
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1831-1838
Microstructure and hydrogen storage properties of as-cast and rapidly solidified Ti-rich Ti-V alloys
Suwarno SUWARNO1, Jan Ketil SOLBERG1, Jan Petter MAEHLEN2, Bente KROGH3,
B?rre Tore B?RRESEN3, Esther OCHOA-FERNANDEZ3, Erling RYTTER3,
Mario WILLIAMS4, Roman DENYS2, Volodymyr A. YARTYS1,2
1. Department of Materials Science and Engineering, Norwegian University of Science and Technology,NO-7491, Trondheim, Norway;
2. Institute for Energy Technology, P.O. Box 40, NO-2027, Kjeller, Norway;
3. Statoil Research Centre, Rotvoll, N-7005, Trondheim, Norway;
4. University of the Western Cape, Private Bag X17, Bellville, 7535, South Africa
Received 10 November 2011; accepted 25 June 2012
Abstract:
The goal of the present work was to optimize the phase-structural composition and microstructure of binary Ti0.8-0.9V0.2-0.1 alloys with respect to their hydrogen sorption properties. Application of these alloys is for hydrogen absorption from gaseous mixtures containing substantial amounts of carbon monoxide (CO) at high temperatures. Irrespective of alloy composition, both α(HCP) and β(BCC) phases in Ti0.8-0.9V0.2-0.1 formed single phase FCC hydrides upon hydrogenation in pure H2. An in situ synchrotron X-ray diffraction study showed that only the β-phase transformed to the corresponding hydride when the alloy was hydrogenated in a mixture of H2+10%CO. Rapid solidification (RS) of the alloy resulted in refined grain sizes both in the Ti0.8V0.2 and Ti0.9V0.1 alloys. Furthermore, RS was found to increase the β-phase fraction in Ti0.9V0.1, being twice larger than that of the as-cast alloy. Ti0.9V0.1 had a platelike microstructure as observed by scanning electron microscopy (SEM), the plates were about 300 nm thick. The microstructure refinement resulted in a faster kinetics of H desorption as observed by temperature desorption spectroscopy (TDS).
Key words:
hydrogen storage; Ti-V alloys; rapid solidification; synchrotron X-ray diffraction; carbon monoxide;
1 Introduction
The hydrogen storage capacity of TiH2 and VH2 is high, up to 4% H (mass fraction) or 150 kg/m3. The volumetric density of hydrogen is twice superior to that of the liquid hydrogen. Alloys of Ti and V form dihydrides which have hydride formation enthalpies between those of the elemental binary hydrides, i.e. between -40 to -130 kJ/mol H2. Ti and V between 2.7% and 80% (mole fraction) vanadium form solid solution alloys [1]. The lattice parameters of these Ti1-yVy alloys are known to be dependent on the composition. Due to larger atomic radius of Ti than that of V, they decrease upon increasing the V content [2,3]. The most common production method of the BCC Ti-based alloy is conventional arc melting. Another production method, such as high energy ball milling of two elements, has also been employed to produce the alloys [4-6]. The as- cast Ti1-yVy alloys can be hydrogenated after activation treatments and form FCC dihydrides in the fully hydrogen-saturated condition.
Rapid solidification is one of the production methods which allow a controllable alloy solidification rate; the latter determines the microstructure and crystal structure. This method has been extensively applied for production of amorphous metals for structural or electronic applications. Recently, it has also been applied for preparation of the alloys for hydrogen storage, characterized by micro- or nano-grained microstructures. For example, promising results of using the rapid solidification technique have allowed production of nanostructured Mg-Mm-Ni composites [7,8] and Ti-V based alloys [9,10]. Some investigators showed that rapid solidification improved cycling properties of the alloy hydrides [11,12].
The goal of the present work is to optimize the phase-structural composition and microstructure of binary Ti-V alloys with 20% and 10% V with respect to their hydrogen sorption properties. The optimized alloy is planned to be used in a hydrogen production process, i.e. in a hydrogen sorption enhanced reactor (HSER) of steam reforming or water-gas shift processes [13]. In such a reactor, the hydrogen is absorbed in-situ from the steam reformate gas mixture, thus increasing the reactor efficiency. Since the reformate gases contain detrimental gases for hydrogen absorbing alloys, such as CO and H2O, electroless deposition of Pd on the metal hydride alloy to increase resistance towards such active gases is also tested out.
2 Experimental
Alloys of Ti0.8V0.2 and Ti0.9V0.1 were synthesized by argon arc melting. About 5 g samples were produced during each melting. The alloy buttons were cut into smaller pieces for hydrogenation in their as-cast condition, and some of them were used as pre-alloys for rapid solidification using the melt spinning technique. Melt spinning is a rapid solidification technique, in which liquid metal is ejected through nozzle onto a rotating wheel (spinner) by which thin metal ribbons are produced. By varying the rotation speed of the spinner, the solidification rate can be controlled. This results in distinct morphology and microstructure of the metallic ribbons. In this work, a copper wheel with diameter of 30 cm and a rotation velocity of 2000 r/min were used, giving a surface velocity of about 21 m/s.
Microstructural characterization and quantification of the alloys and hydrides were performed using a Leica MeF4 light optical microscope, Zeiss Ultra 55LE field emission scanning electron microscope (FE-SEM) and JEOL JXA-8500F electron probe micro-analyzer (EPMA). The SEM and EPMA samples were prepared from both as-cast alloys and ribbons. The samples were hot mounted with conductive resin, ground with abrasive paper down to 2400 grid and mechanically polished with 90% oxide polishing suspension (OPS) +10% H2O2. During the SEM investigation, images were taken in backscattered mode at 20 kV and 120 μm objective aperture size. The hydrogenation properties were studied by using the volumetric thermal desorption spectroscopy (TDS) technique. The saturated dihydrides were used in the dehydrogenation experiments. The sample mass was approximately 1 g. TDS was performed by heating the samples in vacuum at a heating rate of 5 ℃ /min; the temperature range was from 20 ℃ to 800 ℃.
Ex situ and in situ synchrotron diffraction (SR-XRD) studies were applied to probe the phase-structural composition and to yield the crystallographic data of the alloys and their hydrides, which were conducted at the Swiss-Norwegian Beam Lines (SNBL), Grenoble, France. Samples with an electroless deposited Pd metal with a particle size between 40 and 60 nm were used. The method used for Pd deposition has been described in previous publications [14-16]. Small amounts of hydride powders were inserted into a quartz capillary (0.5 mm inner diameter, 0.01 mm wall thickness) and attached to a specially designed setup that was connected to a goniometer head. The in situ SR-XRD experiments were performed in an H2 or H2+10%CO gaseous flows of 20 mL/min (1.1 bar). The data were collected using a MAR2300 image plate detector. The wavelength was 0.6540 ?, and the sample to detector distance was 200 mm. GSAS software was used in the powder profile Rietveld refinements [7,18].
3 Results and discussion
3.1 Microstructure of as-cast and RS alloys
Figure 1 shows the microstructures of the as-cast alloys. Both Ti0.8V0.2 (Fig. 1(a)) and Ti0.9V0.1 (Fig. 1(b)) were composed of large grains with size exceeding 500 μm. The grain size of Ti0.8V0.2 was smaller than that of Ti0.9V0.1. The composition mapping of these as-cast alloys using EMPA indicated that the alloys were macroscopically homogeneous. The composition of Ti0.8V0.2 measured by EMPA was 79%±2% Ti (mole fraction) and 20%±0.5% V while that of Ti0.9V0.1 was 89%±2% Ti and 10%±0.5% V.

Fig. 1 Microstructures of as-cast alloys: (a) Ti0.8V0.2; (b) Ti0.9V0.1
The thickness of the ribbons produced using the melt spinning technique depended on the chemical composition of the alloys as well as the spinner velocity during preparation. By using 2000 r/m spinner velocity, ribbons with thickness of (32±3) μm were produced for Ti0.8V0.2, while thicker ribbons, (49±5) μm, were formed for Ti0.9V0.1. The thickness of the ribbons correlates with the solidification rate [19]. Thinner ribbons are in general expected to be produced at higher cooling rates as compared to the thicker ribbons. However, since the alloys in this work have different compositions, direct comparison of the data is not possible. Nevertheless, it can be seen from Fig. 2(a) that RS Ti0.8V0.2 had a fine-grained microstructure with an average grain size of about 12 μm (Fig. 2(b)). This differs considerably from the RS Ti0.9V0.1 alloy, as shown in Fig. 2(c). The microstructure of the RS Ti0.9V0.1 resembles a typical martensitic type microstructure. A high magnification backscattered image of RS Ti0.9V0.1 microstructure shows a plate-like microstructure with a plate thickness of about 300 nm (Fig. 2(d)).
Element mappings of RS Ti0.8V0.2 and Ti0.9V0.1 alloys examined by EPMA are shown in Fig. 3. It can be seen that both alloys are macroscopically homogenous without macro-segregation. Twenty point analyses were done along a line with 1 μm distance between the points, and gave the following RS alloy composition: for Ti0.8V0.2, Ti content was 80.0%±0.1% and the V content was 19.9%±2%; while for Ti0.8V0.1 the Ti content was 89.3%±1% and the V content was 10.6%±2%. This indicates that macroscopic inhomogeneity was not the case.
3. 2 Hydrogenation properties
The hydrogenation kinetics for both Ti0.8V0.2 and Ti0.9V0.1 was excellent. The activated alloys were able to absorb hydrogen within 1 min with H sorption capacities depending on the hydrogenation temperature. At room temperature, the hydrogen capacity of both alloys was 3.95% H (mass fraction). This value slightly decreased to (3.0%-3.7%) H when the hydrogenation was carried out at 450 ℃. due to the thermodynamic limitations. Rapidly solidified alloys needed a short incubation time prior to hydrogen absorption, as seen in Fig. 4. In the case of the rapidly solidified Ti0.9V0.1, activated ribbons needed about 30 s before hydrogen started to interact with the alloy. The second rehydrogenation kinetics of the RS alloys was very fast and slightly faster than that of the as-cast alloys. Similar incubation time was needed for Ti0.8V0.2. The reason for the incubation time must be the formation of an oxide layer on the ribbon surface preventing hydrogen dissociation at the beginning of the hydrogen absorption process. However, fast kinetics can be achieved after rehydrogenation, when the ribbons have been fractured and new surfaces have been created facilitating hydrogen penetration into the bulk material. However, the difference in absorption kinetics between the as-cast and rapidly solidified alloys seems to be marginal as both cast and RS alloys are able to fully absorb hydrogen within less than 60 s.
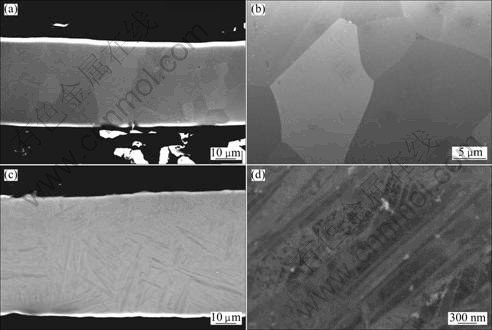
Fig. 2 Microstructures of rapidly solidified alloys: (a) RS Ti0.8V0.2 (cross-section view); (b) High magnification image of RS Ti0.8V0.2; (c) RS Ti0.9V0.1 (cross section view); (d) High magnification view of RS Ti0.9V0.1 showing a plate-like submicron structure

Fig. 3 Microstructures of Ti0.8V0.2 and Ti0.9V0.1 alloys and their vanadium mapping: (a, b) RS Ti0.8V0.2; (c, d) RS Ti0.9V0.1 (The amount of vanadium is indicated by the colour scale on right side of the maps)

Fig. 4 Hydrogen absorption curves for RS Ti0.9V0.1 at 100 ℃. and 4 bar initial hydrogen pressure in comparison with as-cast alloy
TDS traces of hydrogen desorption from the fully hydrogenated samples are shown in Fig. 5. All traces have common features. They contain two well resolved desorption peaks. As expected, the increase of vanadium content reduces the thermal stability of the hydrides; indeed, the temperature of the main desorption peak shifts from around 500 ℃. for the hydride of as-cast Ti0.9V0.1H2 to 460 ℃. for Ti0.8V0.2H2 also synthesized from the as-cast alloy. Rapid solidification significantly destabilizes Ti0.8V0.2H2 hydride, with the main desorption peak shifting by 50 ℃., down to 410 ℃.. In contrast, RS does not affect the stability of the Ti0.9V0.1H2 where the location of the peak does not change, being situated at 500 ℃. for both as-cast and RS hydrogenated samples. From the data given in Fig. 5, it can be concluded that kinetics of hydrogen desorption from the hydrides is improved for the rapidly solidified samples. Indeed, hydrogen desorption flows are always higher for the RS alloy hydrides.

Fig. 5 TDS traces of hydrogen desorption from Ti0.9V0.1H2 and Ti0.8V0.2H2 dihydrides
The destabilization of the RS Ti0.8V0.2H2 hydride observed in the TDS study can be linked to the changes in the thermodynamic and/or kinetic properties. From the thermodynamic point of view, the destabilization of the hydride may be due to a compositional change in the Ti0.8V0.2 alloy during the RS process. Even though, EPMA does not show any evidence of macro-segregation, it is possible that the alloy contains small regions enriched in V, namely Ti0.8-xV0.2+x formed on a micro scale level; corresponding hydride has a lower stability as compared to the equilibrium Ti0.8V0.2H2. This proposal is in agreement with the X-ray diffraction data (see the next section), showing that phase composition and the element distribution in the alloys are altered by the rapid solidification process. The second possible reason is the reduced crystallite size of the RS samples yielding improved kinetics of hydrogen exchange. We suggest that both mentioned factors contribute to the destabilization of the Ti0.8V0.2 hydride. However, the situation is more complex for the RS Ti0.9V0.1. Here, despite lowering of the crystallite size on rapid solidification, a destabilization was not observed as compared to the as-cast sample.
3.3 Crystal structures
Phase-structural composition and crystallographic data derived from the Rietveld refinements of the SR- XRD pattern for the as-cast and RS alloys are listed in Table 1. The as-cast Ti0.8V0.2 alloy after one complete hydrogen absorption-desorption cycle contained mainly the β-BCC solid solution Ti-V (97%, mass fraction) and α-Ti (3%). The RS Ti0.8V0.2 alloy contained a lower amount of the BCC β-phase (90.3%); while the amount of the α-phase increased to 7%. Unit cell parameter of the β phase of the as-cast alloy, 3.2322(4) ?, is higher as compared to that of the RS Ti0.8V0.2, 3.216 ?. This data evidenced lowering of Ti content in the β phase during the rapid solidification, as the amount of α-Ti increased from 3% to 7% (mass fraction). Crystallite size derived from the refinements of the profiles of XRD pattern gave 1.53 μm for the as-cast alloy, while much refined value of 210 nm was observed for the dehydrogenated rapidly solidified sample.
The ex situ SR-XRD powder diffraction pattern of the as-cast Ti0.9V0.1 alloy obtained after one complete hydrogen absorption-desorption cycle is shown in Fig. 6(a). The alloy contained a mixture of the β and α phases in a ratio of 32/67. During the rehydrogenation, the BCC phase formed a FCC dihydride, see SR-XRD pattern in Fig. 6(b). The RS Ti0.9V0.1 contained 63% (mass fraction) β-phase which is almost twice more than that for the corresponding as-cast alloy. The unit cell parameter of the β-Ti0.9V0.1 is lower than that of the β-phase in the Ti0.8V0.2. This must be due to the lower Ti content in the former phase. The unit cell parameter of the β-phase of the RS alloy is higher than that of the as-cast alloy due to the increased content of titanium in the RS alloy. The mean crystallite size derived from the refinements of the profile parameters equals 265 nm which is significantly lower as compared to the as-cast alloy, being 1.4 μm.
All studied alloys, as-cast and rapidly solidified, formed γ-FCC dihydrides, with space group ![]() after interacting with hydrogen gas. Typical powder X-ray diffraction pattern of the FCC dihydride is shown in Fig. 6(b) presenting the data for the hydrogenated as-cast Ti0.9V0.1. The hydrogen content of this sample was close to 2 H/M (3.95% H) (mass fraction), and the unit cell parameter of the FCC dihydride was 4.4390(7) ?. Increased fraction of the β-phase as well as the decreased grain size observed in the RS Ti0.9V0.1 shows a beneficial effects of the rapid solidification on the synthesis of the Ti0.9V0.1 alloy for hydrogen storage applications.
after interacting with hydrogen gas. Typical powder X-ray diffraction pattern of the FCC dihydride is shown in Fig. 6(b) presenting the data for the hydrogenated as-cast Ti0.9V0.1. The hydrogen content of this sample was close to 2 H/M (3.95% H) (mass fraction), and the unit cell parameter of the FCC dihydride was 4.4390(7) ?. Increased fraction of the β-phase as well as the decreased grain size observed in the RS Ti0.9V0.1 shows a beneficial effects of the rapid solidification on the synthesis of the Ti0.9V0.1 alloy for hydrogen storage applications.
Table 1 Phase-structural composition and crystallographic data for dehydrogenated alloys determined from Rietveld refinements of ex situ SR-XRD data
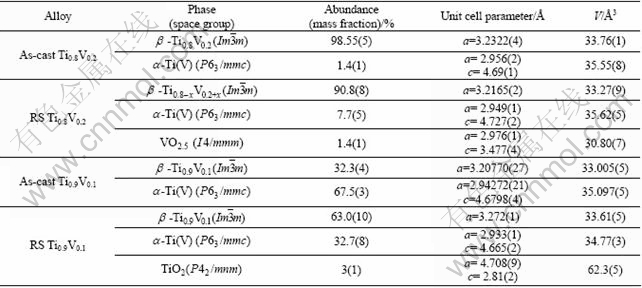
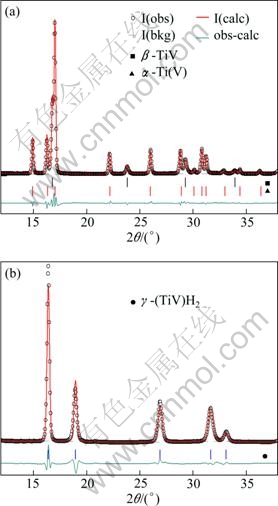
Fig. 6 Powder diffraction pattern and their Reitveld refinements: (a) Dehydrogenated as-cast Ti0.9V0.1; (b) Fully rehydrogenated sample forming FCC dihydrides
3.4 In situ SR-XRD study
Pd-modified dihydride of the hydrogen saturated as-cast Ti0.9V0.1 alloy (Ti0.9V0.1H2 + nano Pd) was used for the in situ SR-XRD studies of hydrogen desorption and absorption. During the diffraction experiments, the sample was heated and cooled with programmed rate of the temperature changes in controlled gas environment. In this experiment, the total time needed to perform one scan was about 95 s, so that the temperature change during that time was about 8 ℃.. Hydrogen desorption experiments were done by heating the sample from RT to 250 ℃ with heating rate of 20 ℃/min and then, with a lower heating rate of 5 ℃/min to reach a setpoint temperature of 800 ℃; the experiments were performed in a flow of hydrogen gas (p = ~1.12 bar).
Figure 7 shows a 2D plot of the diffraction pattern from the sample during the course of experiment as well as the temperature history. During the heating, at 500 ℃ hydrogen started to desorb from the γ-hydride of Ti0.9V0.1H2; the desorption yielded β-phase and a small amount of α-phase. Further heating resulted in enhanced transformation of the γ hydride to β hydride; the decomposition of γ hydride was completed at 700 ℃. Even though the initial sample (at 25 ℃.) can be fitted well with a single phase FCC hydride with a unit cell parameter of 4.4390(7) ?, the formation of α-phase from the γ-hydride at about 500 ℃. indicated that the sample may contain another FCC hydride. This must be the FCC TiH2 decomposing to α-HCP phase on dehydrogenation [20].

Fig. 7 2D X-ray diffraction intensity plot vs scan number from in situ experiments on Ti0.9V0.1H2 (hydrogenated as-cast alloy) during dehydrogenation (heating) and rehydrogenation (cooling) (The dashed line gives a temperature profile)
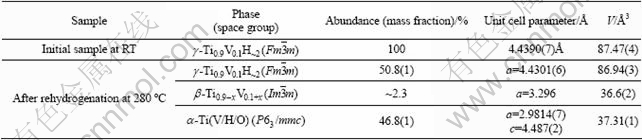
Prior to rehydrogenation, the sample was cooled from 800 ℃ to 640 ℃. During this period the formation of α-phase from β-phase? was observed. At 640 ℃, the sample was subjected to a mixture of H2+CO gases (mixture of 90/10) with flow rate 20 mL/min and outlet pressure 1.12 bar. The sample readily absorbed hydrogen yielding a FCC dihydride. This transformation was completed at about 380 ℃; no further transformations were observed upon continuing cooling. As listed in Table 2, at the end of rehydrogenation, the sample was composed of 51% (mass fraction) γ hydride, 47% α phase and small amount of β phase. It is evident that the β phase was able to absorb hydrogen and form dihydrides. The α phase can be considered to be not active in hydrogen uptake at the applied experimental conditions. The reason for that can be two folds: (a) bulk absorption of oxygen which stabilized the HCP α-phase making its hydrogenation impossible; (b) blocking by CO molecules of the metal surface thus creating barriers for hydrogen adsorption and dissociation, and preventing its diffusion into the bulk.
Table 2 Phase-structural composition and crystallographic data for initial saturated hydride and rehydrogenated Ti0.9V0.1H2 sample obtained from Rietveld refinements of powder X-ray diffraction data
4 Conclusions
The alloy preparation process determined the microstructure, crystal structure and the hydrogen storage properties of the binary Ti0.8V0.2 and Ti0.9V0.1 alloys. As-cast Ti0.8V0.2 alloy was primarily composed of the β phase with BCC structure, while Ti0.9V0.1 contained a mixture of α and β phases with a dominant phase being the α HCP one (67%). Rapid solidification refined the grain size and changed the abundance of the phase constituent. For the Ti0.8V0.2, RS decreased the grain size from an average size of above 500 μm to around 12 μm and destabilized its corresponding hydride. For the Ti0.9V0.1, RS refined the microstructure as well and doubled the β phase fraction as compared to the as-cast Ti0.9V0.1. From Rietveld profile refinements, it was concluded that the crystallite size of the RS alloys significantly decreased from 1.53 μm for the as-cast Ti0.8V0.2 alloy to 210 nm for the RS Ti0.8V0.2 and from 1.4 μm for the as-cast Ti0.9V0.1 to 265 nm for the RS Ti0.9V0.1. In situ SR-XRD experiments showed that when hydrogenation was performed in a mixture of H2+10% CO, the β phase was more reactive towards hydrogen absorption and was transformed into the FCC dihydride. On the other hand, the α phase was inactive towards hydrogen in such a gaseous mixture of H2+10%CO. Thus, microstructures which contain mainly β phase are preferable to be used for hydrogen separation applications from gas mixtures containing hydrogen and CO. From this prospective, rapid solidification improves properties of the alloys by increasing the amount of β phase. Furthermore, RS refined the grain size, which resulted in a faster desorption kinetics from the Ti0.8-0.9V0.2-0.1H2.
Acknowledgements
The authors thank the Norwegian Research Council and Statoil for the financial support. A skilful assistance from the staff of Swiss-Norwegian Beam Lines during the SR XRD experiments is gratefully acknowledged.
References
[1] PREDEL B. Ti-V (Titanium-Vanadium) [M]. MADELUNG O, Editor. Springer-Verlag. DOI:10.1007/b60148.
[2] ONO S, NOMURA K, IKEDA Y. The reaction of hydrogen with alloys of vanadium and titanium [J]. J Less-Common Met, 1980, 72(2): 159-165.
[3] HAYASHI S, HAYAMIZU K, YAMAMOTO O. Structure of Ti1-yVyHx alloys studied by X-ray diffraction and by 1 H and 51 V NMR [J]. J Solid State Chem, 1983, 46(3): 306-312.
[4] AKIBA E, IBA H. Hydrogen absorption by Laves phase related BCC solid solution [J]. Intermetallics, 1998, 6(6): 461-470.
[5] AMIRA S, SANTOS S F, HUOT J. Hydrogen sorption properties of Ti-Cr alloys synthesized by ball milling and cold rolling [J]. Intermetallics, 2010, 18(1): 140-144.
[6] HUOT J, ENOKI H, AKIBA E. Synthesis, phase transformation, and hydrogen storage properties of ball-milled TiV0.9Mn1.1 [J]. J Alloys Compd, 2008, 453(1-2): 203-209.
[7] WU Y, HAN W, ZHOU S X, LOTOTSKY M V, SOLBERG J K, YARTYS V A. Microstructure and hydrogenation behavior of ball-milled and melt-spun Mg-10Ni-2Mm alloys [J]. J Alloys Compd, 2008, 466(1-2): 176-181.
[8] WU Y, LOTOTSKY M V, SOLBERG J K, YARTYS V A. Microstructural evolution and improved hydrogenation– dehydrogenation kinetics of nanostructured melt-spun Mg–Ni–Mm alloys [J]. J Alloys Compd, 2011, 509(S2): S640-S645.
[9] PEI P, SONG X P, LIU J, CHEN G L, QIN X B, WANG B Y. The effect of rapid solidification on the microstructure and hydrogen storage properties of V35Ti25Cr40 hydrogen storage alloy [J]. Int J Hydrogen Energy, 2009, 34(19): 8094-8100.
[10] PAN H, ZHU Y, GAO M, LIU Y, LI R, LEI Y, WANG Q. A study on the cycling stability of the Ti-V-based hydrogen storage electrode alloys [J]. J Alloys Compd, 2004, 364(1-2): 271-279.
[11] ARASHIMA H, TAKAHASHI F, EBISAWA T, ITOH H, KABUTOMORI T. Correlation between hydrogen absorption properties and homogeneity of Ti-Cr-V alloys [J]. J Alloys Compd, 2003, 356-357: 405-408.
[12] ITOH H, ARASHIMA H, KUBO K, KABUTOMORI T, OHNISHI K. Improvement of cyclic durability of BCC structured Ti-Cr-V alloys [J]. J Alloys Compd, 2005, 404-406: 417-420.
[13] BORRESEN B T, RYTTER E, AARTUN I, KROGH B, RONNEKLEIV M. Method and reactor for production of hydrogen. US Patent: 20100047158 [P].
[14] WILLIAMS M, LOTOTSKY M, LINKOV V M, NECHAEV A N, SOLBERG J K, YARTYS V A. Nanostructured surface coatings for the improvement of AB5-type hydrogen storage intermetallics [J]. International Journal of Energy Research, 2009, 33(13): 1171-1179.
[15] WILLIAMS M, NECHAEV A N, LOTOTSKY M V, YARTYS V A, SOLBERG J K, DENYS R V, PINEDA C, LI Q, LINKOV V M. Influence of aminosilane surface functionalization of rare earth hydride-forming alloys on palladium treatment by electroless deposition and hydrogen sorption kinetics of composite materials [J]. Mater Chem Phys, 2009, 115(1): 136-141.
[16] LOTOTSKY M V, WILLIAMS M, YARTYS V A, KLOCHKO Y V, LINKOV V M. Surface-modified advanced hydrogen storage alloys for hydrogen separation and purification [J]. J Alloys Compd, 2011, 509(S2): S555-S561.
[17] LARSON A C, DREELE R B V. General structure analysis system (GSAS) [R]. Los Alamos National Laboratory Report LAUR, 2004.
[18] TOBY B, EXPGUI. A graphical user interface for GSAS [J]. J Appl Crystallogr, 2001, 34(2): 210-213.
[19] CANTOR B, KIM W T, BEWLAY B P, GILLEN A G. Microstructure—cooling rate correlations in melt-spun alloys [J]. J Mater Sci, 1991, 26(5): 1266-1276.
[20] MALACHEVSKY M T, D’OVIDIO C A. Thermal evolution of titanium hydride optimized for aluminium foam fabrication [J]. Scr Mater, 2009, 61(1): 1-4.
快速凝固富钛Ti-V合金的微观组织和储氢性能
Suwarno SUWARNO1, Jan Ketil SOLBERG1, Jan Petter MAEHLEN2, Bente KROGH3,
B?rre Tore B?RRESEN3, Esther OCHOA-FERNANDEZ3, Erling RYTTER3,
Mario WILLIAMS4, Roman DENYS2, Volodymyr A. YARTYS1,2
1. Department of Materials Science and Engineering, Norwegian University of Science and Technology,NO-7491, Trondheim, Norway;
2. Institute for Energy Technology, P.O. Box 40, NO-2027, Kjeller, Norway;
3. Statoil Research Centre, Rotvoll, N-7005, Trondheim, Norway;
4. University of the Western Cape, Private Bag X17, Bellville, 7535, South Africa
摘 要:研究目的在于优化Ti0.8-0.9V0.2-0.1二元合金的相结构成分、微观组织和储氢性能。该合金主要用于从含有大量一氧化碳的高温气态混合物中吸收氢气。Ti0.8-0.9V0.2-0.1合金中的α-(HCP) 和β-(BCC) 相在纯氢气中基于氢化作用,形成单相FCC结构的氢化物,此过程与合金的化学成分无关。同步辐射X射线衍射的原位分析表明,在含有氢气和10%一氧化碳的混合气体中,只有β相转变成相应的氢化物。快速凝固 (RS) 处理细化了Ti0.8V0.2和Ti0.9V0.1合金的晶粒组织,而且,快速凝固处理增加了Ti0.9V0.1合金中的β相,其所占比例是普通熔铸条件下的两倍。扫描电子显微镜 (SEM) 分析表明,Ti0.9V0.1合金含有片状组织,层片的厚度约为300 nm。热脱附谱 (TDS) 显示,微观组织的细化可以加快氢脱附的动力学过程。
关键词:储氢;Ti-V合金;快速凝固;同步辐射X射线衍射;一氧化碳
(Edited by YUAN Sai-qian)
Foundation item: Project “Integrated Process for Hydrogen Production and Separation” supported by Norwegian Research Council and Statoil, Norway
Corresponding author: Suwarno SUWARNO; Tel: +47-73594868; E-mail: suwarno@material.ntnu.no
DOI: 10.1016/S1003-6326(11)61394-0
Abstract: The goal of the present work was to optimize the phase-structural composition and microstructure of binary Ti0.8-0.9V0.2-0.1 alloys with respect to their hydrogen sorption properties. Application of these alloys is for hydrogen absorption from gaseous mixtures containing substantial amounts of carbon monoxide (CO) at high temperatures. Irrespective of alloy composition, both α(HCP) and β(BCC) phases in Ti0.8-0.9V0.2-0.1 formed single phase FCC hydrides upon hydrogenation in pure H2. An in situ synchrotron X-ray diffraction study showed that only the β-phase transformed to the corresponding hydride when the alloy was hydrogenated in a mixture of H2+10%CO. Rapid solidification (RS) of the alloy resulted in refined grain sizes both in the Ti0.8V0.2 and Ti0.9V0.1 alloys. Furthermore, RS was found to increase the β-phase fraction in Ti0.9V0.1, being twice larger than that of the as-cast alloy. Ti0.9V0.1 had a platelike microstructure as observed by scanning electron microscopy (SEM), the plates were about 300 nm thick. The microstructure refinement resulted in a faster kinetics of H desorption as observed by temperature desorption spectroscopy (TDS).


