
Microstructures and properties of aluminum film and its effect on
corrosion resistance of AZ31B substrate
ZHANG Jin(张 津)1, 2, YANG Dong-hua(杨栋华)2, OU Xin-bing(欧信兵)2
(1. School of Materials Science and Engineering, University of Science and Technology Beijing,
Beijing 100083, China;
2. College of Materials Science and Engineering, Chongqing Institute of Technology, Chongqing 400050, China)
Abstract:
Aluminum films with thickness of 8.78-20.82 μm were deposited on the AZ31B magnesium alloys by DC magnetron sputtering. The influences of aluminum film on the micro-mechanical properties and corrosion behavior of the magnesium alloys were investigated. The morphology of aluminum film was examined by scanning electron microscopy and the microstructure of aluminum film was analyzed by X-ray diffractometry. Nanoindentation and nanoscratch tests were conducted to investigate their micro- mechanical properties. Moreover, potentiodynamical polarization test performed in 3.5%NaCl solution was carried out to study their anticorrosion performances. The results show that the surface hardness of AZ31B magnesium alloy with aluminum film is 1.38-2.01 GPa, higher than that of the magnesium alloy substrate. The critical load of Al film/AZ31B substrate is in the range of 0.68-2.77 N. The corrosion current density of AZ31B with aluminum film is 2-3 orders of magnitude less than that of bare AZ31B. And the corrosion potential with aluminum film positively shifts. Thus aluminum film can increase the corrosion resistance of Mg alloys obviously.
Key words:
AZ31B magnesium alloy; aluminum films; micro-mechanical properties; corrosion resistance; magnetron sputtering;
1 Introduction
As the lightest of all the commonly used metals, magnesium alloy has an excellent processing performance, good biocompatibility, high thermal conductivity, high dimensional stability, high damping and good electromagnetic shielding characteristics. These properties make it applied in many fields, such as aerospace, automotive, electronics, telecommunications and even used as an implant metal. However, poor corrosion resistance limits its widespread use in many applications[1]. In addition, magnesium cannot form a self-healing passive surface contrast to aluminum and titanium, because there is a misfit between the hydroxide lattice in the surface region and the lattice of the bulk material. Therefore, the magnesium hydroxide film is loose and the layer is undermined by corrosion process[2].
Because aluminum and its alloy have the ability to self-repair corrosion and, Al element is one of the main compositions of Mg alloys, aluminum coating is designed to protect magnesium alloy from corrosion resistance. Aluminum coating has been given more concerns and studied in the past years. There are many ways to prepare Al coating, such as thermal spray[3-5], physical vapor deposition (PVD)[6-8], chemical vapor deposition (CVD)[9] and electrodeposition[10].
WU et al[6] prepared pure Al and Ni thin films successfully by RF-sputtered on AZ91D substrate. After heat treatment in high vacuum at 350 ℃ for 24 h, the 2 μm thick aluminum film reacted completely with the substrate to form Al12Mg17 phase. Although aluminum film is dense, the intrinsic brittleness of Al12Mg17 phase has a low cohesive strength with the substrate and thus will influence the corrosion resistance.
In this work, pure Al films on AZ31 magnesium alloy have been prepared by DC magnetron sputtering. The main aim is to investigate the properties of these films and find out the better film for the corrosion resistance protection to magnesium alloy. The morphology, chemical composition and microstructure of the films were investigated by scanning electron microscopy and X-ray diffractometry. The mechanical properties were measured by nanoscratch/nano- indentation. The corrosion characteristics of Al films on AZ31 substrate were investigated by a potentiodynamic polarization test in 3.5%NaCl solution at room temperature.
2 Experimental
2.1 Deposition process
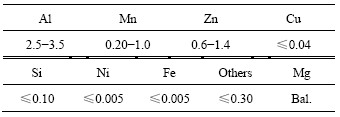
The chemical composition of AZ31B magnesium alloy with size of 25 mm×25 mm×1 mm is shown in Table 1. Firstly the samples were rinsed in 192 g/L CH3COOH+50 g/L NaNO3 solution to eliminate the surface oxides, then were polished to 1000# SiC paper. Then, they were cleaned in acetone for 10 min and in ethanol for 10 min by ultrasonic cleaning. Al films were prepared by DC magnetron sputtering using aluminum target with purity of 99.99% in an ultra-high vacuum system with ion gun. Ar ion cleaning before deposition was carried out to avoid contamination and improve adhesion. The deposition process was performed at room temperature without any heating to substrate additionally. The sputtering power was 350 W with current of 1.0 A, and the distance of substrate-to-target was about 60 mm. The base pressure in the system prior to sputtering was 4.0×10-4 Pa and the Ar gas pressure during sputtering was 0.6 Pa. The deposition time was 120, 150 and 300 min corresponding to samples 1, 2 and 3, respectively.
Table 1 Compositions of AZ31B magnesium alloy (mass fraction, %)
2.2 Film characterization
The top surface morphologies of aluminum film were observed by JSM-6460LV scanning electron microscope operated at 20 kV. X-ray diffractometer with Co Kα radiation was used to study the microstructure of the samples. The operating voltage and current were 40 kV and 200 mA, respectively. The mechanical properties of Al film were characterized at room temperature by Nano-Test system (MML) which is a pendulum-based depth-sensing system, with the sample mounted vertically and the applied electromagnetically. The thickness and critical load were obtained by nano-scratch test. The tests over the same region of the Al film surface can be scheduled in the instrument software. A 25 μm radius spherical diamond was used as the probe in all the tests. The constant loading rate was 5 mN/s during a track length of 2 mm at velocity of 10 μm/s. The 1 N load was applied on the point 500 μm deep from the surface and end at the top surface with 3 N. Pre-scanning was performed under load of 1 mN with which no scratch formed on the surface. All the indentation experiments were performed using Borkovich indenter with the maximal loads of 100 mN at constant loading and unloading rate of 5 mN/s. The indenter holds 60 s at the maximal load. For each sample, an average indentation value was obtained from nine measurements. Before test, the indenter tip shape function and the machine compliance were calibrated using a standard sample of fused quartz.
Polarization measurements were carried out using a potentiostat at 1 mV/s in a 3.5% NaCl solution at room temperature. Potentials were measured versus saturated calomel electrode. The exposure area was 1.0 cm× 1.0 cm.
3 Results and discussion
3.1 Surface morphology and microstructure characterization
The formation of Al films includes nucleation, island growth, impingement and coalescence of islands, formation of polycrystalline islands and channels, development of a continuous structure, and film growth[11-12]. As to island growth, the islands gather and coalescence into clusters of different sizes, and film thickening proceeds through local epitaxy on this individual cluster grains when surface diffusion rates are significant. It can be seen from Fig.1 that the surface morphology of Al films on the magnesium alloy is uniform small grains free of defects. With increasing sputtering time, the aluminum clusters grow gradually during the deposition process, which results in the increase of surface roughness for Al films. Denser films near the substrate can be observed from the cross- sectional image as shown in Fig.2. Therefore, it is suggested that the surface cavities between the grains or clusters do not form the pores breaking through the surface of Al film to the substrate.
Fig.3 shows the XRD pattern of the as-deposited Al film on AZ31B alloy. It can be found that the diffraction peaks of pure Al (111), (200) and (220) are clearly detected from the samples, showing a typical polycrystalline face-centered cubic structure. The signals from the AZ31B alloy substrates are suppressed since their surfaces are covered by the deposited Al films, and Mg peak mainly shows without the second phase Mg17Al12. It can be inferred that the composition of film is primarily aluminum free of oxidation and other impurities as no other oxide diffraction peak appears in Fig.3.
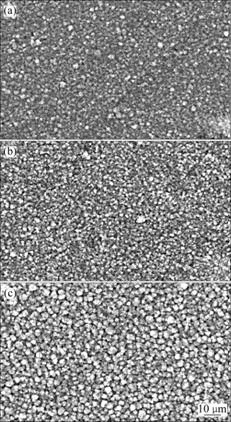
Fig.1 SEM morphologies of Al films on AZ31B alloy: (a) Sample 1; (b) Sample 2; (c) Sample 3

Fig.2 Cross-sectional image of Al film on AZ31B alloy
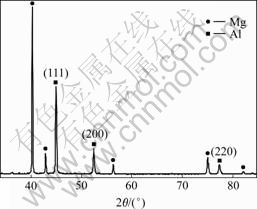
Fig.3 XRD pattern of as-sputtered Al film on AZ31B alloy
3.2 Mechanical properties of Al films on AZ31B alloy
Typical results of Al films deposited on AZ31B substrate from the scratch test are shown in Fig.4. In the initial topography scan, a small load of 1 mN was applied to the probe and the sample was scanned for 2 mm at 5 mN/s (see curve ①), and automatically moved back to its starting position for the scratch test. In the scratch test, the probe was moved along the same track for 500 μm at 1 N, and then the applied load ramped at 10 μm/s to the pre-set maximal load of 3 N until the end of scratch (see curve ②). After automatically moving the sample back to its stating position, the process was repeated, before a final topography scan (at 1 mN applied loads, see curve ③). Curve ④ is the linear loading curve when the displacement starts from 500 μm. The topography curve ① shows the surface structure of aluminum films due to the cluster, which clearly indicate the surface roughness of samples.
Scratching curve ② shows smooth curves (A-B) during the indenter penetrating the aluminum film. When the indenter touched the interface between films and substrate, the slope of scratching curve changed dramatically because of different friction of different materials. The longitudinal coordinates of this inflexion (point B in curve ②) corresponded to the thickness value of Al film. The SEM image (see Fig.4(a)) of Al film confirms the film failure position, where the complete film is removed. In addition, the turning point also means that the film is broken from the substrates, therefore it is usually defined as the critical load of failure film[13], corresponding to the abscissa of point C in the linear loading curve ④. The critical load represented the integrated mechanical properties of Al films to resistance damage, and depended on the properties of films, substrates and its interface.
In comparison to scratching curve ② and final topography scan curve ③, the depth reduced by reason
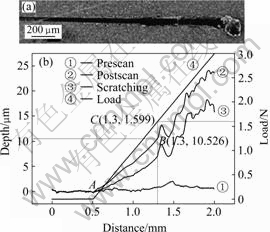
Fig.4 SEM image of Al films(a) and typical depth—displacement curves from scratch tests on Al films
of the elastic recovery of Al films after unloading, therefore it suggested that the Al films has good elastic and plastic properties.
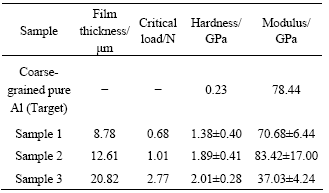
The variations of thickness and the critical load of samples with respect to different sputtering times are listed in Table 2. The thickness of the sample 1 (120 min), sample 2 (150 min) and sample 3 (300 min) are found to be 8.78, 12.61 and 20.82 μm, respectively. As the sputtering time increases, the thickness of aluminum and the critical load also increases gradually.
Indentation tests are performed on each sample and the corresponding loading curves are plotted in Fig.5.

Fig.5 Load—depth curves of Al films on Mg alloys: (a) Sample 1; (b) Sample 2; (c) Sample 3
The test curves were relatively smooth, mainly due to fluctuations in the surface structure. As shown in Fig.5, poor uniformity of surface structure of sample 2 results in the bad consistency of the test curves. The average hardness and modulus values are calculated from nine load—depth curves taken on the films, and the results are summarized in Table 2. It is observed that the hardness of sample 1 (120 min) and sample 2 (150 min) are 1.38 and 1.89 GPa, respectively. The corresponding moduli are 70.68 and 83.42 GPa, respectively. With the sputtering time increasing to 300 min, the hardness increases to 2.01 GPa. Moreover, the hardness of Al films is higher than that of coarse-grained pure aluminum target, which may be attributed to the finer grain of the deposited films. With increasing depth, the hardness of aluminum films reduces gradually to the hardness of magnesium alloy substrates. Al and Mg have similar characteristics, so their performance shows better match. As a result, it is suggested that Al films can improve the surface mechanical properties of magnesium alloy, and the thicker the Al film is, the better protection the film have on the mechanical properties of substrate.
Table 2 Nano-test results of UFG/NC pure Al thick films on AZ31B Mg alloy
3.3 Corrosion resistance of Al films on AZ31B alloy
Fig.6 shows the polarization curves of Al films on AZ31B Mg alloys in 3.5% NaCl solution, and the fitting results are summarized in Table 3.
It can be seen from Fig.6 that, the corrosion potential of bare AZ31B is -1.527 V, and those, after deposited with different depths of aluminum films, positively shift to -1.497 (Sample 1), -1.442 (Sample 2) and -1.361 V (Sample 3), respectively. In addition, both anodic and cathodic current densities of the magnesium alloys after deposited Al films move to lower values. The corrosion rates increase in the following order: Sample 3<Sample 2<Sample 1<bare AZ31B alloy.
The uncoated AZ31B alloy shows no passivation in the solution, which can be seen in the polarization curves. However, it can be seen that the anodic current density of Al films on AZ31B alloy samples is smaller when compared with that of the bare alloy, especially sample

Fig.6 Polarization curves of Al films on AZ31B Mg alloys in 3.5% NaCl solution
Table 3 Parameters and corrosion test results of Al films on AZ31B Mg alloys
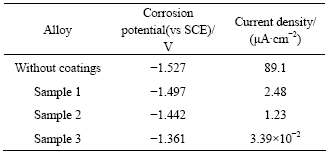
3. As to AZ31B deposited with Al film for sputtering time of 300 min, the anodic current density remains at 0.10-0.08 mA/cm2, and passive-like behavior is observed.
The thickness and critical load of Al film samples vary greatly with sputtering time which affects the corrosion resistance of samples. There is a slight increase of corrosion potential due to the thickness and critical load increase when the sputtering times increase from 120 min to 150 min. With increasing sputtering time, the thickness and critical load increase significantly, especially after deposited aluminum film for 300 min (Sample 3), the corrosion potential increased is 166 mV larger than that of the bare magnesium alloy. Meantime, the corrosion current density also decreases by three orders of magnitude.
In order to investigate the corrosion behavior of the samples, the macroscopic and microscopic images of the sample surfaces were observed as shown in Fig.7. It can be seen that remarkable corrosion appears on Sample 1, but no corrosion attack appears on Sample 3. Some crack and detachment exist in Sample 1 and Sample 2, due to corrosion solution penetrating the film. The large volumes of the corrosion products lead to the film lifts off or even peels off. Only one crack is observed in Sample 2 sputtering with 150 min, while much more cracks and pitting exist on Sample 1. In contrast, an Al film (Sample 3) is still intact and only two small shadow pits appear on the coatings surface. This infers that the thicker the Al film, the better the corrosion resistance. It is observed that there is discontinuous thin passive film or corrosion product film on the surface of Sample 3 (see Fig.7).
Small defects in the film, such as pinholes, pores or cracks, would decrease the corrosion resistance of the alloy. The defect is like a path to allow corrosion media to reach the substrate surface and leads to the formation of a galvanic cell and corrosion pitting[2]. Nevertheless, according to the phenomenon in this study, it can be observed that the thickness and relatively poor adhesion between coating and substrate are the significant reasons for severely peeled off from the AZ31B substrate. Therefore, in order to improve the corrosion resistance AZ31B alloy with Al films, it is necessary to pay attention to the deposition conditions, especially the sputtering power and Ar pressure. Another important way is to deposit thicker films by increasing the sputtering time. Some studies confirmed that the thick film has great film/substrate properties[14-15], therefore, it can better improve the corrosion resistance and protect the magnesium alloy in the process of corrosion. Further developments are required for Al films as coatings to provide AZ31B substrate a long term protection in the aggressive environment.
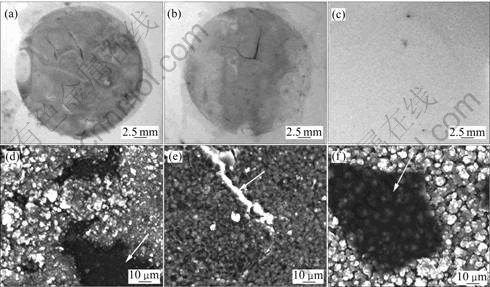
Fig.7 Macroscopic images (a, b, c) and SEM images (d, e, f) of samples after test in 3.5% NaCl solution: (a), (d) Sample 1, 120 min; (b), (e) Sample 2, 150 min; (c), (f) Sample 3, 300 min
4 Conclusions
1) Aluminum films with thickness of 8.78-20.82 μm were deposited on AZ31B magnesium alloy using DC magnetron sputtering. The fine and uniform grain clusters of Al film show typical polycrystalline Al with face-centered cubic structure.
2) The surface hardness of aluminum film on AZ31B magnesium alloy ranges in 1.38-2.01 GPa, higher than that of the magnesium alloy substrate. The critical load of Al film/AZ31B substrate ranges in 0.68-2.77 N. With increasing sputtering time, the thickness of Al films increases. Al films exhibit good adhesion and hardness. Thicker Al films can protect magnesium alloy with good film/substrate properties.
3) Aluminum films deposited on AZ31B magnesium alloy improve the corrosion resistance. The corrosion potential shifts positively, and the corrosion current density is reduced by 2-3 orders of magnitude. The corrosion resistance of AZ31B with Al films is improved distinctly.
References
[1] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—A critical review[J]. Journal of Alloys and Compounds, 2002, 336(1/2): 88-113.
[2] HOLLSTEIN FRANK, WIEDEMANN RENATE, SCHOLZ JANA. Characteristics of PVD-coatings on AZ31HP magnesium alloys[J]. Surface and Coatings Technology, 2003, 162(2/3): 261-268.
[3] ZHANG Jin, WANG Ying, ZENG Rong-chang, HUANG Wei-jiu. Effects of post heat treatment on the interfacial characteristics of aluminum coated AZ91D magnesium alloy[J]. Materials Science Forum, 2007, 546/549: 529-532.
[4] ZHANG Jin, SUN Zhi-fu. Research on magnesium alloy AZ91D surface coating by metallizing aluminum[J]. China Mechanical Engineering, 2002, 23: 2057-2058. (in Chinese)
[5] ZHANG Jin, WANG Ying. Effect of heat treatment on microstructures and properties of zinc-aluminum coating on AZ91D magnesium alloy[J]. Key Engineering Materials, 2008, 373/374: 55-58.
[6] WU S K, YEN S C, CHOU T S. A study of RF-sputtered Al and Ni thin films on AZ91D magnesium alloy[J]. Surface and Coatings Technology, 2006, 200(8): 2769-2774.
[7] WU Guo-song. Fabrication of Al and Al/Ti coatings on magnesium alloy by sputtering[J]. Materials Letters, 2007, 61(18): 3815-3817.
[8] BOHNE Y, MANOVA D, BLAWERT C, STORMER M, DIETZEL W, MANDL S. Influence of ion energy on morphology and corrosion properties of Mg alloys formed by energetic PVD processes[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2007, 257(1/2): 392-396.
[9] CHANG J K, CHEN S Y, TSAI W T, DENG M J, SUN I W. Electrodeposition of aluminum on magnesium alloy in aluminum chloride (AlCl3)-1-Ethyl-3- methylimidazolium chloride (emic) ionic liquid and its corrosion behavior[J]. Electrochemistry Communications, 2007, 9(7): 1602-1606.
[10] LIU Y, OVERZET L J, GOECKNER M J. Chemical vapor deposition of aluminum from methylpyrrolidine alane complex[J]. Thin Solid Films, 2006, 510(1/2): 48-54.
[11] PETROV I, BARNA P B, HULTMAN L. Microstructural evolution during film growth[J]. Journal of Vacuum Science & Technology A, 2003, 21(5): 117-128.
[12] POLOP C, HANSEN H, LANGENKAMP W, ZHONG Z, BUSSE C, LINKE U, KOTRLA M, FEIBELMAN PETER J, MICHELY T. Oscillatory interaction between O impurities and Al adatoms on Al(111) and its effect on nucleation and growth[J]. Surface Science, 2005, 575(1/2): 89-102.
[13] BHUSHAN B. Handbook of micro/nanotribology[M]. 2nd ed. Boca Raton: CRC Press, 1999.
[14] QI Jun, CHAN C Y. Film thickness effects on mechanical and tribological properties of nitrogenated diamond like carbon films[J]. Surface and Coatings Technology, 2001, 145: 38-43.
[15] ZHANG Hua-li, ZHANG Fei-hu. Study of nanoscratch resistance by experiment based on nano indenter[J]. Machinery & Electronics, 2006(3): 3-6. (in Chinese)
Foundation item: Project(2070950) supported by the Key Research Projects of the Ministry of Education, China; Project(KJ070617) supported by the Chongqing Municipal Education Commission, China
Corresponding author: ZHANG Jin; Tel.: +86-10-82377393; E-mail: zhangjin@mater.ustb.edu.cn


