Trans. Nonferrous Met. Soc. China 27(2017) 1143-1149
Microbial oxidation of refractory gold sulfide concentrate by a native consortium
N. MARCHEVSKY1,2, M. M. BARROSO QUIROGA2, A. GIAVENO3, E. DONATI1
1. Centro de 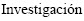 y Desarrollo en Fermentaciones Industriales (CINDEFI, CCT LA PLATA-CONICET, UNLP), Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Calle 50 N° 227, 1900 La Plata, Argentina;
y Desarrollo en Fermentaciones Industriales (CINDEFI, CCT LA PLATA-CONICET, UNLP), Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Calle 50 N° 227, 1900 La Plata, Argentina;
2. Departamento de 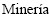 , Facultad de Ciencias
, Facultad de Ciencias 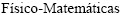 y Naturales, Universidad Nacional de San Luis, Chacabuco 917, 5700 San Luis, Argentina;
y Naturales, Universidad Nacional de San Luis, Chacabuco 917, 5700 San Luis, Argentina;
3. Instituto de 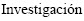 y Desarrollo en
y Desarrollo en  de Procesos,
de Procesos,  Alternativas (PROBIEN, CONICET-UNCo), Departamento de
Alternativas (PROBIEN, CONICET-UNCo), Departamento de  , Facultad de
, Facultad de  , Universidad Nacional del Comahue, Buenos Aires 1400, 8300
, Universidad Nacional del Comahue, Buenos Aires 1400, 8300  , Argentina
, Argentina
Received 9 January 2016; accepted 3 November 2016
Abstract:
A defined mesophilic consortium including an iron oxidizing bacterium and a sulfur oxidizing bacterium was constructed to evaluate its ability for bioleaching a flotation concentrate from Andacollo mine in Neuquén, Argentina. Experiments were performed in shake flasks with a pulp density of 10% (w/v), using a basal salt medium containing ferrous iron at pH 1.8. The leaching solutions were analyzed for pH, redox potential (using specifics electrodes), ferrous iron (by UV-Vis spectrophotometry) and metal concentrations (by atomic absorption spectroscopy). The results showed that the consortium was able to reduce the refractory behavior of the concentrate, allowing 91.6% of gold recovery; at the same time, high dissolution of copper and zinc was reached. These dissolutions followed a shrinking core kinetic model. According to this model, the copper solubilization was controlled by diffusion through a product layer (mainly jarosite), while zinc dissolution did not show a defined control step. This designed consortium, composed of bacterial strains with specific physiological abilities, could be useful not only to optimize gold recovery but also to decrease the leachates metallic charge, which would be an environmental advantage.
Key words:
biooxidation; refractory gold concentrate; native consortium; kinetic analysis;
1 Introduction
Refractory gold-bearing ores are characterized by containing submicroscopic gold particles occluded in sulfide minerals such as pyrite and arsenopyrite. Under these conditions, gold cannot be recovered efficiently without an oxidative pretreatment such as roasting, chemical oxidation, or biooxidation. During these processes, the mineral structures which contain gold are destroyed and the precious metal is subsequently available to be solubilized by a conventional leaching technique like cyanidation [1,2].
Traditionally, roasting has been used to treat these ores despite the high energy consumption and production of pollutant gases that severely damage the environment. Furthermore, chemical oxidation is an expensive process requiring high temperature, high pressure and corrosion- resistant materials [3].
The increasing interest in using biooxidation as alternative technology is due to its numerous advantages as opposed to conventional techniques. This process takes place at atmospheric pressure and low temperatures; in addition, it is easy to operate, has low capital costs and is compatible with the environmental legislation [4,5].
The first experiences with microorganisms were carried out to recover gold from refractory sulfides in the 1980s. Nowadays, iron- and sulfur-oxidizing bacteria are widely used to oxidize sulfur-containing ores and flotation concentrates. Depending on the type of metal sulfides, dissolution of the mineral occurs by a combined action of ferric iron and protons. Microorganisms re-generate ferric iron and create acidic conditions in the systems.
The development of stirred tanks to treat flotation concentrates containing refractory gold was initially done by the Gencor Company [6]. The first commercial plant to use the BIOX process was Fairview Mine (in South Africa), installed by Gold Fields in 1986. The number of biooxidation projects in gold mining increased due to the successful application of this technology during the 1980s. At least 14 active gold projects with biooxidation can be identified in the commercial project databank of the Minerals Economic Group [7] as well as in other sources (BGR databanks) [8].
Leachable gold deposits occurring near the surface have been preferentially exploited in the past and are now due to be depleted. Gold production obtained from gold refractory or low grade ores will significantly increase in the future. Many of the new projects are deeply located and must be considered as refractory in mineralogical terms, since gold is occluded in sulfides [7]. This context leads to thinking about the need to include a pretreatment before leaching the minerals with cyanide, in order to improve the recovery of gold. Previous studies showed that biooxidation seems to be a promising alternative to process these sulfides.
Although the commercial application of biooxidation has increased over time worldwide, only a few regional ores have been studied in Argentina at laboratory scale. This work attempts to contribute to the knowledge of this technology applied to regional ores, using native microorganisms.
In the Andacollo mining district, gold is present as submicroscopic particles contained in a pyrite matrix. Previous studies have distinguished a highly refractory feature in this ore (about 50% of gold was recovered without pretreatment) [9]. Currently, CORMINE SEP and MAG S.A. are exploiting the natural resources of this area. The treatment plant processes 350 t/d. The products obtained are a high-grade sand (1000-3000 g/t Au and 5000-14000 g/t Ag) and a flotation concentrate (50-100 g/t Au and 4000-10000 g/t Ag) [10]. In this context, it is imperative to find some alternative environmentally-friendly technologies, which allow to increase Au and Ag recovery from this concentrate.
The aims of this work are to evaluate an alternative process to improve the recovery of gold contained in a flotation concentrate and to study copper and zinc dissolution as subproducts of this biooxidation process.
2 Experimental
2.1 Mineral sample
A flotation concentrate from Andacollo’s treatment plant was used throughout this study. The concentrate consists of polymetallic sulfides and gold which is mainly occluded in pyrite. In addition, great amounts of zinc and copper are in a sulfide form. X-ray diffraction (XRD) was used to identify the phases present in the mineral sample, which were pyrite, sphalerite, covellite, galena, quartz and feldspar. In addition, the sample could contain remnants of froth flotation collectors like sodium isobutyl xanthate (SIBX), potassium amyl xanthate (PAX), AERO 208, AERO 404 and AERO 3477, since these organic compounds are regularly used in this processing plant. The sample chemical characterization was carried out by Inductively Couple Plasma/Optical Emission Spectrometry (ICP-OES) and the results are shown in Table 1.
Table 1 Main values of metals present in flotation concentrate determined by ICP-OES (mass fraction, %)

2.2 Microorganisms and culture media
The consortium used in these experiments was composed of mesophilic iron and sulfur oxidizing bacteria isolated from La Carolina mining district (Province of San Luis, Argentina). Two different isolates (called OxFe and OxS) were used to constitute the inoculum. OxFe presented iron oxidative capacity, and according to BLAST comparison, its 16S-rDNA gene sequence showed 99% similarity with many Leptospirillum ferrooxidans sequences. OxS was able to oxidize sulfur, and its 16S-rDNA gene sequence manifested similarities of 98%-99% to Acidithiobacillus ferrooxidans and Acidithiobacillus ferrivorans, although it has not shown any iron oxidative capacity up until now [11].
OxFe isolate was regularly grown in a 9K medium at pH 1.8, whereas OxS was cultivated in a 0K medium supplemented with elemental sulfur (10 g/L) at pH 3.0 [12]. After the bacteria reached the exponential growth phase, cultures were filtered using a blue ribbon filter paper (Whatman Schleicher and Schuell, Kent, England, 2 μm retention) to eliminate jarosite and sulfur from OxFe and OxS, respectively. Cells were harvested using a 0.2 μm membrane and then suspended again in an iron-lacking medium. A suspension containing about 5×108 cell/mL from each culture was used as inoculum.
2.3 Biooxidation experiments
Experiments were carried out in 1000 mL Erlenmeyer flasks with 400 mL of 1K medium (containing 1 g of ferrous iron per liter) at pH 1.8 and 40 g of flotation concentrate (10% (w/v) pulp density). Each flask was inoculated with cell suspension containing both microorganisms at 5% (v/v). Sterile controls were also run replacing the inoculum by an equal volume of 2% (w/v) thymol in methanol. The flasks were stirred at 200 r/min and incubated at 30 °C. All experiments were conducted in triplicate.
2.4 Cyanide leaching of gold
After biooxidation, the systems were filtered through a blue ribbon filter paper (Whatman Schleicher and Schuell, Kent, England, 2 μm retention) and then the solid residues were used in cyanide leaching tests. Experiments were carried out in agitated bottles with a pulp density of 10% (w/v), at pH values between 10.5-11.5, and using a solution of 0.15% NaCN. The dissolution kinetics was controlled for 48 h.
2.5 Analytical determination
After compensation for evaporation losses, samples were periodically taken throughout the experiment. The leaching solutions were analyzed for pH, redox potential, ferrous iron, and metal concentrations. pH and potential were measured using two separate electrodes in a multimeter. Ferrous iron was determined by the colorimetric o-phenanthroline method using a UV-Vis spectrophotometer. The concentration of dissolved metal (total iron, zinc, and copper) was measured by atomic absorption spectroscopy (AAS) after performing appropriate dilutions with 0.14 mol/L HNO3. At the end of the cyanide leaching tests, the concentration of gold was determined by Inductively Couple Plasma/Optical Emission Spectrometry (ICP-OES).
2.6 X-ray diffraction
The solid residues from the biooxidation tests were air dried and analyzed by X-ray diffraction (XRD). Diagrams were obtained using a Rigaku Geigerflex, Cu K (λ1=1.5405 nm) equipment, operated at 40 kV, 30 mA, NaCl and quartz as external calibration standards, at 5 (°)/min between 0°-80°. Crystalline phases were identified by comparison with PDF standards (powder diffraction files) from the Joint Committee of Power Diffraction Standards (JCPDS) database.
3 Results and discussion
3.1 Bioleaching experiments
Biooxidation experiments were maintained for 60 d until their metal concentrations reached a steady state. Redox potential values within the range of 330-380 mV were measured in sterile controls, while an important initial increase of these values was detected in cultures, reaching 620 mV after 20 d (see Fig. 1). This significant increase can be explained by the ferrous iron oxidation catalyzed by iron-oxidizing microorganisms present in the inoculum. The following equation shows this reaction:
4Fe2++O2+4H+→4Fe3++2H2O (1)
This behavior was verified by the ferrous iron oxidation kinetics that took place in cultures and sterile controls (Fig. 2). On the other hand, the time required to complete the oxidation of ferrous iron in cultures was longer than that obtained in similar studies employing other mineral samples (without collectors), and with similar iron concentration (data not shown). At first, this result shows a certain inhibition of iron-oxidizing bacteria, probably due to the presence of flotation agents. L. ferrooxidans, the main iron oxidizing bacteria, is usually more sensitive to organic compounds (collectors) than A. ferrooxidans [13].
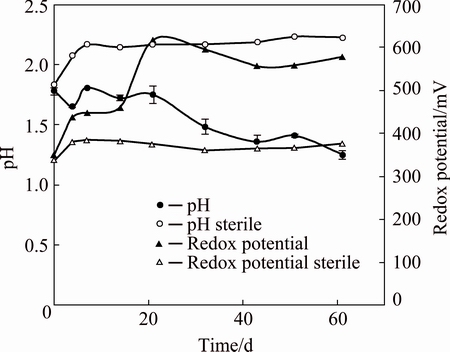
Fig. 1 pH and redox potential during biooxidation using mesophilic native consortium
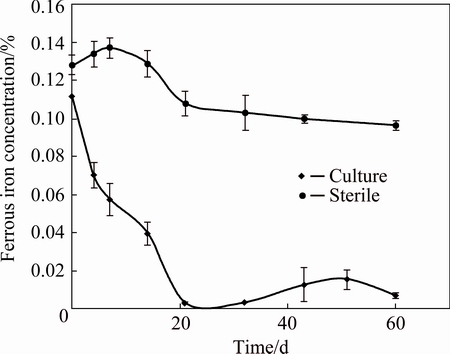
Fig. 2 Ferrous iron concentration during biooxidation of flotation concentrate sample from Andacollo’s processing plant in culture and sterile control
At the beginning, a slight increase in pH values was observed in sterile controls, which could be due to the dissolution of alkaline species contained in the mineral sample. Then, pH values stayed close to 2.1-2.2 throughout the whole experiment (see Fig. 1). In contrast, a decrease of pH values was detected in cultures; this may occur due to the acidic conditions produced by the activity of sulfur oxidizing bacteria (see Eq. (2)). In the end, cultures reached pH values close to 1.2.
S0+H2O+1.5O2→ +2H+ (2)
+2H+ (2)
The dissolution of pyrite takes place through the thiosulfate pathway where the main microbial action is the production of ferric iron which is the oxidizing agent for sulfides [14,15].
Figure 3 shows the total soluble iron profiles for culture and sterile control. Here, it should be mentioned that all systems were initially supplemented with 1 g/L (0.1%) of ferrous iron. During the first 20 d, cultures and abiotic controls exhibited a soluble iron concentration close to 0.14%. Afterwards, the behaviors of culture and sterile control were clearly different: the first one developed an exponential increase that exceeded 0.4% in solution; this result agreed with the values determined in other parameters (redox potential and ferrous iron concentration). Fe3+/Fe2+ is the main redox couple prevailing in the bioleaching/biooxidation systems. Because of this, soluble Fe3+ concentrations are important to mineral sulfide dissolution kinetics [16]. Finally, total iron concentrations remained at constant values between 40-60 d.
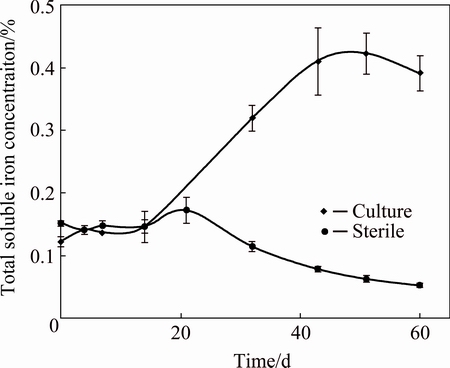
Fig. 3 Total soluble iron concentration during biooxidation of flotation concentrate sample from Andacollo’s plant
On the other hand, soluble iron concentrations decreased progressively in sterile controls after 20 d; the ferric ion hydrolysis along with the consequent precipitation of ferric compounds, might explain this result. Jarosite is one of the most frequent precipitates formed during bioleaching (see Eq. (3)); its appearance is associated with elevated ferric iron concentration and pH values higher than 1.8 [14,17,18]. It is highly likely that the formation of iron precipitates also occurs in cultures, although these systems could sustain an increase of total soluble iron concentration during most of the time (see Fig. 3).
3Fe3++M++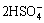 +6H2O→MFe3(SO4)2(OH)6+8H+ (3)
+6H2O→MFe3(SO4)2(OH)6+8H+ (3)
where M represents a monovalent alkali cation, usually K+ or Na+.
The highest solubilization of 21.5% of iron (deducting the initial concentration added) was reached in the cultures, at 40 d. The oxidation degree of pyrite (and consequently the contribution to decrease the mineral refractory character) is surely higher than that calculated from the solubilized iron. This overlooks the solubilized and precipitated iron; however, it would probably not prevent gold leaching. These results were compared with those obtained by CHIACCHIARINI et al [19] using a similar sample of this flotation concentrate with A. ferrooxidans and A. thiooxidans. The experiments were carried out under similar conditions to those of this research, with the exception of the pulp density of 5% (w/v). Cultures achieved similar iron concentrations in solution (20% and 21.5%) after 40 d. The results suggest that the native microorganisms used throughout our studies are at least as efficient as typical leaching strains.
After biooxidation, the solid residues were leached by cyanidation. This test accomplished a 91.6% of gold recovery for cultures and 67.2% for sterile controls. Recoveries higher than 90% are typical of ores containing free gold [20,21]. Our results showed that the biological pre-treatment increased the liberation of gold, and consequently the recovery of metal. Similar results have been reported by other authors [2,22]. Taking into account that abiotic systems were stirred for a long period of time, a significant amount of ferric iron should be formed, improving the ore oxidation. Therefore, the gold recovery percentage was higher than the typical values obtained for a high refractory ore without any treatment (lower than 50%) [21].
Mineral residues from biooxidation had a consumption of 6.6 kg/t of NaCN, while sterile control demanded 3.6 kg/t. Metals, sulfurs and iron complexes are considered “cyanicides”; the presence of these compounds in leaching systems reduces available CN-, so NaCN consumption increased [3,23]. The large iron precipitation that took place in cultures (detected by XRD analysis) was consistent with this result.
3.2 Modeling copper and zinc dissolution
During pyrite biooxidation, other metal sulfides like sphalerite and covellite were also solubilized. Zinc and copper dissolution kinetics profiles are shown in Figs. 4 and 5. Metal recoveries of 55.2% Zn and 47.8% Cu were reached for the cultures, while the sterile controls only reached 33.5% Zn and 12.3% Cu, at 40 d. Although metal recoveries did not reach high enough percentages, physicochemical parameters showed metabolic actions by the native consortium compared to sterile control. In other words, microorganisms were capable of generating ferric iron to oxidize metal sulfides, and at the same time, maintained acidic condition to keep part of the metallic ions in solution.
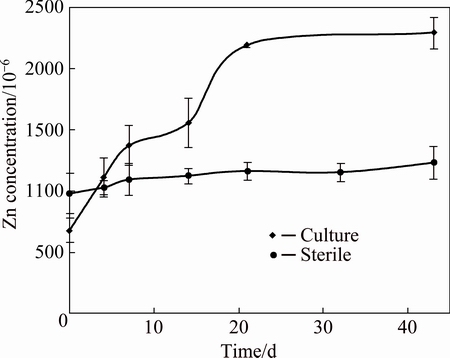
Fig. 4 Kinetics of zinc dissolution in biooxidation for culture and sterile control
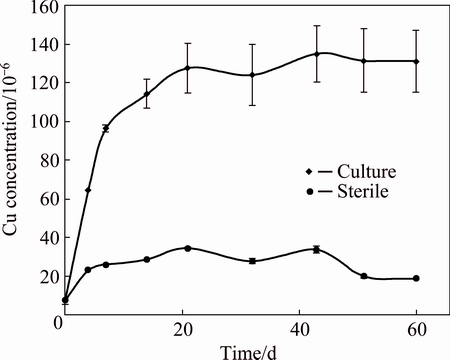
Fig. 5 Kinetics of copper dissolution in biooxidation for culture and sterile control
Sphalerite and covellite dissolutions should take place by the polysulfide pathway. That is, they can be attacked by the action of ferric iron or protons. Equations (4) and (5) show the sphalerite and covellite chemical reactions of dissolution by protons.
0.5ZnS+0.25O2+H+→0.5Zn2++0.5H2O+0.5S0 (4)
CuS+0.5O2+2H+→Cu2++H2O+S0 (5)
At low pH values, the sulfur moiety of metal sulfides is oxidized to elemental sulfur, which can be biologically oxidized to sulfuric acid, according to Eq. (2).
The shrinking core model was applied to study zinc and copper dissolution kinetics. This model proposes that these action kinetics can be controlled by any of the sequential step that occurs in the liquid-solid interface [24]: diffusion through liquid film, diffusion through a product layer or chemical reaction. In bioleaching systems carried out in stirred tanks, it can be assumed that the leaching kinetics is not controlled by diffusion problems through liquid films [25], so the reaction limiting step must obey one of the other two steps.
If the reaction kinetics is controlled by diffusion through the product layer, the results should follow the behavior predicted by
Kpt=1-(2/3)x-(1-x)2/3 (6)
where Kp is the parabolic rate constant (d-1), t is time (d) and x is the fraction of metal reacted.
On the other hand, if the process is controlled by chemical reaction, the mathematical expression is given by
Kpt=1-(1-x)1/3 (7)
A mathematical model was applied to fit the experimental data from cultures. In the case of zinc, the coefficient values were very close to each other (0.969 and 0.971, respectively) for the diffusion through the product layer and chemical reaction. Thus, it seems that it does not have a strict control of the process for any of these steps. Many studies have reported that sphalerite dissolution kinetics by mesophilic bacteria is limited by the diffusion through the product layer [26-30]. In those cases, experiments were carried out at pulp densities lower than 5% (w/v). In this case, a higher pulp density could affect the metal dissolution in a more significant way; as a consequence, the limitation by chemical reaction could have been greater than in the previously mentioned investigations. Many studies have reported decreases in the gas transfer rates at higher pulp density, thus causing a harmful effect to the process [31,32]. In addition, the coating of mineral surfaces, caused by the generation of sulfur and/or precipitates at low pulp densities, seems to be more relevant than that produced at higher values; so the diffusion through the product layer could have a more pronounce contribution in the control of the process.
As opposed to the zinc results, copper dissolution kinetics coefficients were different: 0.986 for the diffusion through the product layer and 0.881 for the chemical reaction. In this way, covellite dissolution was certainly controlled by the generation of a product layer. In order to recognize the main compounds of this layer, XRD analyses were performed in solid residues from bioleaching experiments (sterile and culture). The diffractograms showed peaks of quartz, pyrite, sphalerite, covellite, sulfur (S8), and jarosite (see Fig. 6). Previous studies have demonstrated that the generation of sulfur and jarosite in bioleaching systems reduces the metal sulfur dissolution kinetics [33-36] and that could have harmed the copper dissolution process.
The detection of S8 in culture suggests that the sulfur oxidizing bacteria activity was not entirely efficient, since they did not prevent the accumulation of sulfur on the mineral surface. It is probable that the bacterial activity could have been partially or totally inhibited by the extreme system conditions (high pulp density, high concentration of metals, attrition of cells, etc.).
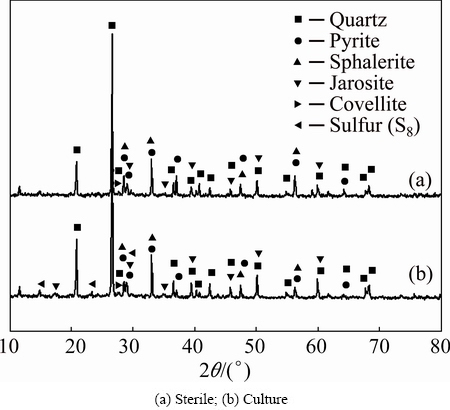
Fig. 6 XRD patterns of residues after biooxidation test
The oxidative mechanism (acceptable for both metal sulfides, sphalerite and covellite) is more effective when bacteria act on the mineral surface and that is even more important when the sulfide is less soluble. Covellite is much less soluble than sphalerite, so that covellite bioleaching requires a microbial activity on its surface. This activity produces higher Fe3+ concentration and high enough pH values (see Eq. (1)) near the surface causing a significant precipitation of jarosite on the surface. This precipitation justifies that the bioleaching of covellite is kinetically controlled by the diffusion through a product layer. Sphalerite is more soluble and the progress of its oxidation takes place mainly into the solution; because of that, its bioleaching is practically not affected by the precipitation of jarosite.
4 Conclusions
A successful biooxidation was performed by a native consortium constituted by iron- and sulfur- oxidizing bacteria. The bacterial activity increased gold recovery up to 91.6% instead of 67.3% reached in the sterile control, showing that biooxidation can be used to enhance gold recovery from this flotation concentrate. In addition, other metals, zinc and copper, contained in the ore could be recovered during the biooxidation process.
The application of the shrinking core model demonstrated that zinc dissolution kinetics lacked a well-defined control step; nevertheless, copper dissolution was clearly limited by the generation of a product layer which could be jarosite.
To sum up, the native consortium used in this work can be used to treat the flotation concentrate from Andacollo mine to enhance the gold recovery and to solubilize zinc and copper at the same time.
Acknowledgments
This work was supported by PIP 0368 from CONICET and PICT 0630 and 0623 from ANPCyT.
References
[1] CIFTCI H, AKCIL A. Biohydrometallurgy in Turkish gold mining: First shake flask and bioreactor studies [J]. Minerals Engineering, 2013, 46-47: 25-33.
[2] KAKSONEN A, MUDUNURU B M, HACKL R. The role of microorganisms in gold processing and recovery—A review [J]. Hydrometallurgy, 2014, 142: 70-83.
[3] UBALDINI S,  F, TORO L, ABBRUZZESE C. Biooxidation of arsenopyrite to improve gold cyanidation: Study of some parameters and comparison with grinding [J]. International Journal of Mineral Processing, 1997, 52: 65-80.
F, TORO L, ABBRUZZESE C. Biooxidation of arsenopyrite to improve gold cyanidation: Study of some parameters and comparison with grinding [J]. International Journal of Mineral Processing, 1997, 52: 65-80.
[4] ANJUM F, SHAHID M, AKCIL A. Biohydrometallurgy techniques of low grade ores: A review on black shale [J]. Hydrometallurgy, 2012, 117-118: 1-12.
[5] BRIERLEY C L. Biohydrometallurgical prospects [J]. Hydrometallurgy, 2010, 104: 324-328.
[6] van ASWEGEN P C, GODFREY M W, MILLER D M, HAINES A K. Developments and innovations in bacterial oxidation of refractory ores [J]. Mineral Metallurgy Processing, 1991, 8: 168-191.
[7] SCHIPPERS A, HEDRICH S, VASTERS J, DROBE M, SAND W, WILLSCHER S. Biomining: Metal recovery from ores with microorganisms [M]//Geobiotechnology I. Berlin: Springer, 2013: 1-47.
[8] BRIERLEY C L, BRIERLEY J A. Progress in bioleaching. Part B: Applications of microbial processes by the minerals industries [J]. Applied Microbiology Biotechnology, 2013, 97: 7543-7552.
[9] CHIACCHIARINI P, de la FUENTE V, DONATI E. Pre-treatment of a low grade refractory gold sulphide ore (Andacollo,  -Argentina) using an airlift reactor [M]//Biohydrometallurgy: Fundamentals, Technology and Sustainable Development. Amsterdam: Elsevier, 2001: 107-114.
-Argentina) using an airlift reactor [M]//Biohydrometallurgy: Fundamentals, Technology and Sustainable Development. Amsterdam: Elsevier, 2001: 107-114.
[10] COUTSIERS N. Technical report. Environmental area [M]. Andacollo: Minera Andacollo Gold S.A, 2010.
[11] MARCHEVSKY N, URBIETA M S, BERNARDELLI C, MAS M, DONATI E R. Zinc recovery during refractory ore biooxidation by an indigenous consortium [J]. International Journal of Mineral Processing, 2015, 138: 30-37.
[12] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Thiobacillus ferrooxidans I. An improved medium and a harvesting procedure for securing high cellular yields [J]. Journal of Bacteriology, 1959, 77: 642-647.
[13] OKIBE N, JOHNSON D B. Toxicity of flotation reagents to moderately thermophilic bioleaching microorganisms [J]. Biotechnology Letters, 2002, 24: 2011-2016.
[14] VERA M, SCHIPPERS A, SAND W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A [J]. Applied Microbiology and Technology, 2013, 97: 7529-7541.
[15] ZHANG R, BELLENBERG S, NEU T R, SAND W, VERA M. The biofilm lifestyle of acidophilic metal/sulfur-oxidizing microorganisms [M]//Biotechnology of Extremophiles. Cham: Springer International Publishing, 2016: 177-213.
[16] RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M L, GONZALEZ F,  J A. New information on the sphalerite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy, 2003, 71: 57-66.
J A. New information on the sphalerite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy, 2003, 71: 57-66.
[17] DAOUD J, KARAMANEV D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans [J]. Minerals Engineering, 2006, 19: 960-967.
[18] GIAVENO A, PETTINARI G,  E, AGUILERA A, URBIETA M, DONATI E.The influence of two thermophilic consortia on troilite (FeS) dissolution [J]. Hydrometallurgy, 2011, 106: 19-25.
E, AGUILERA A, URBIETA M, DONATI E.The influence of two thermophilic consortia on troilite (FeS) dissolution [J]. Hydrometallurgy, 2011, 106: 19-25.
[19] CHIACCHIARINI P, de la FUENTE V, DONATI E. Pre-treatment of refractory gold sulphide ore by means of Acidithiobacilli cells [J]. Latin American Applied Research, 2003, 33: 33-37.
[20] CELEP O, ALP I, DEVECI H, VICIL M. Characterization of refractory behaviour of complex gold/silver ore by diagnostic leaching [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 707-713.
[21] KARTHIKEYAN O P, RAJASEKAR A, BALASUBRAMANIAN R. Bio-oxidation and bio-cyanidation of refractory mineral ores for gold extraction: A review [J]. Critical Reviews in Environmental Science and Technology, 2015, 45: 1611-1643.
[22] TANAKA M, YAMAJI Y, FUKANO Y, SHIMADA K, ISHIBASHI J, HIRAJIMA T, SASAKI K, SAWADA M, OKIBE N. Biooxidation of gold-, silver-, and antimony-bearing highly refractory polymetallic sulfide concentrates, and its comparison with abiotic pre-treatment techniques [J]. Geomicrobiology Journal, 2014, 32: 538-548.
[23] CIFTCI H, AKCIL A. Effect of biooxidation conditions on cyanide consumption and gold recovery from a refractory gold concentrate [J]. Hydrometallurgy, 2010, 104: 142-149.
[24] LEVENSPIEL O. Ingeniería de las reacciones químicas [M]. 2nd ed. Barcelona: Reverté S.A., 1986.
[25] MISHRA M, SINGH S, DAS T, KAR R N, RAO K S, SUKLA L B, MISHRA B K. Bio-dissolution of copper from Khetri lagoon material by adapted strain of Acidithiobacillus ferrooxidans [J]. Korean Journal of Chemical Engineering, 2008, 25: 531-534.
[26] da SILVA G. Kinetics and mechanism of the bacterial and ferric sulphate oxidation of galena [J]. Hydrometallurgy, 2004, 75: 99-110.
[27] GIAVENO A, LAVALLE L, CHIACCHIARINI P, DONATI E. Bioleaching of zinc from low grade complex sulfide ores in an airlift by isolated Leptospirillum ferrooxidans [J]. Hydrometallurgy, 2007, 89: 117-126.
[28] LIAO M X, DENG T L. Zinc and lead extraction from complex raw sulfides by sequential bioleaching and acidic brine leach [J]. Minerals Engineering, 2004, 17: 17-22.
[29] LIZAMA H M A. kinetic description of percolation bioleaching [J]. Minerals Engineering, 2004, 17: 23-32.
[30] MARCHEVSKY N. Studies on the refractory minerals biooxidaation as alternative methodology to increase gold recovery [D]. Corrientes, Argentina: National University of Northeast, 2014.
[31] ACEVEDO F, GENTINA J C, VALENCIA P. Optimization of pulp density and particle size in the biooxidation of a pyritic gold concentrate by Sulfolobus metallicus [J]. World Journal of Microbiology and Biotechnology, 2004, 20: 865-869.
[32] HAGHSHENAS D F, BONAKDARPOUR B, ALAMDARI E K, NASERNEJAD B. Optimization of physicochemical parameters for bioleaching of sphalerite by Acidithiobacillus ferrooxidans using shaking bioreactors [J]. Hydrometallurgy, 2012, 111-112: 22-28.
[33] AHMADI A, SCHAFFIE M, MANAFI Z, RANJBAR M. Electrochemical bioleaching of high grade chalcopyrite flotation concentrate in a stirred tank reactor [J]. Hydrometallurgy, 2010, 104: 99-105.
[34] CHEN P, YAN L, LENG F F, NAN W B, YUE X X, ZHENG Y N, FENG N, LI H Y. Bioleaching of realgar by Acidithiobacillus ferrooxidans using ferrous iron and elemental sulfur as the sole and mixed energy sources [J]. Bioresource Technology, 2011, 102: 3260-3267.
[35]  BALLESTER A. Passivation of chalcopyrite during its chemical leaching with ferric ion at 68 °C [J]. Minerals Engineering, 2009, 22: 229-235.
BALLESTER A. Passivation of chalcopyrite during its chemical leaching with ferric ion at 68 °C [J]. Minerals Engineering, 2009, 22: 229-235.
[36] DONG Y B, LIN H, XU X F, ZHANG Y, GAO Y J, ZHOU S S. Comparative study on the bioleaching, biosorption and passivation of copper sulfide minerals [J]. International Biodeterioration & Biodegradation, 2013, 84: 29-34.
本地混合菌群微生物氧化难处理含金硫化精矿
N. MARCHEVSKY1,2, M. M. BARROSO QUIROGA2, A. GIAVENO3, E. DONATI1
1. Centro de  y Desarrollo en Fermentaciones Industriales (CINDEFI, CCT LA PLATA-CONICET, UNLP), Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Calle 50 N° 227, 1900 La Plata, Argentina;
y Desarrollo en Fermentaciones Industriales (CINDEFI, CCT LA PLATA-CONICET, UNLP), Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Calle 50 N° 227, 1900 La Plata, Argentina;
2. Departamento de  , Facultad de Ciencias
, Facultad de Ciencias  y Naturales, Universidad Nacional de San Luis, Chacabuco 917, 5700 San Luis, Argentina;
y Naturales, Universidad Nacional de San Luis, Chacabuco 917, 5700 San Luis, Argentina;
3. Instituto de  y Desarrollo en
y Desarrollo en  de Procesos,
de Procesos,  Alternativas (PROBIEN, CONICET-UNCo), Departamento de
Alternativas (PROBIEN, CONICET-UNCo), Departamento de 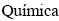 , Facultad de
, Facultad de 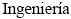 , Universidad Nacional del Comahue, Buenos Aires 1400, 8300
, Universidad Nacional del Comahue, Buenos Aires 1400, 8300 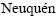 , Argentina
, Argentina
摘 要:采用含铁氧化菌和硫氧化菌的混合嗜中温菌群浸出阿根廷Andacollo矿的含金硫化浮选精矿。实验在摇瓶中进行,矿浆浓度为10%,采用pH 1.8的含亚铁离子的基础盐溶液作为介质。采用不同的方法对浸出液的pH值、氧化还原电位、亚铁离子和总金属浓度进行测定。结果表明,该种混合菌能够很好地还原浸出该难处理金矿,金的回收率可达91.6%,同时,铜和锌的浸出率也较高。动力学研究表明金属的溶解符合收缩核模型。其中,铜的溶解速率受生成产物层的扩散控制,而锌的溶解没有明显的控制步骤。
关键词:生物氧化;难处理金矿;混合菌;动力学分析
(Edited by Sai-qian YUAN)
Corresponding author: N. MARCHEVSKY; E-mail: nmarchevsky@unsl.edu.ar
DOI: 10.1016/S1003-6326(17)60133-X
Abstract: A defined mesophilic consortium including an iron oxidizing bacterium and a sulfur oxidizing bacterium was constructed to evaluate its ability for bioleaching a flotation concentrate from Andacollo mine in Neuquén, Argentina. Experiments were performed in shake flasks with a pulp density of 10% (w/v), using a basal salt medium containing ferrous iron at pH 1.8. The leaching solutions were analyzed for pH, redox potential (using specifics electrodes), ferrous iron (by UV-Vis spectrophotometry) and metal concentrations (by atomic absorption spectroscopy). The results showed that the consortium was able to reduce the refractory behavior of the concentrate, allowing 91.6% of gold recovery; at the same time, high dissolution of copper and zinc was reached. These dissolutions followed a shrinking core kinetic model. According to this model, the copper solubilization was controlled by diffusion through a product layer (mainly jarosite), while zinc dissolution did not show a defined control step. This designed consortium, composed of bacterial strains with specific physiological abilities, could be useful not only to optimize gold recovery but also to decrease the leachates metallic charge, which would be an environmental advantage.


