Trans. Nonferrous Met. Soc. China 26(2016) 3238-3244
Self-magnetization of pyrite and its application in flotation
Xi-qing WU, Xin XIE, Yang-fan CAO
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 18 January 2016; accepted 18 May 2016
Abstract:
Pyrite is a special weakly magnetic mineral containing Fe(II). Its self-magnetization only by adjusting slurry temperature and pH value was able to enhance its magnetism, producing the so-called the magnetized pyrite, which was further used as magnetic seeds in the flotation of pyrite ore to promote flotation recovery. Tests, such as self-magnetization, vibrating sample magnetometer (VSM), XPS, size analysis and flotation were carried out. The optimal conditions of the pyrite self-magnetization were pulp pH of 11.81 and temperature of 65 °C. The magnetized pyrite was characteristic of the valence change of elemental iron, resulting in stronger magnetism of the magnetized pyrite than that of the original pyrite. Then, this magnetized pyrite was applied to the magnetic seeding flotation (MSF) of pyrite ore. It was found that the recovery of pyrite flotation grew with the increase of magnetic susceptibility of the magnetic seeds-the magnetized pyrite; and the proper dosage of the magnetized pyrite was 100 g/t. The reason behind the increased recovery lies in that the magnetized pyrite promoted the magnetic agglomeration between fine pyrite particles; and the fact that the stronger the magnetism of the magnetized pyrite, the larger the aggregate size, indicates that the agglomeration is somewhat in line with the flotation, also confirming that the MSF is more suitable for fine particles than traditional flotation.
Key words:
pyrite; self-magnetization; magnetized pyrite; magnetic agglomeration; magnetic seeding flotation;
1 Introduction
As to magnetism, neither low spinning state ferrous atom nor sulphur atom in pyrite (FeS2) is magnetic whilst pyrite mineral is a weekly magnetic mineral. In general, the ratio of iron to sulphur in natural pyrite deviates from theoretical value, and elements like Mn and Co can easily occur in pyrite with isomorphism. In this way, the magnetism of pyrite stems from these concomitant paramagnetic elements and the specific susceptibility of pyrite is about (11.30-70.36)×10-9 m3/kg [1].
Pyrite is the most widely distributed sulphide in the crust, often intergrowing with copper, lead, zinc and other nonferrous metallic minerals. Therefore, the study of pyrite flotation has been a basic issue for the polymetallic sulfide ores’ separation. Pyrite is a sulfide mineral with a good electric conductivity, and its floatability is heavily related to its semiconductor properties, iron-sulfur ratio, thermoelectric potential and pulp pH value. The actual ratios of iron to sulphur in pyrite are always some departures from the theoretical one of 1:2, and the different values of the ratio lead to varied floatability. What’s more, the reasons for causing the ratio differences also have certain influences on the floatability of pyrite [2-4]. The closer the sulphur-iron ratio is to 2, the better floatability of pyrite will be [5]. When the sulphur-iron ratio is less than 2, the pyrite should be a P-type semiconductor, which has high thermoelectric potential, a better floatability in alkaline medium, a worse floatability in acidity medium and is hard to be depressed by calcium ion and sodium sulphide. In contrast, while suphur-iron ratio being larger than 2, the properties above mentioned will be reversed, i.e., N-type semiconductor with negative thermoelectric potential, bad floatability and being easily depressed by calcium ion and sodium sulphide [6]. Besides, the electrochemical properties and their influence on pyrite floatability have been studied and in highly basic potential control technology [7,8] lime was used as a depressant for pyrite. On the basis of sulphide flotation electrochemistry, BUSWELL et al [9], WOODS [10] and LI et al [11] studied the mechanism of pyrite surface oxidation to form  , Fe(OH)3 and other hydrophilic substances due to the decrease of mineral surface oxidation potential under high alkali conditions. In a pyrite and xanthate system, the electrochemical reaction of xanthate, catalyzed by pyrite, generates dixanthohen on pyrite surface, which is the hydrophobic entity on the pyrite surface [12]. KUOPANPORTTI et al [13] found that dissolved oxygen concentration of the pulp affects the rate of adsorption of xanthate on pyrite surface, resulting in decreased flotation recoveries of pyrite.
, Fe(OH)3 and other hydrophilic substances due to the decrease of mineral surface oxidation potential under high alkali conditions. In a pyrite and xanthate system, the electrochemical reaction of xanthate, catalyzed by pyrite, generates dixanthohen on pyrite surface, which is the hydrophobic entity on the pyrite surface [12]. KUOPANPORTTI et al [13] found that dissolved oxygen concentration of the pulp affects the rate of adsorption of xanthate on pyrite surface, resulting in decreased flotation recoveries of pyrite.
In the flotation process of polymetallic sulphide ores, the widely accepted technology is first recovering the valuable metal elements by depressing pyrite, then activating sulfur. Single pyrite ores are easy to treat with bulk flotation flowsheet, single gravity process or gravity-flotation process to achieve different grades of sulphur concentrates in concentrator [14]. In common sense, it is hard to effectively recover the minerals with particle sizes less than 19 μm, and the size limit for conventional flotation process is 10 μm. CHENG et al [15] used the swirlling jet flotation column to reclaim micro-fine pyrite and found that the flotation column had a better performance than flotation machine dealing with particles less than 19 μm.
The author has disclosed a method of utilizing “self-magnetization method” to prepare magnetic sulphide minerals [16]. The Fe2+ ions on mineral surface were partly oxidized to generate magnetic particles (namely Fe3O4) by controlling pulp pH and temperature. The newly generated magnetic particles could enhance surface magnetism of minerals and the magnetized pyrite mineral was used in magnetic seeding flotation (MSF) to recover micro-sized minerals [17].
This work is aimed to further study the self-magnetization of pyrite, and then find the influence of generated magnetized pyrite on flotation of pyrite ore.
2 Experimental
2.1 Samples and reagents
The pure pyrite mineral was obtained from the YunFu Pyrite Group Limited, Guangdong, China, with purity of 96.43% and mainly used for self-magnetization tests and magnetism measurements.
The pyrite ore does not substantially associate with other non-ferrous metals and its multi-elemental analysis results are shown in Table 1.
Table 1 Sample multi-elemental analysis results (mass fraction, %)

As can be seen from Table 1, the main elements are sulphur, iron and silicon, while the other elements have a very limited content; the main valuable mineral is pyrite and the main gangue mineral is silicate. This raw ore is a pyrite ore with high-sulfur grade and is mainly used for flotation and agglomeration tests.
Butyl xanthate was used as collector, and terpineol was used as frother in the flotation. Besides, sulfuric acid and sodium hydroxide used in all experiments were analytical reagents.
2.2 Methods
2.2.1 Self-magnetization
5 g of pure pyrite mineral was ground to a mean particle size of 21 μm, then put into a 500 mL beaker. After adding 200 mL deionized water, the resultant pulp was kept in agitation in a constant temperature water bath for 8 min. The influences of two variables, pH value and temperature, on pyrite self-magnetization were tested. The magnetized pyrite hereafter refers to the product of self-magnetization with a higher magnetism than the pure pyrite.
2.2.2 Magnetism measurement by VSM
The self-magnetization took place in different pH value and temperature conditions, and the magnetized pyrite went through a magnetic tube separation to get rid of the free Fe3O4 particles with a magnetic field of 0.15 T. After filtration, drying and sampling preparation, the hysteresis loop was obtained by HH-15 vibrating sample magnetometer (VSM).
2.2.3 X-ray photoelectron spectroscopy (XPS)
The magnetized pyrite sample for XPS test was prepared as for VSM described above. Then, the Fe 2p orbital electronic binding energy and mineral surface elemental content variations before and after the self-magnetization were analyzed by Kα X-ray photoelectron spectroscopy.
2.2.4 Magnetic seeding flotation (MSF)
In comparison with traditional flotation, magnetic seeding flotation includes the following sub-stages: adding magnetic seeds into pulp, the subsequent pre-magnetization and aeration of flotation. 200 g of pyrite ore with 82% of grain less than 0.074 mm and some tap water were added into the stirring magnetizer [18] to form pulp with the mass concentration of 40% and conditioning for 3 min, adding concentrated sulfuric acid, the magnetized pyrite (used as magnetic seeds), butyl xanthate and pine oil in order. After pre-magnetizing the suspension, the pulp was transferred into flotation cell (0.5 L) for aeration of flotation process.
2.2.5 Laser particle size analyzer
After putting pyrite ore with an average grain size of 23 μm and water into the stirring magnetizer and keeping stirring for 3 min to make the suspension fully disperse, the magnetized pyrite was added as magnetic seeds, conditioning further for 3 min and then shifting the suspension to a 100 mL beaker as the sample of laser size analyzer. The measuring instruments was the Mastersizer2000 type laser particle size analyzer.
3 Results and discussion
3.1 Self-magnetization of pyrite
3.1.1 Self-magnetization of pyrite and measurement of magnetism
Magnetization level of substance in a magnetic field can be expressed by magnetization intensity. For a certain magnetic field intensity applied, the higher the magnetization intensity is, the larger the specific susceptibility is as well as magnetism of the substance. The hysteresis loops of pyrite before and after magnetization are shown in Fig. 1. It can be seen from Fig. 1 that the original pyrite has a very weak magnetic property and its saturation magnetization intensity (σ) is 0.067 A·m2/kg. The saturation magnetization intensity reaches 0.274 A·m2/kg after magnetization at a pH of 11.81 and a temperature of 65 °C.

Fig. 1 Hysteresis loops of pyrite before and after magnetization
A series of self-magnetization experiments of pyrite were carried out in pulp pH range of 10.36-13.30 and pulp temperature range of 25-85 °C and mean particle size range of 21-50 μm, and then the magnetisms of products were tested by vibrating sample magnetometer (VSM). The results are shown in Figs. 2 and 3.
Figure 2 indicates that the optimum conditions of pyrite self-magnetization are at pH value 11.81 and a pulp temperature of 65 °C in terms of the change of magnetization intensity or their saturation magnetization.
It can be seen from Fig. 3 that at the fixed pH value 11.81 and pulp temperature of 65 °C, the specific saturation magnetization of the magnetized pyrite increases with decreasing the particle size of pyrite.

Fig. 2 Influence of pH and temperature on pyrite magnetization
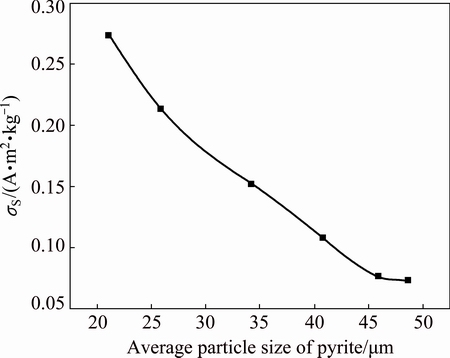
Fig. 3 Influence of particle size of pyrite on pyrite magnetization at pH value 11.81 and pulp temperature of 65 °C
The essence of the magnetization on pyrite surface is to oxidize bivalent iron ions on pyrite surface partially to generate magnetic Fe3O4, which makes the pyrite surface magnetism enhance without destroying the basic shape of mineral particle surfaces. The magnetization process can be divided into two successive stages: the oxidation reaction on pyrite surface to generate Fe(OH)2 in strong alkaline solution as the first stage; Fe(OH)2 being further oxidized to Fe3O4 phase at the second stage. Wherein the reaction equations of the first stage are as follows [19]:
FeS2+5H2O→Fe(OH)2+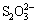 +8H++6e (1)
+8H++6e (1)
FeS2+10H2O→Fe(OH)2+ +18H++14e (2)
+18H++14e (2)
For the second stage [20]:
6Fe(OH)2+O2→2Fe3O4+6H2O (3)
4Fe(OH)2+O2+2H2O→4Fe(OH)3 (4)
2Fe(OH)3+Fe(OH)2→Fe3O4+4H2O (5)
Further, Fe (OH)2 in the oxidation process also likely forms γ-FeOOH and α-FeOOH while the main formation is Fe3O4 on suitable conditions.
3.1.2 Surface characterization of magnetized products by XPS
X-ray photoelectron spectroscopy was used to measure pyrite surface compositions before and after the magnetization in the condition of pH 11.81 and temperature of 65 °C. The Fe 2p orbital electron energy spectra are shown in Fig. 4. A significant offset of Fe 2p electron binding energy can be observed. Fe 2p binding energy of the electron orbit and surface elemental composition are shown in Table 2. There is an augment of Fe 2p orbital electron binding energy after magnetization, which increases from 707.69 eV (before magnetization) to 708.59 ev (after magnetization). The increase of pyrite electronic Fe 2p orbit binding energy indicates that the iron valence on pyrite surface changes before and after treatment, meaning a chemical shift and that Fe2+ is partly oxidized to Fe3+. The contribution from Fe(III)-O typically occurs between 710.8 and 713.8 eV binding energy [21].

Fig. 4 Fe 2p electronic energy spectra on surface of pyrite before and after magnetization
Table 2 XPS data of pyrite before and after magnetization

The molar ratio Fe:S:O in pyrite before treatment is 1:1.739:1.884, and 1:1.739:1.884 after the treatment. The content of Fe element can be considered approximately constant, while the content of O element has increased mainly due to the oxidation of Fe(II)-S and generation of Fe3O4 on pyrite surface after magnetization.
3.2 Application of magnetized pyrite in flotation
3.2.1 Magnetic seeding flotation (MSF)
The author has disclosed a magnetic seeding flotation method [17]. Compared to conventional flotation, the main differences are the adding of magnetic seeds and pre-magnetizing operation. Reagents interact with magnetic seeds to form magnetic seeds having functional groups (active magnetic seeds) and reagents with magnetism (magnetic reagents). The magnetic adsorption and magnetic interaction force between particles and particles along with reagents and particles have been promoted. Therefore, the pre-magnetic field can enhance the oriented adsorption of magnetic reagents on the target minerals or the interaction force between magnetic seeds and particles, resultantly increasing the granularity of mineral particles (agglomeration). What’s more, it can improve flotation efficiency (recovery or selectivity) and reduce reagent dosage. The magnetized pyrite used in this MSF is a kind of special magnetic seeds. The magnetized pyrite was obtained by self-magnetization under the optimal conditions (pH=11.81 and T=65 °C) and pre-magnetic field intensity was 0.05 T unless otherwise noted.
1) Influence of magnetized pyrite dosage on pyrite ore flotation. The magnetism of the magnetized pyrite is larger than pyrite itself. A set of experiments took the magnetized pyrite as magnetic seeds and attempted to find its influence on pyrite ore flotation. The recovery of pyrite flotation increased obviously initially with the dosage increase and then gradually decreased with the continuous increasing of magnetized pyrite dosage,and the optimal dosage is 100 g/t, as shown in Fig. 5.
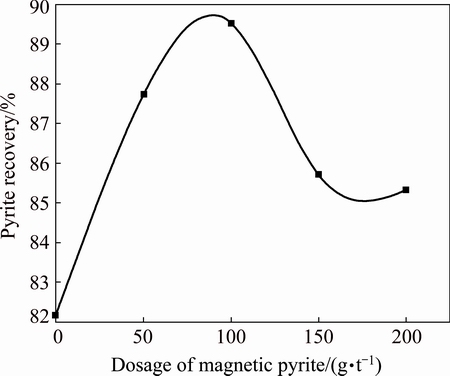
Fig. 5 Influence of magnetized pyrite dosage on pyrite ore flotation
2) Influence of pre-magnetization intensity on pyrite ore flotation. Pre-magnetization is a very important feature of magnetic seeding flotation (MSF). The following experiments were aimed at finding the influence of pre-magnetization field intensity on pyrite ore flotation and the magnetized pyrite dosage was fixed at 100 g/t. The result is shown in Fig. 6. The pre-magnetization field intensity had little effect on pyrite recovery without adding magnetized pyrite, but the pyrite recovery sharply increased and then slowly declined and stabilized in the end with the augment of pre-magnetization field intensity in the presence of magnetized pyrite (Fig. 6). The result is consistent with the conclusion of magnetic seeding flotation of coal slimes studied by DAI [22]. Thus, it can be derived that magnetic seed is a more sensitive factor than pre-magnetization intensity as for flotation system. From this point of view, magnetic seed can be considered as a kind of “physical reagent” in contrast to universally used chemical reagents.

Fig. 6 Influence of pre-magnetization field intensity on pyrite ore flotation
3) Influence of magnetized pyrite with different magnetic susceptibility on pyrite ore flotation. It was seen from the self-magnetization experiments that magnetized pyrite with different magnetization levels was generated under varied conditions (the pulp temperature and pH value). In this section, the experiments were designed to investigate the influence of magnetized pyrite that had different magnetic susceptibility on flotation in a fixed magnetized pyrite dosage of 100 g/t. The result is shown in Fig. 6. The pyrite recovery constantly improved with the increase of magnetic susceptibility (Fig. 7).
3.2.2 Influence of magnetized pyrite on agglomeration of micro-sized pyrite ore
As mentioned above, the floatability of pyrite decreases in an alkaline environment. So, the magnetized pyrite generated by self-magnetization in an alkaline environment could have a weaker floatability than nature pyrite. On the contrary, the flotation experiments presented above suggest that the magnetic seeding flotation (by adding magnetized pyrite into the pulp) can enhance flotation recovery, indicating that the magnetized pyrite particle floatability may not be the factor that matters much. Besides, the magnetic pyrite with an average grain size of 21 μm have a small granularity than pyrite particles, suggesting that the magnetized pyrite is not playing the role of carrier like in carrier flotation. DAI [22] operated magnetic seeding flotation on coal slimes and found that magnetic seeds contributed to the agglomeration of coal slimes. The granularity of pyrite ore with an average grain size of 23.15 μm after being treated by magnetized pyrites with varied magnetic susceptibility was measured by laser particle analyzer. The particle size change was analyzed to find whether the magnetized pyrite has the same agglomeration effect in pyrite suspension. The magnetized pyrite dosage was fixed at 100 g/t. The result is shown in Fig. 8. The average particle size of the ore increases with the increase of magnetic susceptibility.

Fig. 7 Influence of magnetic susceptibility of magnetized pyrite on pyrite ore flotation

Fig. 8 Influence of magnetic susceptibility of magnetized pyrite on ore particle size
LIN et al [23] calculated the magnetic agglomeration interaction force between magnetic particles.
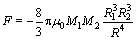 (6)
(6)
where μ0 is the vacuum permeability; M1 and M2 are the magnetizations of the magnetic particles; R1 and R2 are the radii of the magnetic particles; R is distance between the two magnetic particles.
It can be inferred from Eq. (6) that magnetic agglomeration force increases with the increasing of particles magnetization intensity and the increased agglomeration force enables fine particles to agglomerate by magnetic field more easily. In this way, while the magnetic susceptibility of magnetized pyrite increases the magnetic agglomeration force between magnetized pyrite and pyrite particles become larger and contribute to a continuous increase of ore average particle size by magnetic agglomeration.
FANG et al [24] studied the interaction of micro-fine hematite in a very weak magnetic field, and discovered that magnetic agglomeration between weak magnetic mineral particles can occur in a very weak magnetic field. In a magnetic field, magnetic seeds and magnetic minerals can generate magnetic field in a very small distance around them. The surface of magnetic seeds which seems like a kind of steel wool in high gradient magnetic separator has high gradient effect [25]. The magnetic agglomeration occurred between fine pyrite particles and led to an enlargement of size range due to combination of the high gradient effect on the surface of magnetic pyrite and the pre-magnetization field. Therefore, magnetized pyrite can promote the magnetic agglomeration between fine pyrite mineral particles under magnetic field. Comparing Figs. 7 and 8, it is obvious that the variation tendencies of flotation recovery and agglomeration product size are the same with that of magnetized pyrite magnetic susceptibility.
4 Conclusions
1) The optimal conditions for self-magnetization of pyrite were pulp pH of 11.81 and a temperature of 65 °C. After the magnetization of pyrite, the magnetism became stronger and saturation magnetization increased significantly, and the Fe 2p orbital electron binding energy of pyrite mineral increased from 707.69 eV (before magnetization) to 708.59 eV (after magnetization) and chemical shifts of Fe 2p happened, suggesting that iron valence changed, i.e., Fe2+ partly being oxidized to Fe3+, and magnetic Fe3O4 formed on pyrite surface.
2) In an external weakly magnetic field system, the magnetized pyrite significantly improved the flotation recovery of pyrite ore, and the recovery of pyrite flotation grew with the increase of magnetic susceptibility of the magnetic seeds-magnetized pyrite; and the proper dosage of the magnetized pyrite was 100 g/t.
3) In an external weakly magnetic field system with magnetic field intensity of 0.05 T, the addition of magnetized pyrite could facilitate the agglomeration between fine mineral particles, enlarging the average apparent size of pyrite fines in a direct proportion with the magnetic susceptibility of the magnetized pyrite increases. So, the magnetized pyrite promoted the magnetic agglomeration between fine pyrite particles, resulting in proper flotation.
References
[1] TANG Yue-gang, REN De-yi, ZHENG Jian-zhong, GUO Mong-xiong, RONG Xi-lai, NI Yong-ming. The research on magnetism and mechanism on pyrite in coal [J]. Chinese Science Bulletin, 1995, 40(16): 1483-1486. (in Chinese)
[2] SIRKECIA A. The flotation separation of pyrite from arsenopyrite using hexyl thioethylamine as collector [J]. International Journal of Mineral Processing, 2000, 4(60): 263-276.
[3] ABRAITIS P K, PATTRICK R A D, VANSHAN D J. Variations in the compositional, textural and electrical properties of natural pyrite: A review [J]. International Journal of Mineral Processing, 2004, 3(74): 41-59.
[4] TAN H, FENG D, van DEVENTER J S J, LUKEY G C.An electrochemical study of pyrite oxidation in the presence of carbon coatings in cyanide medium [J]. International Journal of Mineral Processing, 2006, 7(80): 153-168.
[5] YU Li-yang. The influence of pyrite on lead-zinc sulphide ore flotation process [J]. Hunan Nonferrous Metals, 1992, 7(4): 219-221. (in Chinese)
[6] CHEN Xu-wen, HU Xi-gen. The relation between pyrite chemical composition inhomogeneity and floatability [J]. Hunan Nonferrous Metals, 1991(5): 278-283. (in Chinese)
[7] CHANDRAA P, GERSON A R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite [J]. Advances in Colloid and Interface Science, 2009, 145(1-2): 97-110.
[8] ZHAO Chun-yan, YU Ke-feng. The technical improvement of recovering sulphur from polymetallic sulphide ores [J]. Non-ferrous Mining and Metallurgy, 2008, 24(1): 17-19. (in Chinese)
[9] BUSWELL A M, BRADSHAW D J, HARRIS P J, EKMEKCI Z. The use of electrochemical measurements in the flotation of a platinum group minerals (PGM) bearing ore [J]. Minerals Engineering, 2002, 15(6): 395-404.
[10] WOODS R. Electrochemical potential controlling flotation [J]. International Journal of Mineral Processing, 2003, 72(1-4): 151-162.
[11] LI Wei-zhong, QIN Wen-qing, SUN Wei, QIU Guan-zhou. Electrodeposition of dixanthogen (TETD) on pyrite surface [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(1): 154-158.
[12] LEPPINEN J, BASILIO C O, YOON R H.Spectroelectrochemical study of ethyl xanthate adsorption on sulphide minerals [J].The Electrochemical Society, 1988(2): 49-65.
[13] KUOPANPORTTI H, SUORSA T, DAHLO. A model of conditioning in the flotation of a mixture of pyrite and chalcopyrite ores [J]. International Journal of Mineral Processing, 2000, 59: 327-338.
[14] LI Bing-rong, QIU Yun-wu, ZHOU Zheng, ZHAO Hua-lun, WANG Heng-feng. Experimental study on the sub-step process for flotation of clean sulphur concentrate from high-grade pyrite [J]. Multipurpose Utilization of Mineral Resources, 2014(4): 52-55. (in Chinese)
[15] CHENG Yu, SONG Yong-sheng, LI Bin, WANG Qin-qin. Experimental research on the column flotation of micro-fine pyrite particles [J]. Metal Mine, 2009(6): 64-68. (in Chinese)
[16] WU Xi-qing, XIE Xin. A preparation method of magnetic sulfide ore minerals: CN 104841549A[P]. 2015-8-19. (in Chinese)
[17] WU Xi-qing, DAI Chuan, DAI Liang. Magnetic seeding flotation (MSF): CN 104117432 A[P]. 2014-7-10. (in Chinese)
[18] WU Xi-qing, CAO Yang-fan. A stirring pre-magnetizer: CN 20449700OU[P]. 2015-7-22. (in Chinese)
[19] FENG Qi-ming, XU Shi. Electrochemistry of sulfide minerals flotation [M]. Changsha: Central South University of Technology Press, 1992: 7. (in Chinese)
[20] JIANG Chao-lan. Theory and technics of magnetic separation [M]. Beijing: Metallurgy Industry Press, 1994: 72-74. (in Chinese)
[21] PRATT A R, MUIR I J, NESBITT H W. X-ray photoelectron and Auger electron spectroscopic studies of pyrrhotite and mechanism of air oxidation [J]. Geochimica et Cosmochimica Acta, 1994, 58(2): 827-841.
[22] DAI Liang. The magnetic seed-polymer agglomeration of coal slime [D]. Changsha: School of Minerals Processing and Bioengineering, Central South University, 2015. (in Chinese)
[23] LIN Chao, SUN Chuan-yao, SUN Jian-ming. Study on magnetic agglomeration force of ferromagnetic particles [J]. Mining and Metallurgy, 2000, 9(1): 25-30. (in Chinese)
[24] FANG Qi-xue, LUO Jia-ke, GAN Jing-chao, LU Shou-ci, GE Chang-xu. Interaction among fine hematite particles in an exceedingly weak magnetic field [J]. Nonferrous Metals, 1998, 50(4): 26-33. (in Chinese)
[25] ZHANG Mao-jun, XU Chun, LUO Jia-ke. The mechanism of aggregation between fine particles of hematite and magnetite [J]. Nonferrous Metals, 1986, 38(3): 26-33. (in Chinese).
黄铁矿的自磁化及其在浮选中的应用
伍喜庆,谢 鑫,曹扬帆
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:黄铁矿是特殊的含2价铁的弱磁性矿物,通过调节矿浆温度和pH值增强其磁性,即所谓“自磁化”,并将自磁化反应产品磁性黄铁矿作为磁种用于黄铁矿石的浮选,提高浮选的回收率。通过自磁化试验、VSM磁性测定、XPS能谱分析、浮选试验以及激光粒度分析,研究黄铁矿的自磁化及其在黄铁矿浮选中的应用。结果表明:黄铁矿最佳自磁化条件为:矿浆pH值为11.81、温度为65 °C;黄铁矿自磁化后磁性变强的原因是自磁化后黄铁矿表面铁的价态发生了变化,部分Fe2+被氧化成了Fe3+,表面有磁性Fe3O4生成;黄铁矿浮选回收率随磁性黄铁矿磁性的增加而提高,磁性黄铁矿最佳用量为100 g/t;磁性黄铁矿能促进微细粒黄铁矿发生磁团聚,且磁性越强,团聚体粒径越大,使其更适合浮选。
关键词:黄铁矿;自磁化;磁性黄铁矿;磁团聚;磁种浮选
(Edited by Yun-bin HE)
Foundation item: Project (51274256) supported by the National Natural Science Foundation of China
Corresponding author: Xi-qing WU; E-mail: xiqingwu@hotmail.com
DOI: 10.1016/S1003-6326(16)64456-4
Abstract: Pyrite is a special weakly magnetic mineral containing Fe(II). Its self-magnetization only by adjusting slurry temperature and pH value was able to enhance its magnetism, producing the so-called the magnetized pyrite, which was further used as magnetic seeds in the flotation of pyrite ore to promote flotation recovery. Tests, such as self-magnetization, vibrating sample magnetometer (VSM), XPS, size analysis and flotation were carried out. The optimal conditions of the pyrite self-magnetization were pulp pH of 11.81 and temperature of 65 °C. The magnetized pyrite was characteristic of the valence change of elemental iron, resulting in stronger magnetism of the magnetized pyrite than that of the original pyrite. Then, this magnetized pyrite was applied to the magnetic seeding flotation (MSF) of pyrite ore. It was found that the recovery of pyrite flotation grew with the increase of magnetic susceptibility of the magnetic seeds-the magnetized pyrite; and the proper dosage of the magnetized pyrite was 100 g/t. The reason behind the increased recovery lies in that the magnetized pyrite promoted the magnetic agglomeration between fine pyrite particles; and the fact that the stronger the magnetism of the magnetized pyrite, the larger the aggregate size, indicates that the agglomeration is somewhat in line with the flotation, also confirming that the MSF is more suitable for fine particles than traditional flotation.


