
Effect of liquid-liquid structure transition on solidification of Sn-Bi alloys
ZU Fang-qiu(祖方遒), ZHOU Bing(周 兵), LI Xian-fen(李先芬),
YI Xun(益 汛), CHEN Yi-ping(陈轶平), SUN Qi-qiang (孙其强)
College of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, China
Received 10 January 2007; accepted 26 April 2007
Abstract:
The effect of the liquid-liquid structure transition(L-LST) on the solidification behaviors and morphologies of Sn-Bi alloys was studied further. The results show that the undercooling of the primary and eutectic phase increases and the microstructure becomes finer after solidifying from the melt experiencing the L-LST. In the meantime, in hypoeutectic alloy, when solidifying from the melt experiencing the L-LST, the morphology of primary phase changes from the fir-tree crystal into the equiaxed crystal, and less primary phase and more eutectic structure are observed. Moreover, in eutectic alloy, the spacing of eutectic phase decreases markedly. These investigations would be beneficial to further exploration of the correlation between the melt structure and the micro mechanism of solidification.
Key words:
liquid-liquid structure transition; undercooling; solidification structure; Sn-Bi alloys;
1 Introduction
It has been accepted that the melt structure has a direct and significant effect on the solidification microstructure and properties of metal materials[1-5]. In recent years, lots of achievements have been made in the solidification theory and technology[6-8]. Many scholars have taken the method of melt overheating to change the solidification behavior[9-11]. It has been found that the solidification structure becomes finer when the melt is held at a high temperature, which can improve the microstructures and properties of casting. These results have a deep significance in engineering practice. To explore the intrinsic reason of this phenomena, in this study, from the new point view of the liquid-liquid structure transition(L-LST), the effect of liquid structure transition on the solidifying behavior and solidification structure of Sn-Bi alloys is investigated, which could avoid the blind choosing of temperature range in overheating treatment.
In our prior investigations, by DC four-probe method, the electrical resistivities of some binary alloys, such as Bi-In, Pb-Sn, In-Sn, Sn-Sb, Sn-Bi and Pb-Bi, were measured[12-17]. The results showed that there were nonlinear changes on the resistivity—temperature curve within a certain range of several hundred degrees above corresponding liquidus. These changes had been confirmed directly and indirectly by the X-ray diffractometry, revised internal friction method, differential scanning calorimetry as well as differential thermal analysis. It is well known that the electrical resistivity is one of the liquid’s structure sensitive parameters, and it is verified to be valid and sensitive to liquid structure[18], so the anomalous change of the electrical resistivity can reflect the melt structure transition. Based on these results, the effect of the L-LST on solidification of Sn-Bi alloy is studied in this study.
2 Experimental
Sn-40%Bi (hypoeutectic alloy) and Sn-57%Bi (eutectic alloy) (mass fraction) were chosen for the investigation. Each sample (20 g) was prepared by melting in corundum crucible (30 mL) from granules of Sn (99.9%) and Bi (99.99%). In order to prevent volatilization and oxidation, the samples were covered with B2O3 during the entire melting and solidification process. According to resistivity experiments, 650 ℃ (below the L-LST temperature) and 1 000 ℃ (above the L-LST temperature) were selected. The prepared samples were respectively melted and held in two electrical resistance furnaces at the temperature set in advance for 1 h and fully agitated. Then the sample held at 650 ℃ was taken out and cooled with the crucible in air, while the sample held at 1 000 ℃ was immediately transferred into the salt bath (650 ℃) (50% BaCl2+30% CaCl2+20% NaCl, 100 g) and kept for 30 min before cooling. And simultaneously, by a NiCr-NiSi thermocouple, the temperature—time curve was recorded by the KEITHLEY-2182 nanovoltmeter and computer collection system. At last, the samples were ground to observe the optical metallographic structure.
3 Results
By DC four-probe method, the same heating rate, 7.5 ℃/min, is used for Sn-40%Bi and Sn-57%Bi alloys in the experiment, and the electrical resistivities of liquid Sn-Bi alloys change nonlinearly within several hundred degrees above liquidus. The change begins at 775 ℃ and ends at 955 ℃ for Sn-40%Bi alloy, and in Sn-57%Bi alloy, the change temperature region is from 740 to 950 ℃. All these can be seen in Fig.1.
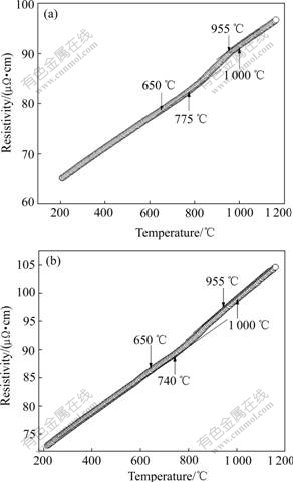
Fig.1 Resistivity—temperature curve of Sn-40%Bi (a) and Sn-57%Bi (b) at heating rate of 7.5 ℃/min
Melting and holding temperature for Sn-Bi alloys is chosen according to Fig.1, and the cooling curves are shown in Fig.2. Curve 1 is the cooling curve of the melt that does not experience the L-LST, and Curve 2 is the cooling curve of the melt experiencing the L-LST.
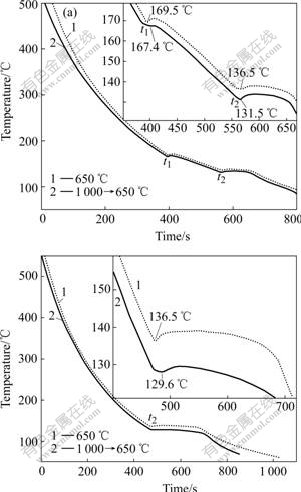
Fig.2 Temperature—time curves of Sn-40%Bi (a) and Sn-57% Bi (b) after 650 ℃, 1 h and 1 000 ℃, 1 h→650 ℃, 30 min treatment
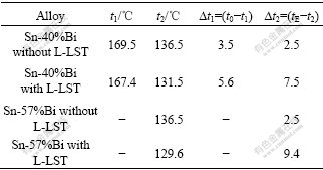
According to Sn-Bi phase diagram, it is easy to find that the balanced eutectic temperature is 139 ℃, and the temperature of balanced primary phase reaction of Sn-40%Bi alloy is 173 ℃. t1 is representation of the temperature, at which the primary phase generates, and t2 is the temperature of the eutectic reaction. The undercooling of the primary and eutectic phase and some key temperature parameters on the temperature—time curves are listed in Table 1, in which t0 is the temperature of balanced primary phase reaction and tE is the temperature of balanced eutectic reaction. According to Table 1 and Fig.2, when solidifying from the melt experiencing the L-LST, the undercooling of the primary phase increases by about 1.5 times and for eutectic phase by about 3-4 times.
Table 1 Key temperatures on temperature—time curve of Sn-40%Bi and Sn-57%Bi after at 650 ℃, 1 h and 1 000 ℃, 1 h→650 ℃, 30 min treatment
After solidifying from the melt experiencing the L-LST, for the Sn-40%Bi alloy, on one hand, the amount of the primary phase decreases a lot, while the amount of the eutectic phase increases obviously. On the other hand, the primary phase is refined obviously, and the microscopic pattern changes from the continuous, fishbone, banding and directional fir-tree crystal into the broken, isolated, finer and equiaxed crystal. In addition, the eutectic structure is also refined a lot, and the spacing of eutectic phase decreases about from 0.75-1.25 μm to 0.3-0.5 μm, and the microscopic pattern changes from the continuous structure into the broken eutectic cluster, as shown in Fig.(3).

Fig.3 Microstructures of Sn-40%Bi alloy: (a) and (c) 650 ℃, 1 h; (b) and (d) 1 000 ℃, 1 h→650 ℃, 30 min
In Fig.4, for Sn-57%Bi alloy, the eutectic structure is refined obviously, and the spacing of eutectic phase decreases approximately from 7.0-10.5 μm to 4.5-6.0 μm.
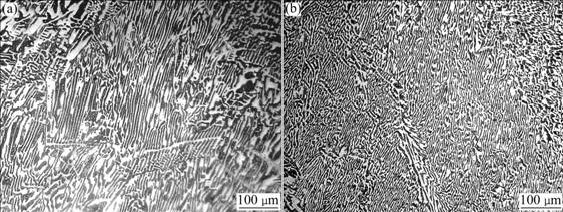
Fig.4 Microstructures of Sn-57%Bi alloy: (a) 650 ℃, 1 h; (b) 1 000 ℃, 1 h→650 ℃, 30 min
4 Discussion
In equilibrium structure, Sn-40%Bi alloy consists of Sn solid solution and Sn-Bi eutectic phase. When the alloy is melted below the L-LST temperature, the atomic bonds of crystals are only partially broken during melting process, and the short-range orders(SROs) similar to the corresponding solid crystal still remain in liquids within certain temperature range. Therefore, the SROs can easily become the core of nucleation, which will promote nucleation during cooling and solidifying process. With temperature rising, the atoms obtain more and more kinetic energy. Up to a certain transfer-temperature, when the energy of these atoms is large enough to overcome the energy barrier, Sn-rich clusters and Bi-rich clusters start to either break up or disintegrate into smaller ones. At the same time, some SROs, deriving from the solid, are broken up and become more uniform and disordered. All these destroy the advantageous conditions for nucleation and produce a lot of free atom that diffuse and rearrange into new clusters of Sn-Bi, and the liquid-liquid structure transition takes place during this transformation process, which makes the liquid atom clusters need to overcome higher potential barrier to change into the solid atom clusters during the solidification process and finally decreases the effective partition coefficient of composition. As a result, the amount of the primary phase decreases, while the amount of the eutectic phase increases. So there is a more distinct temperature back-up at t1 on Curve 1 in Fig.2, about from 169.5 to 172.3 ℃, which is due to the crystal latent heat release of the more primary phase. And there is a higher temperature back-up at t2 on Curve 2 in Fig.2, about from 131.5 to 134.2 ℃, which is caused by the crystal latent heat release of the more eutectic structure.
Therefore, below the L-LST temperature, there are Sn-rich and Bi-rich clusters in the melt, which are SRO structure. This liquid structure is beneficial to nucleation and growth of crystal during the solidification process because of the convenient component fluctuation, thus the nucleation undercooling is small. Whereas, above the L-LST temperature, the melt with smaller and disorder clusters is difficult to nucleate. So, greater undercooling is needed for nucleating under this condition.
According to classical nucleation theory, in the nucleation process, the critical radius is rc[6]:
![]() (1)
(1)
where σ is the interface energy; T0 is the equilibrium solidification temperature; ?H is the variant enthalpy in the solidification and approximately equals to the crystal latent heat; ?T is the nucleation undercooling. And the homogeneous rate of nucleus formation is I[6]:
![]() (2)
(2)
where N1 is the number of single atom cluster in the melt; γ0 is the frequency of the atom cluster vibration, ![]() is the surface area of the critical crystals; nc is the position density that could capture atom cluster on the surface area of the unit crystal; k is the Boltzmann constant; T is the melt temperature; exp[-?Gd/kT] is the piece-rate of the melt atom cluster that has the diffusion activation energy ?Gd.
is the surface area of the critical crystals; nc is the position density that could capture atom cluster on the surface area of the unit crystal; k is the Boltzmann constant; T is the melt temperature; exp[-?Gd/kT] is the piece-rate of the melt atom cluster that has the diffusion activation energy ?Gd.
According to the Eqns.(1) and (2), I is sensitive to ΔT, and it increases markedly with ΔT rising. However, rc diminishes with ΔT increasing, which leads to the result that r exceeds rc easily and turns into stabilization crystal nucleus during the solidification.
The solidification is a dynamic process, in which there is a transition from liquid to solid and a reversible transition from solid to liquid. When the former transition rate exceeds the latter, the solidification process proceeds. The crystal of Sn-40%Bi and Sn-57%Bi alloys grows continuously, so the growing rate of the crystal is R, R=αγnet, where α is the interface impelling distance when an atom cluster adds to the solid of the liquid/solid interface, and γnet is the net frequency of a liquid atom cluster hurdling grain boundary into a solid atom cluster. When solidifying from the melt experiencing the L-LST, a liquid atom cluster has to overcome more energy barrier to transit into a solid atom cluster, since some of the bonds between the congenetic atom clusters are broken and new bonds come into being. As a result, γnet decreases, so R decreases. No matter the decrease of rc, R or the rise of I would all result in finer solidification structure.
5 Conclusions
1) L-LST affects the solidification microstructure remarkably. When solidifying from the melt experiencing the L-LST, the undercooling increases, which would decrease the critical radius, while increase the nucleation rate. At the same time, the effective partition coefficient of composition decreases. As a result, the solidification behaviors and morphologies of the primary and eutectic phase change.
2) For Sn-40%Bi hypoeutectic alloy, the solidification structure is refined a lot, and the amount of the primary phase decreases, while the amount of the eutectic phase increases obviously. The microscopic pattern of the primary phase changes from coarser fir-tree crystal into finer equiaxed crystal. Moreover, the spacing of eutectic phase decreases.
3) For Sn-57%Bi eutectic alloy, the eutectic structure is refined obviously, and the spacing of eutectic phase decreases obviously.
References
[1] SETTE F, KRISCH M H. Dynamics of glasses and glass-forming liquids studied by inelastic X-ray scattering [J]. Science, 1998, 280(5369): 1550-1555.
[2] HERLACH D M. Solidification from undercooled melts [J]. Materials Science and Engineering, 1997, A226/228: 348-356.
[3] FU Heng-zhi, GUO Jing-jie, SU Yan-qing, LIU Lin, XU Da-ming, LI Jin-shan. Directional solidification and lamellar orientation control of TiAl intermetallics [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 797-810. (in Chinese)
[4] LO T S, DOBLER S, PLAPP M. Two-phase microstructure selection in peritectic solidification: from island to coupled growth [J]. Acta Materialia, 2003, 51: 599-611.
[5] WASEDA Y. The structure of non-crystalline materials [M]. New York: Mc Graw Hill, 1980: 57-58.
[6] HU Han-qi. The principle of metal solidification [M]. Beijing: China Machine Press, 2000: 66-86. (in Chinese)
[7] IKEDA T, HAILE S M, RAVI V A, AZIZGOLSHANI H, GASCOIN F, SNYDER G J. Solidification processing of alloys in the pseudo-binary PbTe-Sb2Te3 system [J]. Acta Materialia, 2007, 55(4): 1227-1239.
[8] PANOFEN C, HERLACH D M. Solidification of highly undercooled Si and Si-Ge melts [J]. Materials Science and Engineering, 2007, A449/451: 699-703.
[9] LI Pei-jie, NIKITIN V I, KANDALOVA E G, NITIKIN K V. Effect of overheating, cooling and solidification rates on Al-16wt%Si alloy structure [J]. Mater Sci Eng, 2002, A332: 371-374.
[10] KOH H J, RUDOLPH P, SCH?FER N, UMETSU K, FUKUDA T. The effect of various thermal treatments on supercooling of PbTe melts [J]. Mater Sci Eng, 1995, B34: 199-203.
[11] CHENG Guang, YU Jian-wei, FU Heng-zhi. Influence of the melt heat history on the solid/liquid interface morphology evolution in unidirectional solidification [J]. Journal of Materials Science Letters, 1999, 18(19): 1571-1573.
[12] XI Yun, ZU Fang-qiu, LI Xian-fen, YU Jin, LIU Lan-jun, LI Qiang, CHEN Zhi-hao. High-temperature abnomal behavior of resistivities for Bi-In melts [J]. Physical Letters, 2004, A329(3): 221-225.
[13] ZU Fang-qiu, ZHU Zhen-gang, GUO Li-jun, QIN Xu-bo, YANG Hua, SHAN Wen-jun. Observation of an anomalous discontinuous liquid-structure change with temperature [J]. Physical Review Letters, 2002, 89(12): 125505-01-125505-04.
[14] LI Xian-fen, ZU Fang-qiu, DING Hou-fu, YU Jin, LIU Lan-jun, LI Qiang, XI Yun. Anomalous change of electrical resistivity with temperature in liquid Pb-Sn alloys [J]. Physics Letters B, 2005, 358: 126-131.
[15] ZU Fang-qiu, LI Xian-fen, GUO Li-jun, YANG Hua, QIN Xu-bo, ZHU Zhen-gang. Temperature dependence of liquid structures in In-Sn20 diffraction experimental evidence [J]. Physics Letters A, 2004, 324: 472-478.
[16] ZU Fang-qiu, SHEN Rong-rong, XI-Yun, LI Xian-fen, DING Guo-hua, LIU Hai-ming. Electrical resistivity of liquid Sn-Sb alloy [J]. Jounal of Physics: Condensed Matter, 2006, 18: 2817-2823.
[17] LI Xian-fen, ZU Fang-qiu, DING Hou-fu, YU Jin, LIU Lan-jun, XI Yun. High-temperature liquid-liquid structure transition in liquid Sn-Bi alloys: Experimental evidence by electrical resistivity method [J]. Physics Letters A, 2006, 354: 325-329.
[18] LI Qiang, ZU Fang-qiu, LI Xian-fen, XI Yun. The electrical resistivity of liquid Pb-Bi alloy [J]. Modern Physics Letters B, 2006, 20(4): 151-158.
Foundation item: Projects(50571533, 50371024) supported by the National Natural Science Foundation of China; Project(104106) supported by Chinese Ministry of Euducation
Corresponding author: ZU Fang-qiu; Tel/Fax: +86-551-2905057; E-mail: fangqiuzu@hotmail.com


