Trans. Nonferrous Met. Soc. China 24(2014) 723-728
Effect of vinylene carbonate as electrolyte additive on cycling performance of LiFePO4/graphite cell at elevated temperature
Hai-shen SONG1,2, Zheng CAO1, Zhi-an ZHANG1,2, Yan-qing LAI1,2, Jie LI1, Ye-xiang LIU1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Engineering Research Center of High Performance Battery Materials and Devices, Research Institute of Central South University in Shenzhen, Shenzhen 518057, China
Received 20 March 2013; accepted 25 June 2013
Abstract:
Effects of film-forming additive on stability of electrode and cycling performance of LiFePO4/graphite cell at elevated temperature were studied. Two 18650 cells with and without VC additive were investigated by galvanostatic cycling, electrochemical impedance spectroscopy, scanning electron microscopy, energy-dispersive X-ray analysis and Raman spectroscopy. The results show that in the presence of VC additive, dissolution of Fe from LiFePO4 material is greatly depressed and stability of graphite structure is improved; the additive can not only reduce reaction of electrolyte on surface of LiFePO4 electrode but also suppress reduction of solvent and thickening of the solid electrolyte interface (SEI) layer on graphite surface. Electrolyte with VC is considered to be a good candidate for improving cycling performance of the LiFePO4/graphite cell at elevated temperature.
Key words:
LiFePO4; vinylene carbonate; electrolyte additive; cycling performance;
1 Introduction
Lithium iron phosphate (LiFePO4) based lithium ion battery has attracted considerable attention for large scale applications in the automotive and space industries due to its well-known advantages of excellent chemical and thermal stability and low cost, and it is also one of attractive cells to meet the demand of long cycle life [1-3]. However, gradual capacity fade of the cell at elevated temperature has been observed [4-6]. In the past few years, extensive works have been conducted on capacity fade of LiFePO4/graphite cell [6-9], and numerous studies have been done on improving cycling stability of LiFePO4/grahphite cell, with a lot of methods involved, such as improvement of electrolyte, modification of materials, improved electrode or current collector [10-14]. In our previous work, capacity fade of LiFePO4/graphite cell at elevated temperature had been studied in detail [15]. It seems that stability of anode and cathode materials play importance roles in cycling performance of cells, and the rapid capacity fade of the cells at elevated temperature was attributed to the excessive formation of interfacial films which were produced on the graphite as a result of catalytic effects of the iron particles dissolved from cathode material and the damage to the surface SEI layer due to intrinsic volume changes in graphite; in other words, change of SEI layer due to structure stabilities of cathode and anode materials is the main source of capacity fade. So, in order to improve cycling performance of cell, stability of SEI layer should be enhanced, and at the same time, stability of cathode and anode materials should be also enhanced to decrease their damage to SEI layer.
In this study, cycling performance of LiFePO4/ graphite cell at elevated temperature was improved by addition of a film-forming additive, and effects of the additive on stability of active materials were highlighted by comparing the behavior of cells and structures of electrodes with or without vinylene carbonate (VC).
2 Experimental
2.1 Electrochemical measurements of LiFePO4/ graphite cells
Experiments were conducted on two similar 1.2 A·h 18650 LiFePO4/graphite cells without and with VC additive, respectively. A Land battery tester (CT2001A, Wuhan Land Electronic Co. Ltd., China) was used for cycling tests. The tests were performed at 55 °C. The cells were galvanostatically cycled at the charge current of 1C and discharge current of 3C between 2.0 and 3.85 V. For electrochemical impedance spectroscopy (EIS) tests, the state of charge (SOC) was first adjusted by partially discharging the cell (0.6 A·h of discharge from a fully charged state), after which it rested for 1 h before measurement was performed. The EIS was performed by perturbing the open-circuit potential with an AC sinusoidal potential of 5 mV and in a frequency range from 100 kHz to 10 mHz. The tests were performed using a 1470E Multi-Channel potentiostats.
2.2 Performance of Li/graphite cells
Electrochemical performance of the additive on graphite was investigated by use of two-electrode half-cells (2025 coin-type cell). The graphite electrode consisted of 80% (mass fraction) synthetic graphite (purity of 99 %, average particle size of 10 μm; supplied by Shanghai Shanshan Tech. Co., Ltd), 10% carbon black (average particle size of 40 nm; supplied by TIMCAL Graphite & Carbon) and 10% binder poly vinylidene fluoride (PVdF, HSV900; supplied by Arkema, France). The half-cells were assembled in a glove box and the electrolytes used in the experiments were 1 mol/L LiPF6/EC-DMC-EMC (mass ratio of 1:1:1) with or without 2% VC additive. The cells were evaluated for charge/discharge behavior under constant current conditions, and cycled galvanostatically at 1/20C (16.5 mA/g) over the range of 0.01-2.0 V.
2.3 Characterization of electrodes
To analyze the microstructures of active materials after cycling, the cells were disassembled in a glove-box under argon atmosphere after being fully discharged. The electrodes were extracted and washed in dimethyl carbonate (DMC) and then dried under vacuum, in order to remove the salt and solvent on the electrode. The scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) analyses were acquired on a FEI Quanta-200 system. Raman analysis was acquired on a Jobin-Yvon LabRAM HR-800, Horiba.
3 Results and discussion
3.1 Effect of VC on cycling performance of cells
Cycling performance of the 18650 cells with different electrolytes is shown in Fig. 1. It can be seen from that the discharge capacities of the cells degrade gradually with increasing the cycle number. And capacity of the cell without VC decreases to 70% of the initial value after 600 cycles at 55 °C, while the cell containing VC retains around 90% of the initial capacity after 600 cycles at the same temperature. The capacity fade is improved by addition of VC additive to the electrolyte. This is in consistent with the published results [3,4].
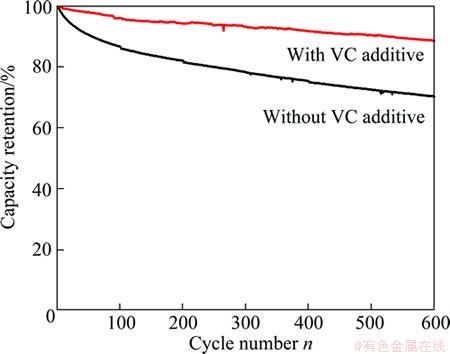
Fig. 1 Cycling performance of LiFePO4/graphite cells at 55 °C
In addition to the cycling tests, impedance measurements were also performed, as shown in Fig. 2. The AC impedances were measured before and after cycling at 55 °C, respectively. A typical spectrum shows two partially overlapped semicircles at high and middle frequencies, and a sloped-line-like diffusion behavior at low frequency. The depressed semi-circular arc is related to the capacitive behavior with a parallel resistance at the electrode/electrolyte interface, for instance, double-layer capacitance with a parallel charge transfer resistance and capacitance of SEI layer in parallel with its resistance [15].
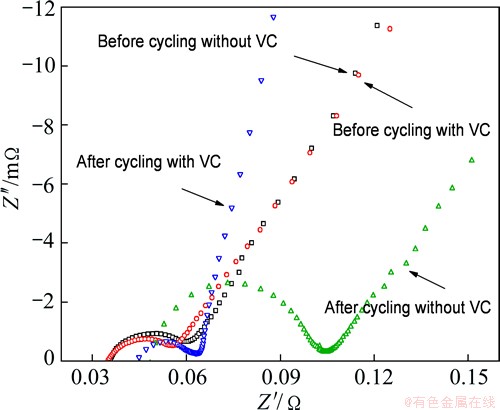
Fig. 2 Impedance spectra of LiFePO4/graphite cells with and without VC additive before and after cycling at 55 °C
The impedances of two cells are very similar before cycling. However, after cycling at 55 °C, the impedance of the cell without VC increases significantly, while that containing VC additive changes slightly. The difference between impedances suggests that the structures of interfacial films are different from each other after cycling [16,17].
3.2 Effect of VC on morphology of electrodes
After cycling at 55 °C, the cells were fully discharged at 1/25C before being disassembled in the glove-box for post-test diagnostic analysis. SEM images of the pristine cathode electrode and the cathode electrodes from cells with and without VC are shown in Fig. 3. Figure 3(a) shows SEM micrograph of the pristine electrode, clearly exhibiting the larger grains of the LiFePO4 particles with an average size of a few micrometers. Figure 3(b) shows the LiFePO4 electrode from cell without VC. Here, a paste like deposited film is observed on surface of the particles, and the deposition virtually buries the large-grain texture of the pristine electrode, effectively sealing the electrode surface from direct contact with the electrolyte. Figure 3(c) shows the LiFePO4 electrode morphology from cell with VC additive. The image shows an appearance between the pristine electrode and the electrode from cell without VC, and most of the large smooth LiFePO4 particles are seen in the pristine electrode. The result indicates that the presence of VC in electrolyte depresses deposition on surface of cathode.
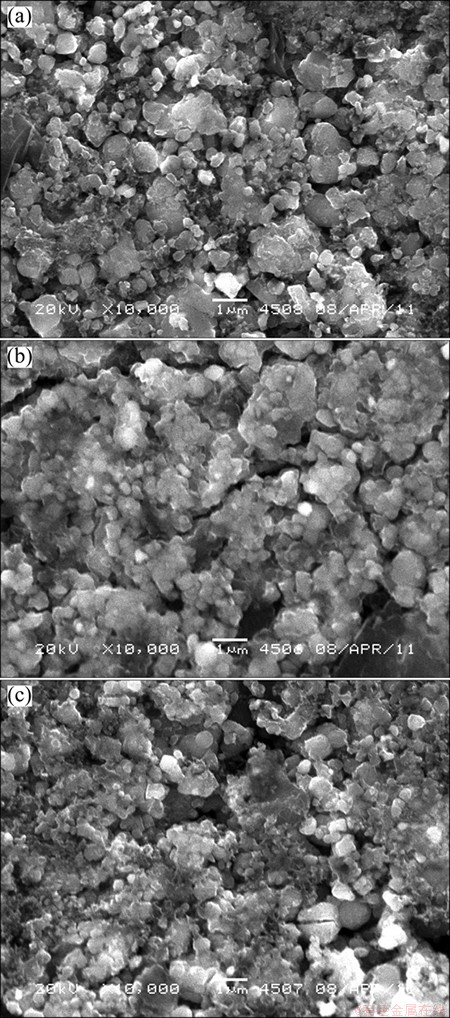
Fig. 3 SEM micrographs of fresh cathode (a) and cathodes cycled without VC (b) and with VC (c) 600 cycles at 55 °C
Figure 4 shows the morphologies of the graphite electrode surface from cells without and with VC additive after cycling tests. The anode from cell without VC, of which the cell shows a faster capacity fading, is covered with a continuous SEI layer so that the graphite particles underneath can no longer be seen. On the contrary, the anode from cell with VC has an amount of SEI layer which is less than the former, and the graphite particles can be partially seen. The results indicate that the presence of VC in electrolyte significantly depresses decomposition of electrolyte on graphite anodes.
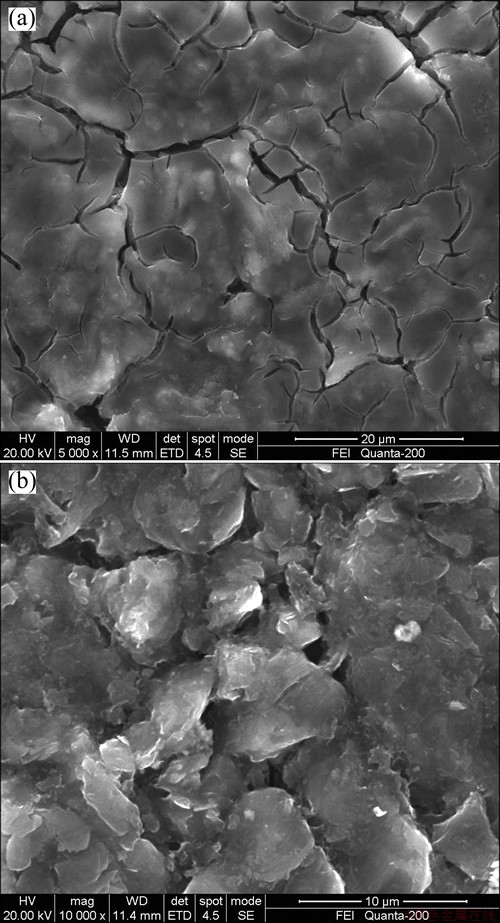
Fig. 4 SEM micrographs of anode in cells without VC (a) and with VC (b) after 600 cycles at 55 °C
3.3 Effect of VC on performance of graphite/Li half-cell
In order to elucidate the effect of VC additive, electrochemical half-cell tests for graphite electrode were carried out. Figure 5 shows the voltage profiles of graphite electrodes with and without VC additive respectively. During the first charging of the battery, electrolyte solvents and salt are reduced at anode/electrolyte interface, and a solid electrolyte interface is formed [18]. The protective layer prevents a substantial reaction between the low voltage negative and the organic electrolyte [2]. The cathodic processes, especially in the first cycle, include both Li insertion into graphite and reduction of solution species to surface films that passivate the electrodes, while the anodic processes relate to lithium deintercalation from graphite [18]. The irreversible capacity implies charges consumed in formation of SEI layer.
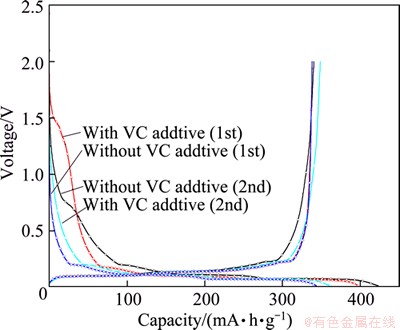
Fig. 5 Voltage profiles of graphite electrodes in VC containing and VC-free electrolytes
For the VC-free cell, an irreversible capacity of 83 mA·h/g is observed at the first cycle, and a coulombic efficiency of 80.4 % is obtained. While for cell with VC additive, the irreversible capacity is about 60 mA·h/g, and a coulombic efficiency of 85% is observed, which is much higher than the former. Obviously, the presence of VC decreases the irreversible capacity involved in the first cathodic process. In the second cycle, the coulombic efficiency is improved to 96.4% for the VC-free cell; and the coulombic efficiency is 98.4% for cell with VC, with still a higher efficiency. The elevated coulombic efficiency implies that the presence of VC suppresses the irreversible charge that relates to the passivation of graphite electrode.
By comparing the first discharge curves of two half-cells in detail, the effect of VC on SEI modification is clarified. The potential of the first turning point of each discharge curve indicates the reduction activity of each electrolyte [19]. For electrolyte with VC, the potential is about 1.4 V, which is in concert with result by SATO et al [19] and ZHANG et al [20]. For electrolyte without VC, the potential is about 0.7 V, which is lower than that of electrolyte with VC. Obviously, the electrolyte with VC additive has much higher reduction activity than the pristine electrolyte, which might be the key effect of VC. Due to reduction of VC on the graphite surface, the excessive reduction of electrolyte is depressed effectively, and coulombic efficiency of cells is elevated.
Surface morphologies of the fresh graphite anode and anodes after 2 cycles are shown in Fig. 6. The fresh anode shows a clear graphite particle with a little adhesive and conductive agent on surface. While surfaces of the anodes after two cycles without and with additive are obviously covered with a SEI film, with an obscure boundary between graphite particles. What’s more, the deposited material on the graphite surface from half-cell with VC is more than that without VC, which would be helpful for formation of a necessary compact SEI film, and this is in concert with Fig. 5.
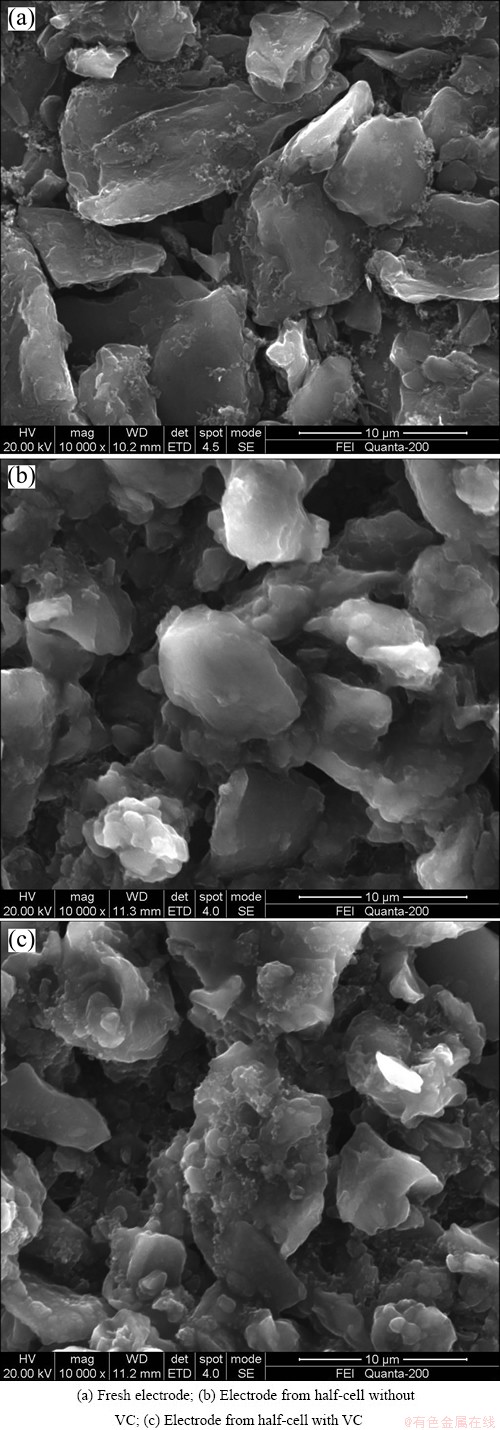
Fig. 6 SEM micrographs of fresh anode and anodes after 2 cycles
3.4 Effect of VC on stability of cathode and anode materials
LiFePO4 was found to release iron ion when aged at high temperature, and subsequently the iron ions deposited on the graphite anode, which accelerates decomposition of electrolyte and results in thick SEI layer [4,10,15]. To investigate the effects of VC on dissolution of Fe from LiFePO4, surfaces of the negatives from full cells were analyzed by EDX technique. The EDX spectra show the presence of Fe species on both graphite surfaces, as shown in Fig. 7. And it can be clearly seen that VC additive decreases the amount of Fe species on surface of graphite. It can be deduced that VC enhances stability of LiFePO4 material.
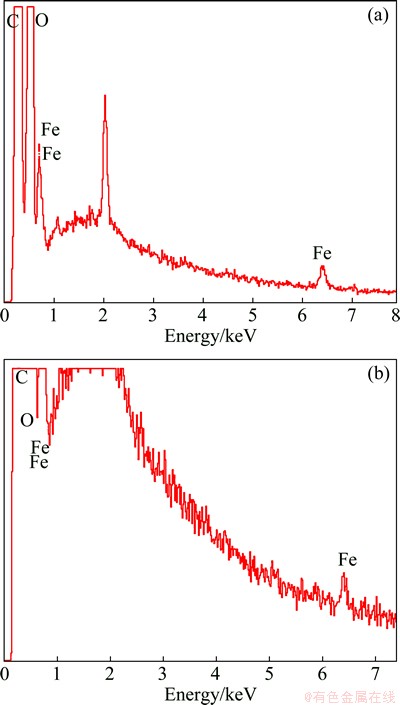
Fig. 7 EDX analyses of graphite electrodes surfaces from full cells without (a) and with (b) VC additive (The contents of Fe species are 0.41% and 0.1%, respectively)
The graphite experiences great change in volume during battery cycling. And the damage to the graphite SEI layer due to the intrinsic volume changes is one of the reasons for consumption of active lithium [2]. In order to investigate the effect of VC on stability of graphite, the structure of graphite was analyzed via Raman spectroscopy. A typical Raman spectrum of graphite consists of two peaks: a strong G band at ~1582 cm-1 and a weak D band at ~1360 cm-1. This D band is associated with the breakage of symmetry occurring at the edges of the graphite sheets. Thus, the more the edges present, the more the phonons that can result in Raman scattering are, and the more intense the D band becomes. The D/G band ratio always increases with cycling for all of the tested anodes, compared with the fresh electrodes. The relative intensity of the D band versus G band is attributed to the level of carbon disorder in microcrystalline graphite and is inversely proportional to the intra-planar microcrystallite distance La [21].
Average Raman spectra of a fresh graphite anode, the cycled anodes from full cells with and without VC additive are shown in Fig. 8. Two broad bands are present at ~1360 and ~1580 cm-1 after cycling. The relative D and G peak heights change noticeably; however, the anode cycled in the electrolyte with VC displays less disordered carbon on the surface at the end of testing than the anode removed from cell cycled in the electrolyte without VC. The D/G band ratio decreases by adding VC in the electrolyte, indicating a smaller structural change occurring in the aonde with VC. Due to the stable SEI layer formed on surface of anode in electrolyte with VC, stability of graphite is improved and excessive consumption of active lithium due to the breakage of SEI layer is prevented, which leads to a lower capacity decreasing.

Fig. 8 Average Raman spectra of graphite anodes
4 Conclusions
1) Capacity of the cell without VC decreases to 70% of the initial value after 600 cycles at 55 °C, while the cell containing VC retains around 90% of the initial capacity at the same temperature.
2) Deposition on surface of LiFePO4 cathode is reduced by addition of VC, with a decreased dissolution of Fe from LiFePO4 material.
3) Due to the reduction of VC, the reduction of electrolyte on graphite surface is reduced, and stability of graphite is also improved by a optimized SEI layer.
4) Enhanced stability of SEI layer and active materials prevent excessive consumption of active lithium and subsequently, cycling performance of the LiFePO4/graphite cells at elevated temperature is improved.
References
[1] GU Yi-jie, ZENG Cui-song, WU Hui-kang, CUI Hong-zhi, HUANG Xiao-wen, LIU Xiu-bo, WANG Cui-ling, YANG Zhi-ning, LIU Hong. Enhanced cycling performance and high energy density of LiFePO4 based lithium ion batteries [J]. Materials Letters, 2007, 61: 4700-4702.
[2] LIU Ping, WANG J, HICKS-GARNER U, SHERMAN E, SOUKIAZIAN S, VERBRUGGE M, TATARIA H, MUSSER J, FINAMORE P. Aging mechanisms of LiFePO4 batteries deduced by electrochemical and structural analyses [J]. Journal of the Electrochemical Society A, 2010, 157: 499-507.
[3] TANG Hao, TAN Long, XU Jun. Synthesis and characterization of LiFePO4 coating with aluminum doped zinc oxide [J]. Transaction of Nonferrous Metals Society of China, 2013, 23: 451-455.
[4] AMINE K, LIU J,
[5] SAFARI M, DELACOURT C. Aging of a commercial graphite/LiFePO4 cell [J]. Journal of the electrochemical Society A, 2011, 158(10): 1123-1135.
[6] CASTRO L, DEDRYVERE R, LEDEUIL J B, BREGER J, TESSIER C, GONBEAU D. Aging mechanisms of LiFePO4//graphite cells studied by XPS: Redox reaction and electrode/electrolyte interfaces [J]. Journal of the Electrochemical Society A, 2012, 159(4): 357-363.
[7] KOLTYPIN M, AURBACH D, NAZAR L, ELLIS B. On the Stability of LiFePO4 Olivine cathodes under various conditions (electrolyte solutions, temperature) [J]. Electrochemical and Solid-State Letters A, 2007, 10(2): 40-44.
[8] ZAGHIB K, RAVET N, GAUTHIER M, GENDRON F, MAUGER A, GOODENOUGH J B, JULIEN C M. Optimized electrochemical performance of LiFePO4 at 60 °C with purity controlled by SQUID magnetometry [J]. Journal of Power Sources, 2006, 163: 560-566.
[9] KIM J H, WOO S C, PARK M S, KIM K J, YIM T, KIM J S, KIMY J. Capacity fading mechanism of LiFePO4-based lithium secondary batteries for stationary energy storage [J]. Journal of Power Sources, 2013, 229: 190-197.
[10] CHANG C C, CHEN T K, HER L J, FEY G T K. Tris (pentafluorophenyl) borane as an electrolyte additive to improve the high temperature cycling performance of LiFePO4 cathode [J]. Journal of the Electrochemical Society A, 2009, 156(11): 828-832.
[11] CHANG H H, WU H C, WU N L. Enhanced high-temperature cycle performance of LiFePO4/carbon batteries by an ion-sieving metal coating on negative electrode [J]. Electrochemistry Communications, 2008, 10: 1823-1826.
[12] STRIEBEL K, SHIM J, SIERRA A, YANG H, SONG X Y, KOSTECKI R, McCARTHY K. The development of low cost LiFePO4-based high power lithium-ion batteries [J]. Journal of Power Sources, 2005, 146: 33-38.
[13] LI W T, LUCHT B L. Inhibition of solid electrolyte interface formation on cathode particles for lithium-ion batteries [J]. Journal of Power Sources, 2007, 168: 258-264.
[14] LIU H, WANG G X, WEXLER D, WANG J Z, LIU H K. Electrochemical performance of LiFePO4cathode material coated with ZrO2nanolayer [J]. Electrochemistry Communications, 2008, 10: 165-169.
[15] SONG Hai-shen, CAO Zheng, CHEN Xiong, LU Hai, JIA Ming, ZHANG Zhi-an, LAI Yan-qing, LI Jie, LIU Ye-xiang. Capacity fade of LiFePO4/graphite cell at elevated temperature [J]. Journal of Solid State Electrochemistry, 2013, 17: 599-605.
[16] JOACHIN H, KAUN T D, ZAGHIB K, PRAKASH J. Electrochemical and thermal studies of carbon-coated LiFePO4 cathode [J]. Journal of the Electrochemical Society A, 2009, 156(6): 401-406.
[17] SHIM J, STRIEBEL K A. Cycling performance of low-cost lithium ion batteries with natural graphite and LiFePO4 [J]. Journal of Power Sources, 2003, 119-121: 955-958.
[18] HERSTEDT M, STJERNDAHL M, GUSTAFSSON T, EDSTOM K. Anion receptor for enhanced thermal stability of the graphite anode interface in a Li-ion battery [J]. Electrochemistry Communications, 2003, 5: 467-472.
[19] SATO K, ZHAO L, OKADA S, YANAKI J I. LiPF6/methyl difluoroacetate electrolyte with vinylene carbonate additive for Li-ion batteries [J]. Journal of Power Sources, 2011, 196: 5617-5622.
[20] ZHANG X, KOSTECKI R, RICHARDSON T J, PUGH J K, ROSS P N. Electrochemical and infrared studies of the reduction of organic carbonates [J]. Journal of the Electrochemical Society A, 2001, 148(12): 1341-1345.
[21] TAKAHASHI M, TOBISHIMA S I, TAKEI K, SAKURAI Y. Reaction behavior of LiFePO4 as a cathode material for rechargeable lithium batteries [J]. Solid State Ionics, 2002, 148: 283-289.
碳酸亚乙烯酯添加剂对LiFePO4/石墨电池高温循环性能的影响
宋海申1,2,曹 政1,张治安1,2,赖延清1,2,李 劼1,刘业翔1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 深圳研究院 深圳高性能电池材料与器件工程研究中心,深圳 518057
摘 要:研究成膜添加剂对材料结构稳定性及LiFePO4/石墨电池高温循环性能的影响。分别测试添加和未添加碳酸亚乙烯酯(VC)的18650电池的高温循环性能,并通过充放电测试、交流阻抗、扫描电镜、X射线能量色散光谱以及拉曼光谱等方法研究VC对电池正、负材料结构的影响。结果表明:VC添加剂在提高石墨结构稳定性的同时显著抑制LiFePO4材料中的溶铁行为;此外,VC添加剂阻止电解液在负极表面还原分解及负极表面SEI膜的增厚,也阻止电解液在LiFePO4电极表面的分解;含有VC添加剂的电解液可以有效改善LiFePO4/石墨电池在高温下的循环稳定性。
关键词:LiFePO4;碳酸亚乙烯酯;电解液添加剂;循环性能
(Edited by Chao WANG)
Foundation item: Project (2007BAE12B01) supported by the National Key Technology Research and Development Program of China; Project (20803095) supported by the National Natural Science Foundation of China
Corresponding author: Zhi-an ZHANG; Tel: +86-731-88830649; E-mail: zza75@163.com
DOI: 10.1016/S1003-6326(14)63117-4
Abstract: Effects of film-forming additive on stability of electrode and cycling performance of LiFePO4/graphite cell at elevated temperature were studied. Two 18650 cells with and without VC additive were investigated by galvanostatic cycling, electrochemical impedance spectroscopy, scanning electron microscopy, energy-dispersive X-ray analysis and Raman spectroscopy. The results show that in the presence of VC additive, dissolution of Fe from LiFePO4 material is greatly depressed and stability of graphite structure is improved; the additive can not only reduce reaction of electrolyte on surface of LiFePO4 electrode but also suppress reduction of solvent and thickening of the solid electrolyte interface (SEI) layer on graphite surface. Electrolyte with VC is considered to be a good candidate for improving cycling performance of the LiFePO4/graphite cell at elevated temperature.


