
J. Cent. South Univ. (2019) 26: 2953-2960
DOI: https://doi.org/10.1007/s11771-019-4227-z
Preparation of low-temperature sintered high conductivity inks based on nanosilver self-assembled on surface of graphene
LIU Piao(刘飘)1, HE Wen-qiang(贺文强)1, LU An-xian(卢安贤)2
1. Hunan LEED Electronic Ink Co., Ltd., Zhuzhou 412007, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
Finer nanoplates of silver are prepared by self-assembly on the surface of graphene, and the low-temperature sintered high conductivity ink containing the silver nanoplates is prepared. Most importantly, graphene is added to the solution before the chemical reduction reaction occurs. Firstly, it is found that silver nanoplates have self-assembly phenomenon on the surface of graphene. Secondly, the Ag nano hexagonal platelets (AgNHPs) with small particle sizes (10 nm), narrow distribution and good dispersion are prepared. Especially, smaller sizes (10 nm) and narrower particle size distribution of AgNHPs particles can be easily controlled by using this process. Finally, the conductivity of the ink is excellent. For example, when the printed patterns were sintering at 150 °C, the resistivity of the ink(GE: 0.15 g/L) reached the minimum value of 2.2×10-6 W·cm. And the resistivity value was 3.7×10-6 Ω·cm, when it was sintered at 100 °C for 30 min. The conductive ink prepared can be used for the field of printing electronics as ink-jet printing ink.
Key words:
Ag nanoparticles; graphene; self-assembly; conductive ink; electrical properties;
Cite this article as:
LIU Piao, HE Wen-qiang, LU An-xian. Preparation of low-temperature sintered high conductivity inks based on nanosilver self-assembled on surface of graphene [J]. Journal of Central South University, 2019, 26(11): 2953-2960.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4227-z1 Introduction
Ink for printed electronics is a key material that produces conducting circuits on flexible substrates, such as poly (ethylene terephthalate) (PET), polyimide and paper [1-3], using the printing techniques. For instance, it has high optical transparency, excellent conductivity and thermal stability, and it also has a flexible design for printing. Most researches have been devoted to finding the appropriate constituent materials for the inks, including conductive polymers, nanoscale metals, and carbon nanotubes [4-9]. GAO et al [10] reported that nanoscale Ag shows high conductivity, interesting chemical and physical properties and has potential for using in areas, such as SERS substrate, optical probe [11-15]. Preparation of nanoparticles with different morphologies has been reported [16]. Ag nanoplates [17-20] have the advantages of large contact area and good electrical performance over spherical nano silver particles. Graphene has high electron conductivity due to the special structure; Especially, it does not scatter electrons when it moves in the submicron range [21, 22]. Thus, graphene(GE) exhibits very good electron-transfer properties, making it an ideal material for nanometer-scale circuits [23]. There have been some reports of conductive ink for nanomaterials and carbon materials[24-26]. However, most of the researches are mainly on graphene oxides (GO) or composites with other materials [27, 28]. The surface of GO contains a large number of oxygen-containing groups. These functional groups can adsorb metal nanoparticles. However, because of the presence of oxygen-containing groups, the conductivity of GO is poor. At present, it was rarely reported adding GE to improve the conductivity of conductive ink.
In this work, we demonstrated a simple method to prepare conductive inks with high electrical properties. The AgNHPs conductive inks are prepared by chemical reduction of silver nitrate on the surface of graphene or graphene oxide, for which graphene was added into the ink before the synthesis of AgNHPs. Interestingly, AgNHPs with small particle size and narrower particle size distribution are obtained and are distributed uniformly on the surface of graphene because of possible interaction between conjugate π-electrons of graphene and the low-energy {111} plane of Ag nanoparticles. The printed patterns resulting from the as-obtained GE-AgNHPs ink exhibit excellent electrical conductivity even though they are sintered at relatively low temperatures. The conductive ink prepared herein can be used for the field of printing electronics as ink-jet printing ink.
2 Experimental
2.1 Materials
GO and GE were purchased from Hunan Element Password Graphene Institute. Methylpyrrolidone (NMP), polyvinypyrrolidone (PVP), silver nitrate, anhydrous ethanol, sodium hydroxide (NaOH) and glucose were purchased from Shanghai Aladdin Company. They were all analytical and not further purified before using. The deionized water used in the experiment was homemade.
2.2 Syntheses of graphene-AgNHPs
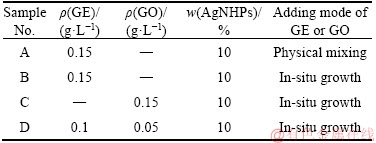
The experimental preparation process is shown in Table 1. First, 50 mL of 0.35 mol/L silver nitrate solution was prepared. Next, PVP with the same mass ratio was added to the silver nitrate aqueous solution. 8 mL of 2 mol/L NaOH was added into the resultant solution by dropping under ultrasonic stirring. The mixing solution was mixed for 0.5 h under ultrasonic agitation in 60 °C water bath. Then, 8 mL of 4 mol/L glucose solution was added to the reaction solution drop by drop with 0.5 mL/min at 60 °C. After ultrasonic processing for 2 h, the reaction was complete. Subsequently, the nano silver was cleaned. At the end, AgNHPs were mixed with GE. Then, the composite conductive ink, containing GE 0.15 g/L, silver 10 wt%, was prepared and marked sample A.
20 mL of 0.875 mol/L silver nitrate solution was prepared. Next, PVP with the same mass ratio was added to the silver nitrate aqueous solution. After stirring well, 30 mL of 0.5 g/L GE-NMP solution was added into above solution. 8 mL of 2 mol/L NaOH was added into the resultant solution by dropping under ultrasonic stirring. The mixing solution was mixed for 0.5 h under ultrasonic agitation in 60 °C water bath. Then, 8 mL of 4 mol/L glucose solution was added into the reaction solution droplet by drop with 0.5 mL/min at 60 °C. After ultrasonic processing for 2 h, the reaction was complete, and the nano silver was cleaned. At the end, GE-AgNHPs were added into the NMP solution, The composite conductive ink, containing GE 0.15 g/L, silver 10 wt%, was prepared and marked sample B.
The experimental procedures of sample C and sample D were the same as sample B. But the GE-NMP solution (GE: 0.5 g/L, 30 mL) was replaced by the GO-deionized water solution (GO: 0.09 g/L, 30 mL) for sample C. And the GE-NMP solution (GE: 0.09 g/L, 30 mL) was replaced by the GE-NMP solution (GE: 0.5 g/L, 20 mL) and GO-deionized water solution (GO: 0.5 g/L, 10 mL) for sample D.
Table 1 Composition and preparation process of inks
2.3 Fabrication of conductive films
The prepared ink was distributed by ultrasound for 1 h before using. The viscosity of the prepared ink was 2-5 mPa·s, and the surface tension was 35-40 mN/m.
The type of equipment used for inkjet printing was EPSON R300 with the minimum droplet of 10 pL. The printing temperature was controlled at 30°C. The substrates were glass substrates. The graph of the ink printed was a rectangular line (0.6 cm×5 cm). Finally, the substrates were dried and sintered to obtain conductive films.
2.4 Characterization
The morphologies of AgNHPs and conductive films were characterized with field emission scanning electron microscope (MIRA3 produced by TESCAN, Czech; 20.0 kV, working distance (14.1±0.1) mm), and TEM system (JEOL-2100, Japan). The resistivity was tested using a four-probe needle (ST2258C, Suzhou Lattice Electronic Co., LTD). The Raman spectrometer was LabRAM HR800 (Japan). The UV absorption spectroscopy was measured using ultraviolet-visible absorption spectrometer (UV-2501PC, Shimadu, Japan).
3 Results and discussion
3.1 Morphological analysis
The morphologies of the graphene-AgNHPs inks prepared are shown in Figure 1. As shown in Figure 1(a), most of the AgNHPs were distributed in the range of 20-30 nm and small particle sizes were 50-80 nm in the sample A. At the same time, the nanoplates were arranged in orderly manner, which indicates that there were interactions between graphene and Ag nanoplates. The average particle size of AgNHPs in the sample B was about 10 nm,which were absorbed on the GE surface, and the particles arranged densely (Figure 1(b)). In the lower left corner of Figure 1(b), it was the TEM image of GE. The average grain diameter of AgNHPs was approximately 20 nm in the sample C , and most of Ag nanoplates were absorbed on the GO surface uniformly (Figure 1(c)). In the lower left corner of Figure 1(c), it was the TEM image of GO. But there were a small number of nano silver plates of 100-200 nm, even greater than 200 nm appearing in the sample D (Figure 1(d)). As shown in Figure 1(a), it can be seen that the nanoplates were arranged close and orderly because the surface energies were high significant between the Ag nanoplates and GE, resulting in a greater number of surface active centers. In addition, an electrophoresis experiment showed that the Ag nanoplates were positively charged. The zeta potential of Ag colloid was 21.3 mV [26]. Given the two-dimensional honeycomb crystal structure of GE, its planar structure contained a large number of conjugate benzenes. The Ag nanoplates underwent bonding interactions with the conjugate π-electrons in these benzenes. As a result, parts of the Ag nanoplates were arranged in a more order manner. When the graphene was added into the solution before the synthesis of AgNHPs, the Ag nanoplates and GE combined strongly because of the surface effect. And, the {111} facet of AgNHPs was easier oriented towards the {001} facet of GE because the {111} plane of the AgNHPs was the low-energy face [26]. As result, the surfaces of Ag nanoplates were adsorbed by GE (Figure 1(b)). But, graphene had space steric effect, which hindered the growth of nano-Ag crystals. Also, the number of silver crystal nucleus in solution was decreased because of nano-Ag crystals adsorbed on the surface of graphene. So, the crystal growth was blocked and the nucleation rate was greater than the growth rate of nano crystals in the solution. The particle size of the generated nano silver particle decreased while the number increased.
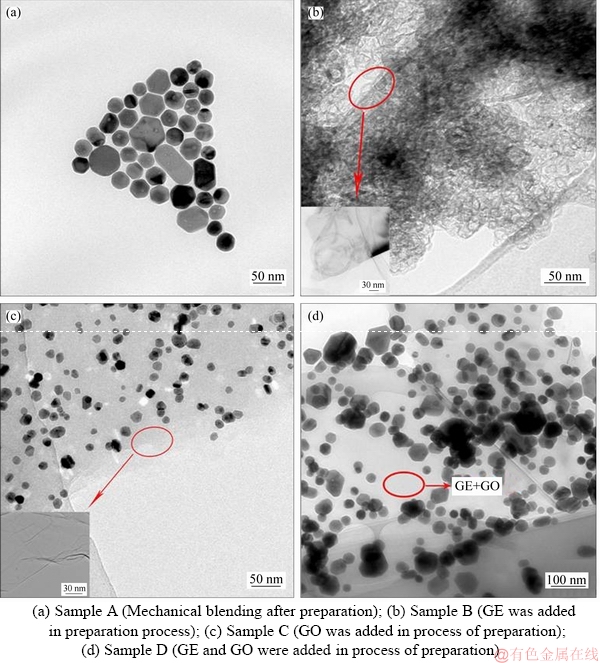
Figure 1 TEM images of samples:
As shown in Figure 1(c), most of Ag nanoplates were absorbed on the GO surface uniformly. Because the structure of graphene oxide was destroyed, there are a lot of the surface active groups (—OH and —O—) on the surface of GO. The absorbability of the surface active groups(—OH and —O—) is stronger than that of the conjugated π bond. Therefore, the AgNHPs were preferentially adsorbed by the active group. Compared to the sample B, there were more crystal silver nanoparticles continuing to grow after nucleation, and the growth rate and nucleation rate competed with each other. The end result was that the particle size of AgNHPs in the sample C was bigger than that of the sample B. In the sample D, graphene and a few graphene oxides were added into the solution before the synthesis of AgNHPs. Because of differences of graphene and graphene oxide in adsorption to silver nanoparticles, nano silver crystals were more likely to be adsorptive with the oxygen group on the surface of graphene oxide than the graphene. According to the theory of Ostwald ripening, when particles coexist, small particles disappeared and large particles continued to grow up, leading to particle size varying and uneven distribution in Figure 1(d).
Metal nanoparticles have LSPR effect [28] and they have a strong spectrum absorption in the ultraviolet-visible band. It is related to the size, distribution and morphology. The UV absorption spectroscopy of the samples is shown in Figure 2. The UV absorption peak at 414 nm is the main absorption peak, corresponding to the characteristic absorption peak of silver nanoparticles (in Figure 2(a)). But as shown in Figure 2(b), the peak position of nano silver-graphene resonance absorption is shifted to 402 nm, indicating that the silver nanoparticle size became small in the sample C. Meanwhile, in Figure 2(b), the absorption spectrum of half peak width was significantly narrowed, indicating that the distribution of silver nanoparticles becomes narrow when graphene oxide was added into the solution before the synthesis of AgNHPs, which is consistent with the result in Figure 1(c). The resonance absorption peak position of nano silver-graphene was blueshifted, and the absorption spectrum of half peak width was narrowed in Figure 2(c), but the difference was not so obvious as that in Figure 2(b), indicating that the effect of graphene on silver particle size is not so obvious as graphene oxide.
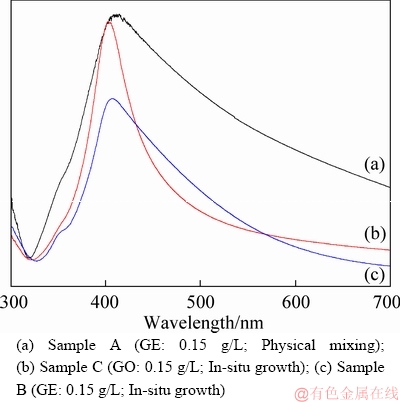
Figure 2 UV absorption spectroscopy of inks:
3.2 Electrical conductivity analysis
The addition method of graphene led to the dispersion and particle sizes of nanoparticles were different, which also affected the conductivity. The resistivity of the samples is shown in Figure 3.
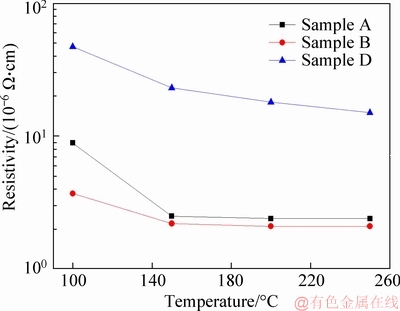
Figure 3 Resistivities of inks after sintering at different temperatures (sample A: GE 0.15 g/L, physical mixing; sample B: GE 0.15 g/L, in-situ growth; sample D: GE 0.1 g/L, GO 0.05 g/L, in-situ growth)
The sample C was obtained by adding the graphene oxide into the solution before the synthesis of AgNHPs. But, the sample C was non-conductive while it was coated on the glass substrate and sintered at different temperatures because the conductivity of graphene oxide was poor.
As shown in Figure 3, the resistivity of the sample D was obviously higher than that of the sample A and the sample B. It was proved again that the graphene oxide reduced the conductivity of the coating sintered. Besides, when the sintering temperature increased, the resistivity decreased, it was especially true to the conductive ink sample A at 150°C. The resistivity value of the sample A was 8.9×10-6 Ω·cm, and that of the sample B was 3.7×10-6 Ω·cm, after sintering at 100°C for 30 min. But, the resistivity was 2.5×10-6 Ω·cm and 2.2×10-6 Ω·cm after sintering at 150°C for 30 min, respectively (in Figure 3). In addition, while the graphene was added into the solution before the synthesis of AgNHPs, the resistivity of ink was less affected by the temperature and changed a little with the temperature. It was because the graphene was added into the solution before the synthesis of AgNHPs and nano silver was prepared by self- assembly in graphene surface; therefore,the particle size of the AgNHPs was smaller. So, the fusion of AgNHPs was faster at lower temperatures. As a result, the resistivity was lower and changed a little with the temperature. As shown in the TEM of the samples in Figure 1(b), it was found that the contact area of AgNHPs with AgNHPs,AgNHPs with GE was large in conducting layer of the sample B, and the stacking density was also high. So, it was more beneficial to the electrical conductivity of the conducting layer. In order to contrast the differences between the samples A and B in the melting at different temperature, the SEM figures of the samples A and B morphology are shown in Figure 5 after being sintered at 150 °C and 250 °C, respectively.
The Raman spectra of graphene, graphene oxide, GE-AgNHPs and GO-AgNHPs at 530 nm excitation wavelength are shown in Figure 4. At 1580 cm-1, it was the characteristic peak for GE which was called G peak. It was the surface motion of carbon atoms in graphene. Between 1340 to 1350 cm-1, it was called the D peak. The intensity ratio of D peak and G peak(ID/IG) was an important indicator of the quality of graphene [29-31]. Obviously, the ID/IG value of graphene oxide was greater than that of graphene. It was indicated that there were structure defects in graphene oxide and some groups such as—O—,—OH and —COOH randomly distributing on the GO after oxidation.
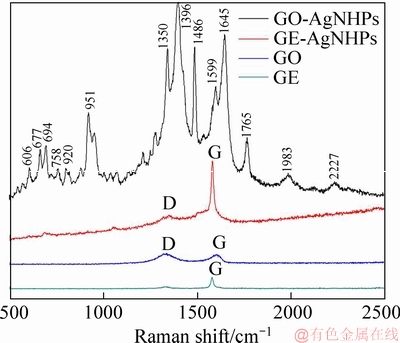
Figure 4 Raman spectra of graphene, graphene oxide, GE-AgNHPs and GO-AgNHPs with 530 nm excitation wavelength
The ID/IG of GE was small (in Figure 4). It was indicated that the graphene sample had no obvious flaws and the structure of it was still relatively complete. The Raman spectra were significantly enhanced when GE (or GO) and AgNHPs were composited. The same results were reported in Ref. [31]. They reported that AgNHPs and GO compounds had obvious SERS effect. The electromagnetic enhancement mechanism (EM) and the chemical enhancement mechanism (CM) were two main types of SERS enhancement mechanism. The studies had shown that end functional groups in GO were an important factor for SERS effect. In Figure 4, it was indicated that there are obvious interactions between GE (or GO) and AgNHPs. It could also be found that GO-AgNHPs on Raman spectra of enhancement effect were better than GE-AgNHPs. Many peaks existed in the GO- AgNPs no in GE-AgNPS. It was indicated that the oxygen group of the surface of GO had stronger adsorption ability to the AgNHPs. The peaks were enhanced significantly at 606, 677, 694, 920, 951, 1350, 1396, 1486, 1599, 1645, 1675, 1983 and 2227 cm-1. Among them, the vibration peaks belonging to the C—C—C ring are 606, 677, 694, 920, 951, 1350, 1486, 1599, 1675, 1983 and 2227 cm-1. However, the frequency displacement in the SERS spectrum was large. It was shown that the GO structure has been greatly damaged; there were many surfactant groups, and the interaction between active groups and nano silver was very strong. The vibration peaks belonging to the carbonyl group were at 1396 and 1645 cm-1. There was a significant frequency shift compared to the characteristic peaks of carbonyl groups in the SERS spectrum. It was indicated that the C=O bond was destroyed because of the strong interaction with silver surface. It was also confirmed in Ref. [32]. At 1350 and 1599 cm-1, the peaks correspond to the characteristic peaks D and G of GO, respectively. However, compared with the GO Raman spectrum, the peak strength was significantly enhanced. It was shown that there was a strong interaction between GO and AgNHPs.
Figure 5(a) shows SEM image of sample A sintered at 150 °C for 0.5 h. Figure 5(c) shows the enlarged photograph of Figure 5(a). Figure 5(b) the sample B sintered at 150 °C for 0.5 h. Figure 5(d) shows the enlarged photograph of Figure 5(b). The melting degree of the sample B was better than that of the sample A at 150 °C. It was indicated that the AgNHPs in the sample B were easier sintered melting when GE was added into the solution before the synthesis of AgNHPs. Because of the smaller particle size of AgNHPs in the sample B, its melting temperature was lower. As a result, the melting degree of the sample B was better. So, the resistivity was lower.

Figure 5 SEM images of GE-AgNHPs after sintering at 150 °C for 0.5 h:
4 Conclusions
In this study, GE-AgNHPs composite ink was produced by adopting the dynamically controlled synthesis reaction. The graphene (GE) was added into the solution before the synthesis of AgNHPs. It was found that AgNHPs were self-assembled on the surface of GE. Especially, it was also found that the smaller sizes (10 nm) and narrower particle size distribution can be easily controlled by using this process. Compared to physical mixing, the GE-AgNHPs conductive ink showed good electrical conductivity. The resistivity of the composite ink was 2.2×10-6 Ω·cm after sintering at 150°C. When the GE content was 0.15 g/L,it was better than the results of the literature(2.5×10-6 Ω·cm); Especially, when it was sintered at 100°C, the resistivity of the sample B was 3.7×10-6 Ω·cm.
Thus, the preparation of nano silver ink with smaller particle size and more stability will bring more extensive application.
References
[1] NGE T T, NOGI M, SUGNUMA K. Electrical functionality of inkjet-printed silver nanoparticle conductive tracks on nanostructured paper compared with those on plastic substrates [J]. Journal of Materials Chemistry C,2013,1(34): 5235-5243.
[2] XU L, LI H, XIA J, WANG L, XU H. Graphitic carbon nitride nanosheet supported high loading silver nanoparticle catalysts for the oxygen reduction reaction [J]. Materials Letters,2014,128(6): 349-353. DOI: 10.1016/j.matlet.2014. 04.110.
[3] PANDEY P A, BELL G R, ROURKE J P, WILSON N. Physical vapor deposition of metal nanoparticles on chemically modified graphene: Observations on metal- graphene interactions [J]. Small, 2011, 7(22): 3202-3210. DOI: 10.1002/smll.201101430.
[4] AN B, CAI X H, WU F S, WU Y P. Preparation of micro-sized and uniform spherical Ag powders by novel wet-chemical method [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1550-1554. DOI: 10.1016/S1003-6326(09)60337-X.
[5] JOHNSON H, AROCKIASAMY A, GANESAN S, KANNUSAMY M. A new approach for deposition of silver film from AgCl through successive ionic layer adsorption and reaction technique [J]. Journal of Central South University, 2017, 24: 2793-2798. DOI: 10.1007/s11771- 017-3693-4.
[6] LIU P, MA J, DENG S, ZENG K, DENG D Y, XIE W, LU A X. Graphene–Ag nanohexagonal platelets-based ink with high electrical properties at low sintering temperatures [J]. Nanotechnology, 2016, 27: 385603. DOI: 10.1088/ 0957-4484/27/38/385603.
[7] CHI K, ZHANG Z, XI J, HUANG Y, XIAO F, WANG S, LIU Y. Freestanding graphene paper supported three- dimensional porous graphene-polyaniline nanocomposite synthesized by inkjet printing and in flexible all-solid-state supercapacitor [J]. ACS Applied Materials & Interfaces, 2014, 6: 16312. DOI: 10.1039/C4TA02721C.
[8] XIAO F, YANG S X, ZHANG Z Y, LIU H F, XIAO J W, WAN L, LUO J, WANG S, LIU Y Q. Scalable synthesis of freestanding sandwich-structured graphene/polyaniline/ graphene nanocomposite paper for flexible all-solid-state supercapacitor [J]. Scientific Reports, 2015, 5: 9359. DOI: 10.1038/srep09359.
[9] SUN Y G, XIA Y N. Shape-controlled synthesis of gold and silver nanoparticles [J]. Science, 2002, 298(5601): 2176- 2179. DOI: 10.1126/science.1077229.
[10] GAO Y, JIANG P, SONG L, WANG J X, LIU L F, LIU D F, XIANG Y J, ZHANG Z X, ZHAO X W, DOU X Y, LUO S D, ZHOU W Y, XIE S S. Studies on silver nanodecadrons synthesized by PVP-assisted N,N-dimethylformamide (DMF) reduction [J]. Journal of Crystal Growth, 2006, 289: 376-380. DOI: 10.1016/j.jcrysgro.2005.11.123.
[11] WILEY B J, XIONG Y J, LI Z Y, Y Y D, XIA Y N. Right bipyramids of silver: A new shape derived from single twinned seeds [J]. Nano Lett, 2006, 6: 765-768. DOI: 10.1021/nl060069q.
[12] GILBERTSON L M, ZIMMERMAN J B, PLATA D L, HUTCHISON J E, ANASTAS P T. Designing nanomaterials to maximize performance and minimize undesirable implications guided by the principles of green chemistry [J]. Journal of Materials Chemistry C, 2015, 44: 5758-5777. DOI: 10.1039/c4cs00445k.
[13] ISABEL P S, LUIS M L M. Synthesis of silver nanoprisms in DMF [J]. Nano Letters, 2002, 2(8): 903-905. DOI: 10.1021/nl025638i.
[14] BAI L, MA X J, LIU J F, SUN X M, ZHAO D Y, EVANS D G. Rapid separation and purification of nanoparticles in organic density gradients [J]. Journal of the American Chemical Society, 2010, 132(7): 2333-2340. DOI: 10.1021/ ja908971d.
[15] LIU P, TANG Q Z, LIU H, LU A X. Low electrical resistivity of graphene-AgNHPs based ink with a new processing method [J]. RSC Advances, 2017, 7: 15228-15235. DOI: 10.1039/c7ra00309a.
[16] XU Z, GAO H, HU G. Solution-based synthesis and characterization of a silver nanoparticle-graphene hybrid film [J]. Carbon, 2011, 49: 4731-4738. DOI: 10.1016/j.jcis.2011. 05.009.
[17] LIU Y, CHANG Q, HUANG L. Transparent, flexible conducting graphene hybrid films with a subpercolating network of silver nanowires [J]. Journal of Materials Chemistry C, 2013, 1: 2970-2974. DOI: 10.1039/ C3TC30178H.
[18] NERSISYAN H H, LEE J H, SON H T, WON C W, MAENG D Y. A new and effective chemical reduction method for preparation of nanosized silver powder and colloid dispersion [J]. Materials Research Bulletin, 2003, 38(6): 949-956. DOI: 10.1016/S0025-5408(03)0078-3.
[19] CHUN K Y, OH Y, RHO J, AHN J H, KIM Y J, CHOI H R, BAIK S. Highly conductive, printable and stretchable composite films of carbon nanotubes and silver [J]. Nature Nanotechnology, 2010, 5: 853-857. DOI: 10.1038/NNANO. 2010.232.
[20] CHARCOSSET C, KIEFFER R, MANGIN D, PUEL F. Coupling between membrane processes and crystallization operations [J]. Industrial & Engineering Chemistry Research, 2010, 49(12): 5489-5495. DOI: 10.1021/ie901824x.
[21] KIM T Y, KWON S W, PARK S J, YOON D H, SUH K S, YANG W S. Self-organized graphene patterns [J]. Advanced Materials, 2011, 23: 2734-2738. DOI: 10.1002/adma. 201100329.
[22] KIM Y H, YOO B, ANTHONY J E, PARK S K.Controlled deposition of a high performance small molecule organic single crystal transistor array by direct ink jet printing [J]. Advanced Materials, 2012, 24(4): 497-502. DOI: 10.1002/ adma.201103032.
[23] HE Yuan, LIU Yun-guo. Direct fabrication of highly porous grapheme/TiO2 composite nanofibers by electrospinning for photocatalytic application [J]. Journal of Central South University, 2018, 25: 2182-2189. DOI: 10.1007/s11771- 018-3906-5.
[24] ZHANG L, LIU H, ZHAO Y, SUN X, WEN Y, GUO Y, GAO X, DI C, YU G, LIU Y.Inkjet printing high-resolution, large-area graphene patterns by coffee-ring lithography [J]. Advanced Materials, 2012, 24(3): 436-440. DOI: 10.1002/ adma.201103620.
[25] LIU J H, HU Y, ZHANG Y W, CHEN W. Graphene- stabilized silver nanoparticle electrochemical electrode for actuator design [J]. Advanced Materials, 2013, 25: 1270-1274. DOI: 10.1002/adma.201203655.
[26] BADAWY A M E, LUXTON T P, SILVA R G, SCHECKEL K G, SUIDAN M T, TOLAYMAT T M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions [J]. Environmental Science & Technology, 2010, 44(4): 1260-1266. DOI: 10.1021/ es902240k.
[27] DONG X Y, GAO Z W, YANG K F, ZHANG W Q, XU L W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals [J]. Journal of Materials Chemistry, 2015, 5: 2554-2574. DOI: 10.1039/C5CY00285K.
[28] LU G, LI H, LIUSMAN C, YIN Z, WU S, ZHANG H. Surface enhanced Raman scattering of Ag or Au nanoparticle-decorated reduced graphene oxide for detection of aromatic molecules [J]. Chemical Science, 2011, 2: 1817-1821. DOI: 10.1039/C1SC00254F.
[29] LEE J, SHIM S, KIM B, SHIN H S. Surface-enhanced Raman scattering of single- and few-layer graphene by the deposition of gold nanoparticles [J]. Chemistry-A European Journal, 2011, 17: 2381-2387. DOI: 10.1002/chem. 201002027.
[30] ISRAELACHVILI J. Intermolecular & surface force [M]. London: Academic Press, 1997.
[31] XU Y X, WU Q, SUN Y, BAI H, SHI G. Three- dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels [J]. ACS Nano, 2010, 4(10): 7358-7362. DOI: 10.1021/nn1027104.
[32] SCHWEINSBERG D P, HOPE G A, TRUEMAN A, OTIENO-ALEGO V. An electrochemical and SERS study of the action of polyvinylpyrrolidone and polyethylenimine as inhibitors for copper in aerated H2SO4 [J]. Corrosion Science, 1996, 38(4): 587-599.
(Edited by YANG Hua)
中文导读
基于纳米银在石墨烯表面自组装制备低温固化高导电纳米银墨水
摘要:采用石墨烯诱导,制备出六角片状纳米银,并最终制备出在低烧结温度下导电性能好的纳米银墨水。其中核心之处是将石墨烯以原料的方式提前加入反应液中制备纳米银颗粒。研究发现,制备过程中纳米银颗粒在石墨烯表面具有自组装现象。其次,制备出的六角片状纳米银颗粒粒径小(10 nm)、分布窄、分散性好。特别是,本文采用的制备粒径小、分布窄的纳米银片的工艺更容易控制,更有利于批量生产。最后,制备的石墨烯-纳米银混合导电墨水具有优良的导电性。如,当石墨烯含量为 0.15 g/L,在150 °C烧结后,印刷制成的导电线路的电阻率达到最小值为2.2×10-6 Ω·cm。在100 °C烧结30 min后,电阻率为3.7×10-6 Ω·cm。制备的混合导电墨水可用于印刷电子喷墨打印领域。
关键词:纳米银颗粒;石墨烯;自组装;导电油墨;导电性能
Foundation item: Project(2018GK4015) supported by the Hunan Provincial Strategic Emerging Industry Project, China
Received date: 2018-08-01; Accepted date: 2019-01-17
Corresponding author: LU An-xian, PhD, Professor; Tel: +86-731-88830351; E-mail: axlu@mail.csu.edu.cn; ORCID: 0000-0001-8051- 4375
Abstract: Finer nanoplates of silver are prepared by self-assembly on the surface of graphene, and the low-temperature sintered high conductivity ink containing the silver nanoplates is prepared. Most importantly, graphene is added to the solution before the chemical reduction reaction occurs. Firstly, it is found that silver nanoplates have self-assembly phenomenon on the surface of graphene. Secondly, the Ag nano hexagonal platelets (AgNHPs) with small particle sizes (10 nm), narrow distribution and good dispersion are prepared. Especially, smaller sizes (10 nm) and narrower particle size distribution of AgNHPs particles can be easily controlled by using this process. Finally, the conductivity of the ink is excellent. For example, when the printed patterns were sintering at 150 °C, the resistivity of the ink(GE: 0.15 g/L) reached the minimum value of 2.2×10-6 W·cm. And the resistivity value was 3.7×10-6 Ω·cm, when it was sintered at 100 °C for 30 min. The conductive ink prepared can be used for the field of printing electronics as ink-jet printing ink.


