
J. Cent. South Univ. (2018) 25: 526-533
DOI: https://doi.org/10.1007/s11771-018-3757-0
Preparation of α-calcium sulfate hemihydrate whiskers with high aspect ratios in presence of a minor amount of CuCl2·2H2O
GUAN Qing-jun(管青军), SUN Wei(孙伟), LIU Run-qing(刘润清),YIN Zhi-gang(殷志刚), ZHANG Chen-hu(张谌虎)
School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
In order to produce α-calcium sulfate hemihydrate (α-CaSO4·0.5H2O) whiskers with high aspect ratios, a minor amount of CuCl2·2H2O was used as the modifying agent in the process of hydrothermal treatment of calcium sulfate dihydrate (CaSO4·2H2O) precursor. The presence of 2.60×10–3 mol/L CuCl2·2H2O resulted in the increase of the aspect ratios of α-CaSO4·0.5H2O whiskers from 81 to 253. The preferential adsorption of Cu2+ on the negative {110} and {100} facets of α-CaSO4·0.5H2O crystal structures was confirmed by EDS and XPS. And ATR-FTIR demonstrated the ligand adsorption of Cu2+ on the surface of α-CaSO4·0.5H2O whiskers. The experimental results reveal that the whiskers with high aspect ratios are attributed to the adsorption of Cu2+, which promotes the 1-D growth of α-CaSO4·0.5H2O whiskers along the c axis.
Key words:
α-CaSO4·0.5H2O whisker; CuCl2·2H2O; hydrothermal treatment; aspect ratio;
Cite this article as:
GUAN Qing-jun, SUN Wei, LIU Run-qing, YIN Zhi-gang, ZHANG Chen-hu. Preparation of α-calcium sulfate hemihydrate whiskers with high aspect ratios in presence of a minor amount of CuCl2·2H2O [J]. Journal of Central South University, 2018, 25(3): 526–533.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3757-01 Introduction
Crystal whiskers are usually regarded as filamentary crystals with regular cross section and almost perfect crystallographic structure, which has attracted tremendous interests of researchers in recent years due to their excellent performance in thermal stability, chemical resistance and mechanical strength [1–4]. The whiskers are proposed as more effective reinforcements than traditional fibers, such as carbon fibers and glass fibers. However, the unaffordable cost of some whiskers, such as SiC whiskers, is becoming a barrier for their widespread use. And the inexpensive α-CaSO4·0.5H2O whiskers attract material scientists attention. α-CaSO4·0.5H2O whiskers, which are traditionally prepared by calcic minerals such as natural gypsum, phosphogypsum, and flue gas desulfurization gypsum [5–7] by means of autoclave technology, mixed salts solution technology and alcohol-water method, can obviously enhance the mechanical properties of rubbers, plastics, adhesives and papers [8–13]. Therefore, calcium sulfate whiskers have great potential to become ideal reinforcing materials.
The mechanical strength of α-CaSO4·0.5H2O whiskers tends to improve with an increase in the aspect ratio [14, 15], which is one of the most important evaluation criterions of calcium sulfate whiskers. And much effort has been made to control the crystallization and morphology of α-CaSO4·0.5H2O whiskers to improve the aspect ratios [16, 17]. For example, the active CaSO4·2H2O, which is produced by the calcination-hydration treatment, can promote the formation of α-CaSO4·0.5H2O whiskers with high aspect ratios [18]. And the use of some crystal growth modifiers can significantly improve the aspect ratios, for example, due to the preferential adsorption of Mg2+ on the side facets of α-CaSO4·0.5H2O, α-CaSO4·0.5H2O whiskers with high aspect ratios can be prepared using the hydrothermal treatment in the presence of Mg2+[5, 19]; some organic molecules, such as cetyltrimethyl ammonium bromide (CTAB) [20] and potassium sodium tartrate [21] can also have the same effect. The change of crystallization environment, including the adjustment of pH and electrolyte concentration [6] or addition of ethanol[22], is also beneficial to the formation of α-CaSO4·0.5H2O whiskers.
In this work, α-CaSO4·0.5H2O whiskers with high aspect ratios were produced by the hydrothermal reaction of calcium sulfate dihydrate precursor using CuCl2·2H2O as the modifying agent, and the corresponding mechanism was studied.
2 Experimental
2.1 Materials and experimental procedure
CaSO4·2H2O (AR) and CuCl2·2H2O (AR) were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
CaSO4·2H2O was calcinated at 150 °C for 4.0 h in a muffle furnace, then mixed with deionized water and stirred (100 r/min) at room temperature for 1.0 h to obtain the suspension containing 2.5% CaSO4·2H2O. Suspension with a certain amount of CuCl2·2H2O was treated in an autoclave at 130 °C for 4.0 h. When the hydrothermal reaction was finished, the slurries were filtrated and washed with ethanol, and then dried at 90 °C for 4.0 h.
2.2 Characterization
The structures of the samples were determined by X-ray diffraction (XRD D8 Advanced, Bruker, Germany) using Cu Kα radiation (λ=1.54178  ), with a scanning rate of 5 (°)/min and a scanning 2θ range of 5° to 90°. The concentration of Cu2+ in the solution was determined by the inductively coupled plasma-atomic emission spectrometry (ICP-AES, PS-6, Baird, USA). The morphology of the samples was characterized with the field-emission scanning electron microscopy (SEM, JSM-6490LV, JEOL, Japan) and the high resolution transmission electron microscopy (HRTEM, JEM-2100F, JEOL, Japan) equipped with the selected area electron diffraction (SAED). The surfaces of the whiskers were analyzed by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher, USA) with a Al Kα photon energy of 1486.6 eV. C 1s peak at 284.8 eV, which is related to the carbon adsorbed on the surface during the exposure of the samples to the ambient atmosphere, was used as reference for all spectra. The interaction between Cu2+ and SO42- was analyzed by Flourier Translation Infrared Spectroscopy (FT-IR, IRAffinity-1, Shimadzu, Japan) with a resolution of 2 cm-1 over the frequency range of 400-4000 cm-1. The average lengths, diameters and aspect ratios of the whiskers for each sample were counted using around 150 crystal particles from the SEM images with magnifications of 250–4000.
), with a scanning rate of 5 (°)/min and a scanning 2θ range of 5° to 90°. The concentration of Cu2+ in the solution was determined by the inductively coupled plasma-atomic emission spectrometry (ICP-AES, PS-6, Baird, USA). The morphology of the samples was characterized with the field-emission scanning electron microscopy (SEM, JSM-6490LV, JEOL, Japan) and the high resolution transmission electron microscopy (HRTEM, JEM-2100F, JEOL, Japan) equipped with the selected area electron diffraction (SAED). The surfaces of the whiskers were analyzed by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher, USA) with a Al Kα photon energy of 1486.6 eV. C 1s peak at 284.8 eV, which is related to the carbon adsorbed on the surface during the exposure of the samples to the ambient atmosphere, was used as reference for all spectra. The interaction between Cu2+ and SO42- was analyzed by Flourier Translation Infrared Spectroscopy (FT-IR, IRAffinity-1, Shimadzu, Japan) with a resolution of 2 cm-1 over the frequency range of 400-4000 cm-1. The average lengths, diameters and aspect ratios of the whiskers for each sample were counted using around 150 crystal particles from the SEM images with magnifications of 250–4000.
3 Results and discussion
3.1 Influence of CuCl2·2H2O on morphology of α-CaSO4·0.5H2O whiskers formed by hydrothermal reaction
The influence of CuCl2·2H2O on morphology and XRD patterns of the products formed at 130 °C for 4.0 h in an autoclave are shown in Figure 1, and the diameter and aspect ratio distributions of the products are shown in Figure 2. In the absence of CuCl2·2H2O, α-CaSO4·0.5H2O whiskers with a length of 20–140 μm, an average diameter of 1.60 μm and an average aspect ratio of 81 were formed (Figures 1(a) and 2(a)). When the concentration of CuCl2·2H2O was 1.32×10–3 mol/L, the length of whiskers was significantly increased along the c-axis direction with a length of 130–240 μm and an average diameter of 1.03 μm, which resulted in a larger aspect ratio of 213 (Figures 1(b) and 2(b)). Further increasing the concentration of CuCl2·2H2O to 2.60×10–3 mol/L led to longer whiskers of 160–300 μm in length, and the average diameter and aspect ratio could reach to 0.87 μm and 253, respectively (Figures 1(c) and 2(c)). TEM, high-resolution TEM (HRTEM) and SAED (Figures 1(d) and (e)) have been performed to explore the morphology and structure of the hydrothermal products further. Figures 1(d) and (e) show that the interplanar spacing of the lattice fringes along the growth direction of the whiskers was 0.560 nm, which corresponds to the (002) plane (d(002)=0.599 nm) of α-CaSO4·0.5H2O. This indicates that the crystals grow along the (001) direction. The electronic diffraction spots in the SAED pattern (Figure 1(e)) can be indexed to the 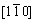 zone axis of α-CaSO4·0.5H2O, further confirming the preferential growth of the crystals along the (001) direction. Figure 1(f) exhibits XRD patterns of the hydrothermal products. The XRD peaks all belong to α-CaSO4·0.5H2O, indicating the sole existence of α-CaSO4·0.5H2O.
zone axis of α-CaSO4·0.5H2O, further confirming the preferential growth of the crystals along the (001) direction. Figure 1(f) exhibits XRD patterns of the hydrothermal products. The XRD peaks all belong to α-CaSO4·0.5H2O, indicating the sole existence of α-CaSO4·0.5H2O.
EDS patterns of α-CaSO4·0.5H2O whiskers prepared with different concentrations of CuCl2·2H2O are shown in Figure 3. The atomic ratios of different atoms are labeled at the corresponding peaks. Compared with the whiskers prepared without CuCl2·2H2O(curve a), 0.86% and 1.38% of Cu are detected in the presence of 1.32× 10–3 mol/L(curve b) and 2.60×10–3 mol/L(curve c), while no Cl was detected in all cases. And the surface composition of α-CaSO4·0.5H2O whiskers analyzed by XPS (Table 1) also shows that there is no Cl in all cases. All these indicated that Cl has no effect on the morphology of α-CaSO4·0.5H2O whiskers in the hydrothermal process.
3.2 Adsorption of Cu2+ on surface of α-CaSO4·0.5H2O whiskers
The surface characteristics of the hydrothermal products were analyzed by XPS (Figure 4). In Figure 4(a), the peaks of 2p1/2(953.9 eV) and 2p3/2(933.9 eV) correspond to Cu2+[23, 24] and these peaks occur in curves b and c, which implies the adsorption of Cu2+ on surface of the whiskers formed in the presence of CuCl2 2H2O. Figure 4(b) displays that a single S 2p peak is detected at the band energy of 169.4 eV in curve a, while double S 2p peaks are observed in curves b and c: one peak is located at 169.4 eV, which is indexed to the interaction between Ca2+ and SO42+, and the other peak is located at 170.3 eV, which indicate the interaction between Cu2+ and SO42+[23, 25]. The occurrence of double S 2p should be attributed to the adsorption of Cu2+ on the surface of α-CaSO4·0.5H2O whiskers. And owing to the higher electronegativity of Cu(1.90) than Ca(1.00), the band energy between Cu and S is stronger than that between Ca and S, which leads to the shift of S 2p in CuSO4 to the right side [26]. Table 1 shows the atomic ratios of the surface elements of α-CaSO4·0.5H2O whiskers.
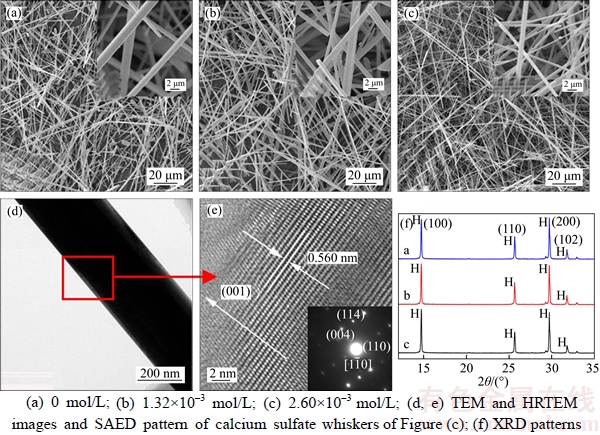
Figure 1 Influence of CuCl2·2H2O on morphology and XRD patterns of α-CaSO4·0.5H2O whiskers:
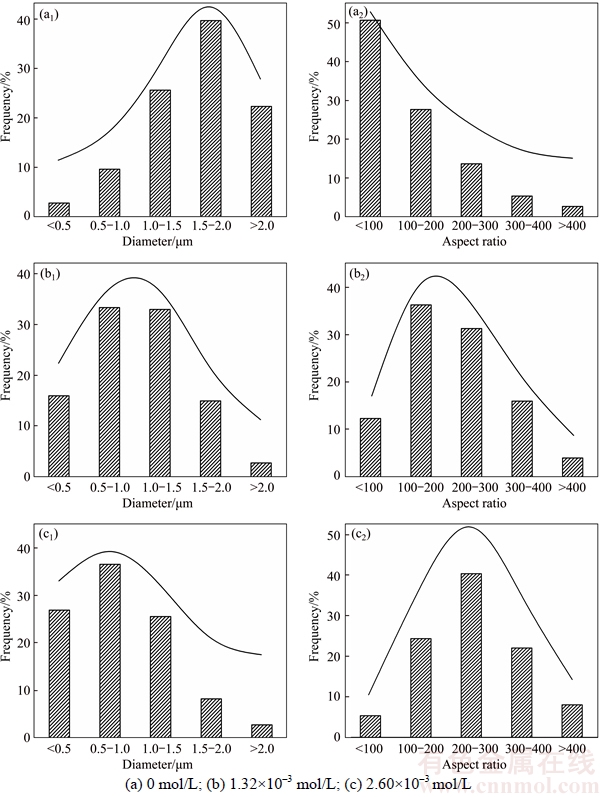
Figure 2 Diameter (a1, b1, c1) and aspect ratio distributions (a2, b2, c2) of α-CaSO4·0.5H2O whiskers prepared with different concentrations of CuCl2·2H2O:
The adsorption of Cu2+ on the surfaces of α-CaSO4·0.5H2O whiskers may be closely related to the properties of α-CaSO4·0.5H2O crystal surfaces. As shown in Figure 5, the α-CaSO4·0.5H2O lattices are composed of repeating, ionically bonded Ca and SO4 atoms in chains of —Ca—SO4—Ca—SO4—, and SO4 is a tetrahedral structure in which each S atom is covalently bonded to four O atoms [27, 28]. The chains structure may account for the fact that α-CaSO4·0.5H2O normally grows in the 1D shape. These chains are hexagonally and symmetrically arranged and form a framework parallel to the c axis, and along the c axis there exist continuous channels with a diameter of about 4.5  , where one water molecule is attached to every two calcium sulfate molecules [29]. The crystal structure makes the distribution of Ca2+ denser on the top facets of {111} and the distribution of SO42– denser on the side facets of {110} and {100}, which makes {111} facets positively charged and {110} and {100} facets negatively charged as shown in Figure 6 [27, 29]. Therefore, the positive Cu2+ is adsorbed more easily on the side facets of α-CaSO4·0.5H2O crystal structures, which promotes the 1D growth of α-CaSO4·0.5H2O whiskers along the c axis.
, where one water molecule is attached to every two calcium sulfate molecules [29]. The crystal structure makes the distribution of Ca2+ denser on the top facets of {111} and the distribution of SO42– denser on the side facets of {110} and {100}, which makes {111} facets positively charged and {110} and {100} facets negatively charged as shown in Figure 6 [27, 29]. Therefore, the positive Cu2+ is adsorbed more easily on the side facets of α-CaSO4·0.5H2O crystal structures, which promotes the 1D growth of α-CaSO4·0.5H2O whiskers along the c axis.
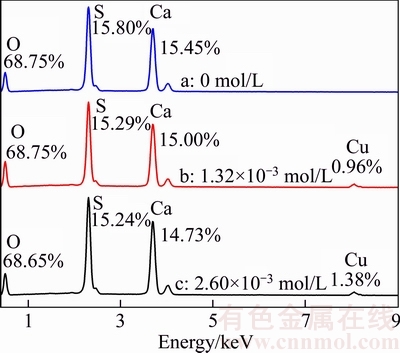
Figure 3 EDS patterns of α-CaSO4·0.5H2O whiskers prepared with different concentrations of CuCl2·2H2O
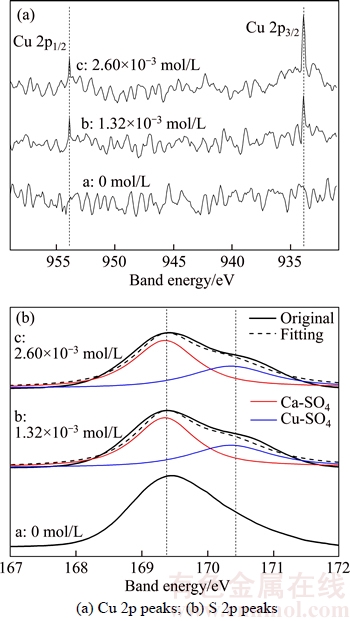
Figure 4 XPS spectra of α-CaSO4·0.5H2O whiskers formed in presence of different concentrations of CuCl2·2H2O:
Table 1 Surface composition of α-CaSO4·0.5H2O whiskers prepared with different concentrations of CuCl2·2H2O
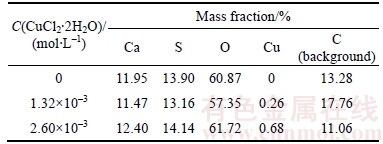
Figure 5 Crystal structure of α-CaSO4·0.5H2O lattice along c axis
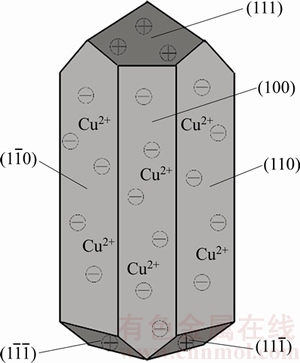
Figure 6 Schematic representation of α-CaSO4·0.5H2O whiskers in presence of CuCl2·2H2O
On the foundation of above discussion that Cu2+ may be adsorbed preferentially on the side facets of α-CaSO4·0.5H2O whiskers which are negatively charged, the specific adsorption styles between Cu2+ and α-CaSO4·0.5H2O are explored by ATR-FTIR spectroscopy. Figure 7 shows the ATR-FTIR spectra of α-CaSO4·0.5H2O whiskers prepared in the presence of different concentration of CuCl2·2H2O. The triple bands at 1097, 1111, and 1153 cm–1 can be assigned to the asymmetric stretching vibration of υ3 SO42–, which are attributed to the inter-sphere complexation between Ca2+ and SO42– [30, 31]. In curve a, the band at 1005 cm–1 should be assigned to the distorted symmetric stretching vibration of υ1 SO42– because of Ca2+ adsorption, however, in the curves b and c, the υ1 band shifts towards higher wavenumbers of 1009 cm–1 and the corresponding intensity also increases, which indicates the existence of the outer-sphere complex structure formed by Cu2+ and SO42– when Cu2+ is adsorbed on the side facets of α-CaSO4·0.5H2O [20, 32–34]. And the higher electronegativity of Cu (1.90) than Ca (1.00) can result in stronger induction effect on the symmetric stretching vibration of υ1 SO42– , which leads to the shift towards higher wavenumbers [35].
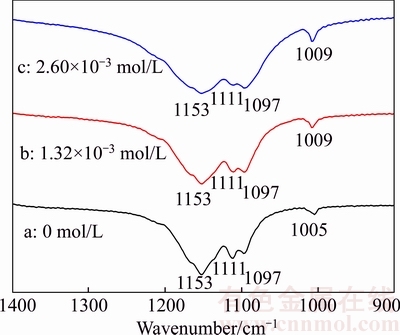
Figure 7 ATR-FTIR spectra of α-CaSO4·0.5H2O whiskers formed in presence of different concentration of CuCl2·2H2O
The adsorption of Cu2+ on the side of facets of α-CaSO4·0.5H2O lattices formed a covering layer of CuSO4, and the layer may inhibit the cross growth of the whiskers and promote the vertical growth along the c axis.
4 Conclusions
1) α-CaSO4·0.5H2O whiskers with an average aspect ratio of 81 could be prepared in the absence of CuCl2·2H2O. When the concentration of CuCl2·2H2O is 1.32×10–3 mol/L, the length of whiskers is significantly increased along the c-axis direction, which leads to a larger aspect ratio of 213. And further increasing the concentration of CuCl2·2H2O to 2.60×10–3 mol/L could result in the increase of the average aspect ratio of α-CaSO4·0.5H2O whiskers from 213 to 253.
2) The preferential adsorption of Cu2+ on the more electronegative facets of {110} and {100} by formation of the outer-sphere complex structure between Cu2+ and SO42– restrains the facets growth, which should be responsible for the formation of α-CaSO4·0.5H2O whiskers with high aspect ratios.
References
[1] CAO Yan, GALOPPINI E, REYES P I, DUAN Zi-qing, LU Yi-cheng. Morphology effects on the biofunctionalization of nanostructured ZnO [J]. Langmuir, 2012, 28(21): 7947–7951.
[2] SPANO F, MASSARO A, BLASI L, MALERBA M, CINGOLANI R, ATHANASSIOU A. In situ formation and size control of gold nanoparticles into chitosan for nanocomposite surfaces with tailored wettability [J]. Langmuir, 2012, 28(8): 3911–3917.
[3] TSAGGEOS K, MASIERA N, NIWICKA A, DOKOROU V, SISKOS M G, SKOULIKA S, MICHAELIDES A. Crystal structure, thermal behavior, and photochemical reactivity of a series of co-crystals of trans-1, 2-bis (4-pyridyl) ethylene with dicarboxylic acids [J]. Crystal Growth and Design, 2012, 12(5): 2187–2194.
[4] LIU Cheng-jun, ZHAO Qing, WANG Ye-guang, SHI Pei-yang, JIANG Mao-fa. Surface modification of calcium sulfate whisker prepared from flue gas desulfurization gypsum [J]. Applied Surface Science, 2015, 360: 263–269.
[5] WANG Li, MA Ji-hong, GUO Zeng-wei, DONG Bao-sheng, WANG Gao-min. Study on the preparation and morphology of calcium sulfate whisker by hydrothermal synthesis method [J]. Materials Science and Technology, 2006, 14(6): 626–629. (in Chinese)
[6] MIAO Miao, FENG Xin, WANG Gang-ling, CAO Shao-mei, SHI Wen, SHI Li-yi. Direct transformation of FGD gypsum to calcium sulfate hemihydrate whiskers: Preparation, simulations, and process analysis [J]. Particuology, 2015, 19(2): 53–59.
[7] WANG Xiao, YANG Liu-shuan, ZHU Xin-feng, YANG Jia-kuan. Preparation of calcium sulfate whiskers from FGD gypsum via hydrothermal crystallization in the H2SO4-NaCl- H2O system [J]. Particuology, 2014, 17: 42–48.
[8] LIU Ling, YIN Ning, KANG Mao-qing, WANG Xin-kui. Study on CaSO4 whisker reinforcing and toughening mechanisms for polyurethane elastomer [J]. Acta Polymerica Sinica, 2001, 80(2): 245–249. (in Chinese)
[9] HU Xiao-lan, YU Mou-fa, Study of calcium sulfate whiskers modified bismaleimide resin by friction and wear properties [J]. Acta Polymerica Sinica, 2006, 6(5): 686–691. (in Chinese)
[10] LIU Jin-yan, REN Li, WEI Qiang, WU Jian-ling, LIU Sa, WANG Ying-jun, LI Guo-yuan. Microstructure and properties of polycaprolactone/calcium sulfate particle and whisker composites [J]. Polymer Composites, 2012, 33(4): 501–508.
[11] WANG Jin-cheng, TANG Li-juan, WU Ding, GUO Xi, HAO Wen-li. Application of modified calcium sulfate whisker in methyl vinyl silicone rubber composites [J]. Polymers & Polymer Composites, 2012, 20(5): 453–462.
[12] WANG Jin-cheng, XUE Ye, CANG Shi-jiao. Studies on the application properties of calcium sulfate whisker in silicone rubber composites [J]. Journal of Elastomers & Plastics, 2012, 44(1): 55–66.
[13] FENG Xin, ZHANG Ying, WANG Gang-ling, MIAO Miao, SHI Li-yi. Dual-surface modification of calcium sulfate whisker with sodium hexametaphosphate/silica and use as new water-resistant reinforcing fillers in papermaking [J]. Powder Technology, 2015, 271: 1–6.
[14] BACON D J, BARNETT D M, SCATTERGOOD R O. Anisotropic continuum theory of lattice defects [J]. Progress in Materials Science, 1978, 23: 51–262.
[15] GURTIN M E, MURDOCH A L. A continuum theory of elastic material surfaces [J]. Archive for Rational Mechanics and Analysis, 1975, 57(4): 291–323.
[16] WANG Xiao, JIN Biao, YANG Liu-shuan, ZHU Xin-feng. Effect of CuCl2 on hydrothermal crystallization of calcium sulfate whiskers prepared from FGD gypsum [J]. Crystal Research & Technology, 2015, 50(8): 633–640.
[17] MAO Xiu-long, SONG Xin-fu, LU Gui-min, SUN Yu-zhu, XU Yan-xia, YU Jian-guo. Effects of metal ions on crystal morphology and size of calcium sulfate whiskers in aqueous HCl solutions [J]. Industrial & Engineering Chemistry Research, 2014, 53(45): 17625–17635.
[18] HOU Si-chao, XIANG Lan. Influence of activity of CaSO4·2H2O on hydrothermal formation of CaSO4·0.5H2O whiskers [J]. Journal of Nanomaterials, 2013(2013): Article ID 237828.
[19] HOU Si-chao, WANG Jing, WANG Xiao-xue, CHEN Hao-yuan, XIANG Lan. Effect of Mg2+ on hydrothermal formation of α-CaSO4·0.5H2O whiskers with high aspect ratios [J]. Langmuir, 2014, 30(32): 9804–9810.
[20] KONG Bao, GUAN Bao-hong, YATES M Z, WU Zhong-biao. Control of α-calcium sulfate hemihydrate morphology using reverse microemulsions [J]. Langmuir, 2012, 28(40): 14137–14142.
[21] SHEN Zhuo-xian, GUAN Bao-hong, FU Hai-lan, YANG Liu-chun. Effect of potassium sodium tartrate and sodium citrate on the preparation of α-calcium sulfate hemihydrate from flue gas desulfurization gypsum in a concentrated electrolyte solution [J]. Journal of the American Ceramic Society, 2009, 92(12): 2894–2899.
[22] PAN Zong-you, YANG Guang-yong, LOU Yi , XUE En-xing, XU Hua-zi, MIAO Xi-geng, LIU Jian-li, HU Chun-feng, HUANG Qing. Morphology control and self-setting modification of α-calcium sulfate hemihydrate bone cement by addition of ethanol [J]. International Journal of Applied Ceramic Technology, 2013, 10(s1): E219–E225.
[23] SIRIWARDANE R V, JR J A P, FISHER E P, SHEN M S, MILTZ A L. Decomposition of the sulfates of copper, iron (II), iron (III), nickel, and zinc: XPS, SEM, DRIFTS, XRD, and TGA study [J]. Applied Surface Science, 1999, 152(s3, 4): 219–236.
[24] HAYEZ V, FRANQUET A, HUBIN A, TERRYN H. XPS study of the atmospheric corrosion of copper alloys of archaeological interest [J]. Surface and Interface Analysis, 2004, 36(8): 876–879.
[25] COMYN J. Practical surface analysis—by Auger and X-ray photoelectron spectroscopy [J]. International Journal of Adhesion and Adhesives, 1984, 4(3): 142.
[26] WEAVER J H, CHAI Y, KROLL G H, JIN C, OHNO T R, HAUFLER R E, GUO T, ALFORD J M, CONCEICAO J, CHIBANTE L P F. XPS probes of carbon-caged metals [J]. Chemical Physics Letters, 1992, 190(5): 460–464.
[27] BALLIRANO P, MARAS A, MELONI S, CAMINITI R. The monoclinic I2 structure of bassanite, calcium sulphate hemihydrate (CaSO4·0.5H2O) [J]. European Journal of Mineralogy, 2001, 13(5): 985–993.
[28] BEZOU C, NONAT A, MUTIN J C, CHRISTENSEN A N, LEHMANN M S. Investigation of the crystal structure of γ-CaSO4, CaSO4·0.5H2O, and CaSO4·0.6H2O by powder diffraction methods [J]. Journal of Solid State Chemistry, 1995, 117(1): 165–176.
[29] FREYER D, VOIGT W. Crystallization and phase stability of CaSO4 and CaSO4–based salts [J]. Monatshefte Fuer Chemie/chemical Monthly, 2003, 134(5): 693–719.
[30] GUAN Qing-jun, TANG Hong-hu, SUN Wei, HU Yue-hua, YIN Zhi-gang. Insight into influence of glycerol on preparing α-CaSO4· H2O from flue gas desulfurization gypsum in glycerol-water solutions with succinic acid and NaCl [J]. Industrial & Engineering Chemistry Research, 2017, 56: 9831–9838.
H2O from flue gas desulfurization gypsum in glycerol-water solutions with succinic acid and NaCl [J]. Industrial & Engineering Chemistry Research, 2017, 56: 9831–9838.
[31] WANG Yu, PENG Ying-lin, ZHENG Ya-jie. Recovery of magnetite from FeSO4·7H2O waste slag by co-precipitation method with calcium hydroxide as precipitant [J]. Journal of Central South University, 2017, 24(1): 62–70.
[32] HUG S J. In situ Fourier transform infrared measurements of sulfate adsorption on hematite in aqueous solutions [J]. Journal of Colloid and Interface Science, 1997, 188(2): 415–422.
[33] PEAK D, FORD R G, SPARKS D L. An in situ ATR-FTIR investigation of sulfate bonding mechanisms on goethite [J]. Journal of Colloid and Interface Science, 1999, 218(1): 289–299.
[34] NAKAMOTO K. Infrared and Raman spectra of inorganic and coordination compounds [M]. Hoboken, New Jersey: John Wiley & Sons, INC., 1986: 119–123.
[35] COLTHUP N B, DALY L H, WIBERLEY S E. Introduction to infrared and Raman spectroscopy [M]. San Diego: Academic Press, 2012: 375–380.
(Edited by FANG Jing-hua)
中文导读
少量CuCl2·2H2O作用下制备高长径比α-半水硫酸钙晶须
摘要:为了制备高长径比的α-半水硫酸钙(α-CaSO4·0.5H2O)晶须,在以二水硫酸钙(CaSO4·2H2O)前驱体为原料的水热反应过程中加入CuCl2·2H2O作为媒晶剂。当反应体系中加入2.60×10–3 mol/L的CuCl2·2H2O时可以使产物α-半水硫酸钙晶须的长径比由81增加到253。EDS和XPS检测结果表明, CuCl2·2H2O能够显著增加α-半水硫酸钙晶须的长径比主要是因为Cu2+优先吸附于α-CaSO4·0.5H2O晶体带负电的{110}和{100}面,从而阻碍了侧面的生长,最终导致晶体长径比的增加。ATR-FTIR进一步证实了Cu2+主要通过配位吸附的形式与α-CaSO4·0.5H2O晶体表面进行作用。
关键词:α-半水硫酸钙晶须;CuCl2·2H2O;水热处理;长径比
Foundation item: Project(B14034) supported by the National 111 Project, China; Project(2015CX005) supported by the Innovation Driven Plan of Central South University, China; Project(2016zzts104) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2016-06-26; Accepted date: 2016-08-25
Corresponding author: SUN Wei, PhD, Professor; Tel: +86–731–88876697; E-mail: sunmenghu@csu.edu.cn; ORCID: 0000-0002-9173- 2682; LIU Run-qing, PhD, Associate Professor; Tel: +86–731–88830482; E-mail: liurunqing@126.com; ORCID: 0000-0002-7600-5452
Abstract: In order to produce α-calcium sulfate hemihydrate (α-CaSO4·0.5H2O) whiskers with high aspect ratios, a minor amount of CuCl2·2H2O was used as the modifying agent in the process of hydrothermal treatment of calcium sulfate dihydrate (CaSO4·2H2O) precursor. The presence of 2.60×10–3 mol/L CuCl2·2H2O resulted in the increase of the aspect ratios of α-CaSO4·0.5H2O whiskers from 81 to 253. The preferential adsorption of Cu2+ on the negative {110} and {100} facets of α-CaSO4·0.5H2O crystal structures was confirmed by EDS and XPS. And ATR-FTIR demonstrated the ligand adsorption of Cu2+ on the surface of α-CaSO4·0.5H2O whiskers. The experimental results reveal that the whiskers with high aspect ratios are attributed to the adsorption of Cu2+, which promotes the 1-D growth of α-CaSO4·0.5H2O whiskers along the c axis.


