggregation among three cationic surfactants, DDA, 1227 and CTAB at pH 7.
References
[1] WANG En-fu, MA Chao-jian, LU Qin-fang, LI Yao-wu. Discussion of disposal high silica bauxite ore by ore-dressing Bayer process in alumina production of China [J]. Light Metals, 1996(7): 3-6. (in Chinese)
[2] JIANG Hao, HU Yue-hua, WANG Dian-zuo, QIN Wen-qing, GU Guo-hua. Structure of the adsorbed layer of cationic surfactant at diaspore-water interface [J]. Journal of China University of Mining and Technology, 2005, 34(4): 500-501. (in Chinese)
[3] LI Long-feng. Desilication and iron removal research for diasporic-hauxite by mineral processing [J]. Journal of Central South Institute of Mining and Metallurgy, 1980(4): 82-84. (in Chinese)
[4] LIANG An-zhen, LI Ting-hui. Discussion on reasonable technology flowsheet of bauxite processing [J]. Light Metal, 1982(11): 1-6. (in Chinese)
[5] FENG Qi-ming, ZHANG Guo-fan, LU Yi-ping. The 90’s research and outlook of bauxite on impurity removing by mineral processing [J]. Light Metal, 1998(4): 9-13. (in Chinese)
[6] ZHANG Guo-fan. Theory and technology of flotation on bauxite desilication [D]. Changsha: Central South University, 2001. (in Chinese)
[7] LU Yi-ping, ZHANG Guo-fan, FENG Qi-ming, OU Le-ming. A novel collector RL for flotation of bauxite [J]. J Cent South Univ Technol, 2002, 9(1): 21-24.
[8] JIANG Hao, HU Yue-hua, QIN Wen-qing, WANG Yu-hua, WANG Dian-zuo. Mechanism of flotation for diaspore and aluminium-silicate minerals with alkyl-amine collectors [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 688-692. (in Chinese).
[9] LI Hai-pu, HU Yue-hua, WANG Dian-zuo, XU Jing. Mechanism of interaction between cationic surfactant and kaolinite [J]. Journal of Central South University: Natural Science, 2004, 35(12): 228-233. (in Chinese)
[10] WANG Yu-hua, HU Yue-hua, LIU Xiao-wen. Flotation desilicating from diasporic-bauxite with cetyl trimethylammonium bromide [J]. J Cent South Univ Technol, 2003, 10(4): 324-328.
[11] WANG Yu-hua, HU Yue-hua, CHEN Xiang-qing. Alum inum-silicates flotation with quaternary ammonium salts [J]. Trans Nonferrous Met Soc China, 2003, 13(3): 715-719.
[12] HU Yue-hua, SUN Wei, JIANG Hao, MILLER J D, FA Ke-qing. The anomalous behavior of kaolinite flotation with dodecyl amine collector as explained from crystal structure considerations [J]. Mineral Processing, 2005, 76: 163-172.
[13] TARL G, BOBOS J. Modification of surface charge properties during kaolinite to halloysite-7A transformation [J]. Journal of Colloid and Interface Science, 1999, 210(2): 360-366.
[14] CUI Ji-rang, FANG Qi-xue, HUANG Guo-zhi. Crystal structures and surface properties of diaspore and kaolinite [J]. Nonferrous Metals, 1999, 51(4): 25-30. (in Chinese)
[15] SADOWSKI Z., Study on hydrophobic aggregation of calcite aqueous suspensions [J]. Powder Technol, 1994, 80(2): 93-98.
[16] HU Yue-hua, SUN Wei, LIU Xiao-wen, WANG Dian-zuo. Cleavage nature, electrokinetics, aggregation and dispersion of kaolinite [J]. Trans Nonferrous Met Soc China, 2003, 13(6): 1430-1434.
[17] LUO Zhao-jun, HU Yue-hua, WANG Yu-hua, QIU Guan-zhou. Mechanism of dispersion and aggregation in reverse flotation for bauxite [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 680-683. (in Chinese)
[18] MAREK Z, HORN R G. Hydrophobic attraction may contribute to aqueous flocculation of clays [J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2003, 222: 323-328.
(Edited by YANG Hua)
Foundation item: Project(2005CB623701) supported by the Major State Basic Research Development Program of China
Received date: 2007-11-17; Accepted date: 2008-01-09
Corresponding author: HU Yue-hua, Professor; Tel: +86-731-8879815; Fax: +86-731-8879815; E-mail: hyh@mail.csu.edu.cn
- Hydrophobic aggregation of ultrafine kaolinite


