Trans. Nonferrous Met. Soc. China 24(2014) 3682-3687
Effects of particle size and particle interactions on scheelite flotation
Wan-zhong YIN, Ji-zhen WANG
College of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China
Received 24 November 2013; accepted 26 June 2014
Abstract:
Effects of size distribution (particle size and content of fine fraction (<10 μm)) on scheelite flotation were studied using flotation tests and theoretical calculations. The results show that particle size influences the scheelite recovery and the performance of combined reagents. The scheelite recovery is lowered by adding fine particles (<10 μm) into the pulp containing coarse particles. Extended DLVO (EDLVO) theory confirms that the fine fractions (<10 μm) could interface with the coarse fractions. The interaction energy and fluid forces are relative to the particle size, which might explain why the fine fractions influence the scheelite flotation. The highest recovery of scheelite using combined reagents as collector and optimum ratio of combined reagents were determined by scheelite particle size and reagent performance. However, the optimum adding order was only determined by reagent performance, which has nothing to do with particle size.
Key words:
scheelite; flotation; sodium oleate; benzohydroxamic acid; particle size; combined reagents;
1 Introduction
Scheelite is a calcium tungstate mineral (CaWO4), and it is one of the most important tungsten-bearing minerals. A scheelite concentrate is recovered from scheelite ore using a froth flotation process which involves grinding the ore sufficiently to liberate scheelite grains. Flotation is based on the surface-chemistry. In order to understand the surface properties of commonly exposed surface of scheelite, some theoretical and experimental studies have been conducted on surface broken bonds property of mineral crystal [1-3]. These researches showed that the broken bonding density and wettability of scheelite crystals exhibited anisotropy. Scheelite is often associated with other calcium-bearing minerals such as calcite, fluorite and apatite. The flotation separation of scheelite from calcium-bearing minerals is difficult because of similar surface properties of calcium minerals and high reactivity with their conventional reagents [4,5], and the investigate focus of scheelite flotation is collector and depressant with good selectivity.
Reagents, including collector and regulator, are key factors that determine the flotation. The most widely used collector in scheelite flotation is carboxylic acid collector [6]. The common opinion is that chemisorptions occurred by the interaction between —COO— and Ca2+ on the surface of scheelite. In recent years, hydroxamate has obtained more and more attention [7], and the extensive research has been carried out in a variety of mineral flotations such as monazite, bastnaesite [8], malachite, azurite [9], cassiterite [10], and wolfram [11]. Recently, benzohydroxamic acid (BHA), a kind of hydroxamates, has been applied to scheelite flotation [12]. It was worth mentioning that BHA was usually combined with carboxylic acid collector in scheelite flotation. To our knowledge, however, few studies were conducted on the factors that determine the performance of combined reagents of BHA and carboxylic acid collector. These findings will be helpful to improve the scheelite separation.
Flotation studies showed that the formation of slime coating, caused by the fine fractions, was related to the surface potential, which decreases mineral recovery [13-15]. The research focus of scheelite flotation is the separation of scheelite from calcium- bearing minerals. To our knowledge, however, few studies have examined the effect of scheelite particle size and particle interactions on the scheelite recovery. These findings will be helpful to the research of floation scheelite.
2 Experimental
2.1 Materials and reagents
The minerals used in this study containing scheelite (CaWO4) were obtained from Malipo in Yunnan province, China. Hand-selected scheelite crystals were crushed and ground in a laboratory porcelain ball-mill to <10 μm, 10-38.5 μm, 38.5-74 μm, 74-106 μm, and 106-125 μm, respectively. The scheelite purity was 95.98%. The X-ray powder diffraction analysis confirmed that the scheelite sample was of high purity (Fig. 1).
Analytical pure sodium oleate and BHA were used as collectors for scheelite. Dilute solution of hydrochloric acid (HCl) and sodium hydroxide (NaOH) were employed as pH adjustment agents. The distilled water produced by automatic adsorption-type ultrapure water systems was used.
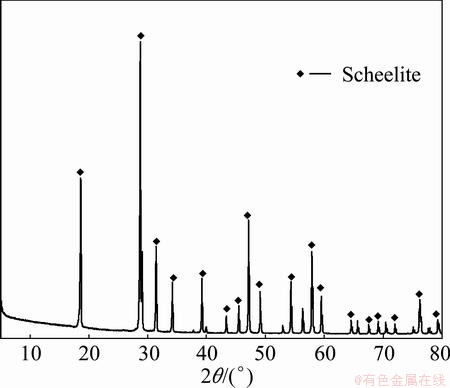
Fig. 1 XRD pattern of scheelite
2.2 Methods
2.2.1 Micro-flotation tests
Single mineral flotation tests were carried out on a mechanical agitation flotation machine. The impeller speed was fixed at 2000 r/min. The micro-flotation tests involved the following procedures: 1) wetting the single minerals (2.0 g) with 40 mL deionized water for 1.0 min; 2) adding the pH regulator for required pH and agitating for 3.0 min; 3) adding the collectors and agitating for 5.0 min; 4) collecting the floated materials for 5.0 min; 5) filtering the collected materials, drying and weighing them for recovery. The recovery rate (ε) was calculated as follows:
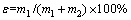 (1)
(1)
where m1 is the mass of floated products (including the coarse scheelite and fine scheelite); m2 is the mass of tailing products (including the coarse scheelite and fine scheelite).
In order to study the influence of fine fractions on the flotation of coarse particles, a theoretical recovery (εT) was defined and it was calculated as follows:
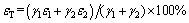 (2)
(2)
where ε1 and γ1 are recovery and mass fraction of fine fractions in the absence of coarse fractions, respectively; ε2 and γ2 are recovery and mass fraction of coarse fractions in the absence of fine fractions, respectively.
2.2.2 Zeta potential measurements
Isoelectric point (IEP) values of scheelite samples were determined by measuring the electrophoretic mobility of aqueous dispersions as a function of pH using a zeta potentiometer. For these measurements, 30 mg of scheelite sample was added to desire 1 mmol/L KNO3 solution and ultrasonicated for 30 min, and magnetically stirred for 10 min; finally, the pH was adjusted using HCl or KOH.
3 Results and discussion
3.1 Effect of pH on scheelite flotation
The pulp pH value has strong effects on the water-scheelite interface and reagent performance. In order to determine the optimum pH of scheelite flotation,floatation tests were carried out at different pH and the results are shown in Fig. 2.
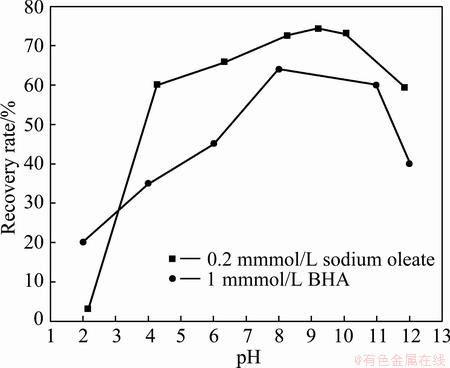
Fig. 2 Effect of pH on scheelite flotation with sodium oleate or BHA
As shown in Fig. 2, the recovery of scheelite using sodium oleate increases with increasing pH from 2 to the maximum at about 4, then nearly levels off in a wide pH range (4-10), and decreases slightly with further pH increasing to 12. The flotation results indicate that the preferable pH for recovery of scheelite with BHA is 8-10. From Fig. 2, it can also be seen that both sodium oleate and BHA collect scheelite well at pH 8-10. In order to research the effects of particle size on the performance of combined reagent of sodium oleate and BHA, pH of 9.0 was used in this study.
3.2 Effect of particle size and fine fractions on scheelite flotation
Reagent concentration and pH are two main factors determining mineral recovery. In our opinion, particle size is also responsible for scheelite flotation. To support this hypothesis, we studied the effects of particle size and fine particle (<10 μm) percent on scheelite flotation recovery at pH 11.0 (Figs. 3 and 4).
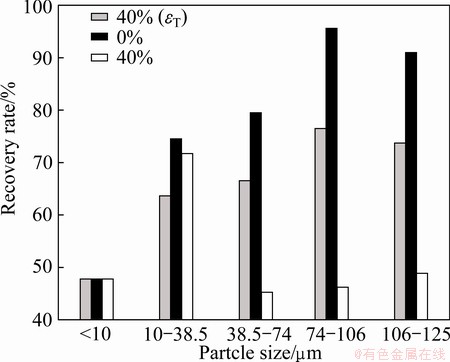
Fig. 3 Effect of fine fractions (<10 μm) on coarse scheelite recovery at sodium oleate concentration of 0.1 mmol/L
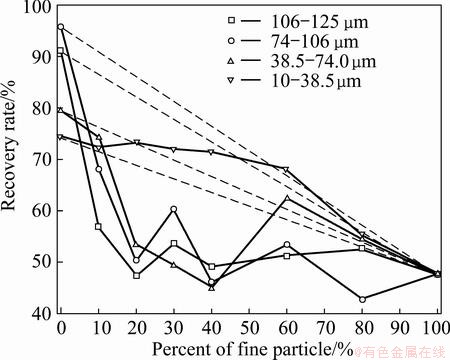
Fig. 4 Effect of fine scheelite (<10 μm) on 10-125 μm scheelite recovery at sodium oleate concentration of 0.1mmol/L
The grey histogram in Fig. 3 and dashed lines in Fig. 4 represent the theoretical recovery (εT). In Fig. 3, it can be seen that the floatability of coarse scheelite is better than that of fine particles (<10 μm). Therefore, it is reasonable to conclude that the recovery of scheelite decreases with adding fine fractions (<10 μm) if the recovery is lower than εT.
As shown in Fig. 3, particle size markedly affects scheelite recovery. Scheelite recovery increases from 47.71% to 95.77% as the maximum particle size increases from 10 to 106 μm, and then decreases slightly as particle size increases further. The recovery of sample containing coarse scheelite (d>38.5 μm) decreases when fine particles (<10 μm) is added into pulp. However, the recovery of 10-38.5 μm scheelite is almost uninfluenced by fine particles (<10 μm) (Fig. 3). In Fig. 4, it can be seen that the recovery of coarse scheelite (d>38.5 μm) steadily decreases as the percentage of fine particles (<10 μm) increases from 0 to 10%, then changes slightly as percentage of fine particles (<10 μm) increases further. However, the recovery of 10-38.5 μm scheelite is almost uninfluenced by fine particles (<10 μm) when the percentage of fine particles (<10 μm) increases from 0 to 60% (Fig. 4). The highest recovery of scheelite for each particle size in Fig. 4 is determined by particle size and percent of fine particles (<10 μm), which is in agreement with earlier observation [16].
3.3 Effect of particle size on scheelite flotation with combined reagents
Reagent concentration, pH, and order of addition are important factors determining the properties of the combined reagents. As particle size and fine fractions influence scheelite flotation, we explore the effects of reagent concentration and adding order of combined reagents on scheelite flotation (Fig. 5 and Table 1).
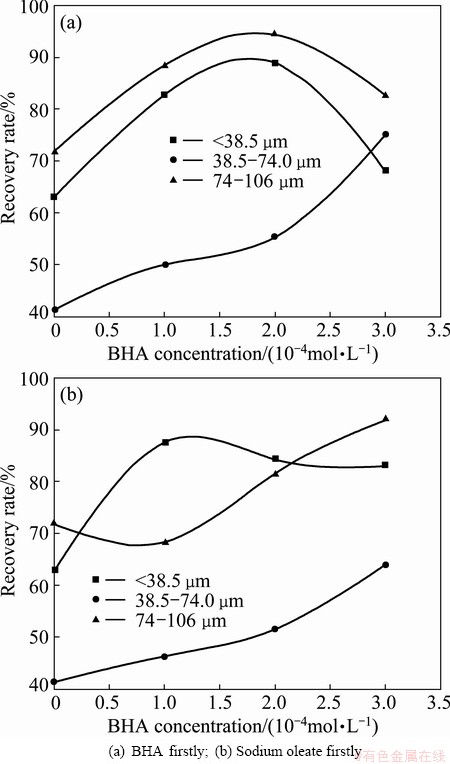
Fig. 5 Effect of BHA concentration on scheelite flotation at sodium oleate concentration of 0.2 mmol/L
Table 1 Correlation between particle size and properties of combined reagents

Figure 5 shows that the combined reagents of sodium oleate (NaOL) and BHA recover more scheelite than sodium oleate. More importantly, it is obvious that the scheelite is determined by reagent concentration, adding order and particle size. Table 1 shows that the highest recovery of scheelite and optimum ratio of combined reagents are determined by scheelite particle size and reagent performance, while the preferable order of addition is only determined by reagent performance. This gives insight into how to regulate the performance of combined reagents.
3.4 Discussion
3.4.1 Calculation of interactions between scheelite particles
Flotation tests showed that fine fraction content influenced scheelite flotation. This might be caused by scheelite particle interactions, which resulted in the fine particles adhering to coarse particles. Based on the EDLVO theory [16,17], the total energy between scheelite particles can be described as
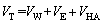 (3)
(3)
where VW and VE are Vander Waals energy and electrostatic energy, respectively; VHA represents hydrophobic energy. And VW is calculated as
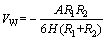 (4)
(4)
where
A=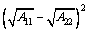 (5)
(5)
where the Hamaker constant A11 of scheelite is 10×10-20 J; A22 of sodium oleate is 4.70×10-20 J; R1 and R2 represent the radii of fine particle and coarse particle, respectively; H is the separation distance. VE can be calculated as
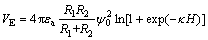 (6)
(6)
where κ is the thickness of the electric double-layer, κ=0.180 nm-1; εa is the relative dielectric constant of the continuous phase; ψ0 is the surface potential (when contact time between the particles is short, the assumption of constant surface charge is appropriate). VHA is calculated as
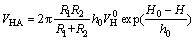 (7)
(7)
where VH0 is the energy constant between two polar surfaces; h0 is the attenuation length. And VH0 is calculated as
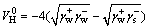 (8)
(8)
where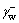 and
and 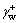 represent electron donor and recipient component of the surface free energy of water, respectively;
represent electron donor and recipient component of the surface free energy of water, respectively; 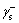 is electron recipient component of the surface free energy of scheelite surface. In addition,
is electron recipient component of the surface free energy of scheelite surface. In addition, 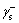 is calculated as follows:
is calculated as follows:
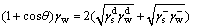 (9)
(9)
where θ is the contact angle between the solid surface and the liquid; 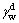 and
and 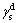 are dispersive components of the surface free energy of water and scheelite, respectively. And
are dispersive components of the surface free energy of water and scheelite, respectively. And
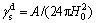 (10)
(10)
The components of the surface free energy of water are listed in Table 2.
Table 2 Component of surface free energy of water
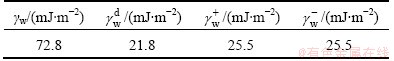
Figure 6 shows the curves of total energy (VT) vs distance (H) at pH 11.0 on the basis of Eqs. (3)-(10). All the curves were carried out in sodium oleate solution at the surface potential ψ0 of -49.12 mV, and θ0 in sodium oleate solution is 80°.
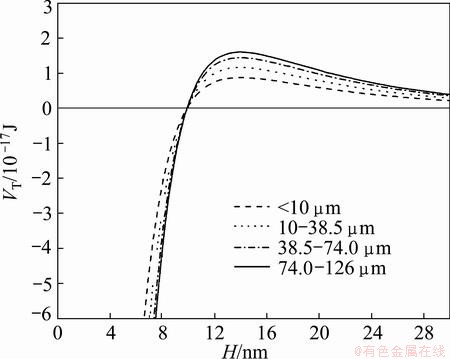
Fig. 6 Total energy between fine scheelite particles and coarse particles at pH 11.0
The scheelite recovery was lowered by adding fine particles (<10 μm) into the pulp containing coarse particles (see Figs. 3 and 4). The flotation speed of the hydrophobic and fine particles is usually slow [18], and the recovery of fine scheelite (<10 μm) is lower than that of coarse particles (see Fig. 3). In addition, fine scheelite (<10 μm) particles that are dispersed in solution greatly reduce the collector concentration in solution. Hence, the recovery is lowered by adding fine particles into the coarse particles if they do not interface with the coarse particles, and it is also reasonable to conclude that coarse particles, used as the carrier, have interfaced with fine particles if the recovery is higher than the theoretical recovery.
In Fig. 6, it can be seen that the fine scheelite particles (<10 μm) could interface with the coarse particles when the value of H is less than 10 nm. As mentioned previously, the adhesion of fine particles to coarse particles would increase the recovery of fine particles, as a result, the recovery of sample containing 40% particles (<10 μm) and 60% particles (10-38.5 μm) is almost equal to that of 100% particles (10-38.5 μm) (see Fig. 3), and the recovery of samples containing 10-38.5 μm particles and <10 μm particles is higher than theoretical recovery (see Fig. 4). In Fig. 6, it also can be seen that the value of VT increases as particle size increases from 10 to 125 μm when the value of H is more than 10 nm, which indicates that the adhesion of fine fractions to 38.5-125 μm particles is less obvious than that to 10-38.5 μm particles. In addition, the shear force increases as particle size increases [16]. Therefore, the strength of particle interaction becomes weak as coarse particle size increases. This is why the samples containing 10-38.5 μm particles and <10 μm particles have the highest recovery in Fig. 3 and the samples containing 38.5-74 μm particles and <10 μm particles have the highest recovery in the percent range of 40%-100% of fine particles when the coarse particle size is more than 38.5 μm in Fig. 4.
3.4.2 Correlation between performance of combined reagents and activity of reagents
The contact angle θ between the solid surface and liquid is directly determined by the activity of reagent. Mulliken net charge and group electronegativity (χg) can be used to evaluate the activity of flotation agents. Mulliken net charge could be calculated by Gaussian 03 software. The formula for χg is
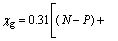
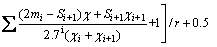 (11)
(11)
where r and N represent the covalent radius and valence electron number of bonding atom, respectively; P represents the valence electron number of atom adjacent to the bonding atom; mi and Si are numbers of chemical bonds containing two electrons and non-bonding electrons of atoms that are separated by i bonds from bonding atoms.
The calculated Mulliken net charge, χg, and solubility product are listed in Table 3.
Table 3 Structure-activity parameters of sodium oleate and BHA
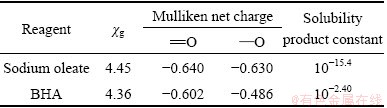
As shown in Table 3, the Mulliken net charge and χg of sodium oleate are greater than those of BHA, indicating that sodium oleate has a stronger collecting ability, in accordance with the solubility product constant listed in Table 3.
The specific surface area of the cleaved surface, floatability and flotation speed of scheelite are related to the particle size. Therefore, the highest recovery of scheelite using combined reagents as collector is related to the scheelite particle size.
The broken bonding density and wettability of scheelite crystals exhibited anisotropy [2]. Since the activities of adsorption sites on the surface of scheelite are different, the first reagent added would react with the adsorption sites that have the highest activity. If sodium oleate is added first, the number of adsorption sites that have higher activity for BHA would be decreased. This results in a reduction of the adsorption of BHA on the surface of scheelite. If BHA is added firstly, although the number of adsorption sites that have higher activity for sodium oleate is decreased, the adsorption of sodium oleate on the scheelite surface is less affected because of its strong collecting ability. So, the optimal order of addition is to add BHA first, which is in accordance with the flotation tests. Optimal order of addition is directly the related to reagent activity and has nothing to do with the particle size.
In addition, it is worth noticing that the optical order of addition is also related to the reagent concentration. Too much BHA would decrease the adsorption sites for sodium oleate. In this case, the addition of BHA first might decrease the scheelite recovery, which needs to be further studied.
4 Conclusions
1) Particle size and fine fractions are significant factors that determine the scheelite flotation quality. The recovery of coarse scheelite (d>38.5 μm) steadily decreases as the percent of fine particles (<10 μm) increases from 0 to 10%, and then changes slightly as the percent of fine particles (<10 μm) increases further. The highest recovery of simples containing coarse scheelite (10-126 μm) and fine scheelite (<10 μm) increases as coarse particle size decreases when the fine particles (<10 μm) content is equal to or above 10%.
2) EDLVO calculation confirms that fine scheelite particles (<10 μm) could interface with the coarse particles. The total energy, shear forces and collision strength increase as particle size increases. The recovery is lowered by adding fine particles (<10 μm) into the coarse particles if fine particles do not interface with the coarse particles. These are the reasons why the sample containing 10-38.5 μm particles and fine particles (<10 μm) has a higher recovery than that containing 38.5-126 μm particles and fine particles (<10 μm), and the sample containing 38.5-74 μm particles and fine particles (<10 μm) has the highest recovery in the percent range of 10%-100% of fine particle when the coarse particle size is 38.5-126 μm.
3) The highest recovery of scheelite and optimum ratio of combined reagents of sodium oleate and BHA are determined by scheelite particle size and reagent performance, while the preferable order of addition is only determined by the reagent performance.
References
[1] COOPER T G, de LEEUW N H. A computer modeling study of the competitive adsorption of water and organic surfactants at surfaces of the mineral scheelite [J]. Langmuir, 2004, 20(10): 3984-3994.
[2] GAO Zhi-yong, SUN Wei, HU Yue-hua, LIU Xiao-wen. Anisotropic surface broken bond properties and wettability of calcite and fluorite crystals [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1203-1208.
[3] HU Y H, GAO Z Y, SUN W, LIU X W. Anisotropic surface energies and adsorption behaviors of scheelite crystal [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 415: 439-448.
[4] PRADIP, RAI B, RAO T K, KRISHNAMURTHY S, VETRIVEL R, MIELCZARSK J, CASES J M. Molecular modelling of interactions of diphosphonic acid based surfactants with calcium minerals [J]. Langmuir, 2002, 18(3): 932-940.
[5] PRADIP, RAI B, RAO T K, KRISHNAMURTHY S, VETRIVEL R, MIELCZARSK J, CASES J M. Molecular modeling of interactions of alkyl hydroxamates with calcium mineral [J]. Journal of Colloid and Interface Science, 2002, 256: 106-113.
[6] BULATOVIC S M. Handbook of flotation reagents: Chemistry theory and practice [M]. Amsterdam: Elsevier, 2007: 25-38.
[7] ASSIS S M, MONTENEGRO L C M, PERES A E C. Utilization of hydroxamates in minerals froth flotation [J]. Minerals Engineering, 1996, 9(1): 103-114.
[8] LEE K, ARCHIBALD D, MCLEAN J, REUTER M A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors [J]. Minerals Engineering, 2009, 22(4): 395-401.
[9] PAVEZ O, BRANDAO P R G, PERES A E C. Adsorption of oleate and octyl-hydroxamate onto rare-earths minerals [J]. Minerals Engineering, 1996, 9(3): 357-366.
[10] WU X Q, ZHU J G. Selective flotation of cassiterite with benzohydroxamic acid [J]. Minerals Engineering, 2006, 19(14): 1410-1417.
[11] HU Y H, WANG D Z, XU Z. A study of interaction and flotation of wolfamite with octyl hydroxamate [J]. Minerals Engineering, 1997, 10(6): 623-633.
[12] XIA Qi-bin, LI Zhong, QIU Xian-yang, DAI Zi-lin. Quantum chemical study on benzyhydroxamic acid flotation agent [J]. Mining and Metallurgical Engineering, 2004, 24(1): 30-33. (in Chinese)
[13] EDWARDS G R, KIPKIE W B, AGAR G E. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite on pentlandite flotation [J]. International Journal of Mineral Processing, 1980, 7(1): 33-42.
[14] LI Zhi-hua. The effect of gangue minerals containing magnesium on pentlandite flotation [J]. Journal of Central South Institute of Mining and Metallurgy, 1993, 24(1): 36-44. (in Chinese)
[15] LU Yi-ping, ZHANG Ming-qiang, FENG Qi-ming, LONG Tao, OU Le-min, ZHANG Guo-fan. Effect of sodium hexametaphosphate on separation of serpentine from pyrite [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(1): 208-213.
[16] QIU Guan-zhou, HU Yue-hua, WANG Dian-zuo. Interaction of particles and flotation techniques of fine particles [M]. Changsha: Central South University of Technology Press, 1993. (in Chinese)
[17] MISSNAN T, ADELL A. On the applicability of DLVO theory to the prediction of clay colloids stability [J]. Colloid Interface Sci, 2000, 230: 150-156.
[18] YOON R H. The role of hydrodynamic and surface forces in bubble-particle interaction [J]. International Journal of Mineral Processing, 2000, 58(1-4): 129-143.
粒度大小和颗粒间相互作用对白钨矿浮选的影响
印万忠,王纪镇
东北大学 资源与土木工程学院,沈阳 110819
摘 要:通过浮选试验和理论计算研究白钨矿粒度分布(粒径小于10 μm的微细粒含量)对浮选的影响。结果表明,颗粒粒径对白钨矿浮选回收率以及组合药剂性能都有影响;扩展的DLVO(EDLVO)理论证明白钨矿颗粒之间存在相互吸引力。颗粒之间的相互作用能、流体剪切力大小都与颗粒粒径有关,这是小于10 μm粒级白钨矿对粗粒级白钨矿回收率的影响随着粗粒级粒度的改变而发生变化的主要原因。以组合药剂为捕收剂时,白钨矿的最高回收率和组合药剂的最佳配比与颗粒粒度以及药剂性能有关,而组合药剂的最佳添加顺序与颗粒粒度无关,只与药剂性能有关。
关键词:白钨矿;浮选;油酸钠;苯甲羟肟酸;粒径;组合药剂
(Edited by Xiang-qun LI)
Foundation item: Project (51074037) supported by the National Natural Science Foundation of China
Corresponding author: Ji-zhen WANG; Tel: +86-15702412118; E-mail: jizhenwang@126.com
DOI: 10.1016/S1003-6326(14)63515-9
Abstract: Effects of size distribution (particle size and content of fine fraction (<10 μm)) on scheelite flotation were studied using flotation tests and theoretical calculations. The results show that particle size influences the scheelite recovery and the performance of combined reagents. The scheelite recovery is lowered by adding fine particles (<10 μm) into the pulp containing coarse particles. Extended DLVO (EDLVO) theory confirms that the fine fractions (<10 μm) could interface with the coarse fractions. The interaction energy and fluid forces are relative to the particle size, which might explain why the fine fractions influence the scheelite flotation. The highest recovery of scheelite using combined reagents as collector and optimum ratio of combined reagents were determined by scheelite particle size and reagent performance. However, the optimum adding order was only determined by reagent performance, which has nothing to do with particle size.


