锡阳极泥硫化焙烧分离锑的热力学研究
云南锡业集团有限责任公司研究设计院
摘 要:
对锡阳极泥硫化焙烧过程进行了系统的热力学计算和趋势分析, 重点研究了硫化剂的选择、Sn-S-O系与Sb-S-O系优势区相图的对比、碳对硫化反应的影响。研究结果表明:在同一温度下, FeS2的离解压比SnS和Sb2S3的离解压大得多, 故可将锡和锑硫化, 而黄铁矿很适合作为锡阳极泥焙烧分离锑工艺所需的硫化剂;高温有利于锡、锑硫化物的生成, 而在同一温度下, 锑比锡更容易硫化;还原剂碳能促进Sb2O4和SnO2硫化反应的进行, 且Sb2O4比SnO2硫化的趋势更强。此外, 由于Sb2S3的饱和蒸汽压比较大, 故锡阳极泥在硫化焙烧过程中锑以Sb2S3的形式挥发, 达到锑开路的目的。
关键词:
中图分类号: TF814
作者简介:卢红波 (1985-) , 男, 贵州安顺人, 学士;研究方向:有色金属冶炼研究与设计 E-mail:hblu0506@163.com;
收稿日期:2013-04-16
基金:云南锡业集团有限责任公司一类科研项目;
Thermodynamics of Separation Stibium from Tin Anode Slime by Sulfidation Roasting
Lu Hongbo Yin Jiufa Zhang Qiwang Zhang Min
Research and Design Institute of Yunnan Tin Group Company Limited
Abstract:
Thermodynamic calculation and trend analysis of sulfidation roasting process for tin anode slime were studied, focusing on the sulfidizer selection, the comparison of predominance area phase diagram of Sn-S-O system and Sn-S-O system, and the influence of carbon on sulfidation reaction. The results showed that FeS2could vulcanize tin and antimony as the value of the dissociation pressure of FeS2was greater than that of SnS and Sb2S3at the same temperature, so pyrite was suitable for using as sulfidizer for separation of stibium from tin anode slime by sulfidation roasting; high temperature benefited for producing tin sulfide and antimony sulfide, while antimony was easier to be vulcanized than tin at the same temperature; carbon as the reducing agent could promote vulcanizing of Sb2O4and SnO2, moreover, Sb2O4sulfidation trend was stronger than that of SnO2. In addition, owing to Sb2S3saturation vapor pressure was relatively larger, so removing antimony in the form of Sb2S3from tin anode slime by sulfidation roasting process is feasible.
Keyword:
tin anode slime; sulfidation roasting; predominance area phase diagram; thermodynamics;
Received: 2013-04-16
锑对锡冶炼是极其有害的杂质, 尤其是在锡的电解工艺, 它使锡的回收率大大降低[1]。当阳极板中含锑大于1%时, 由于锑和锡会形成致密的合金网状结构, 导致锡的电化学溶解困难, 产生阳极钝化效应, 从而引起槽电压升高、能耗增加、电流效率降低等一系列问题[2,3]。
随着锡矿的大量开采, 锡精矿的品位逐年下降, 杂质含量越来越高, 目前处理的粗锡或粗焊锡中锑的含量均在1%以上[4]。而在锡冶炼工艺中, 锑的开路主要是生产巴氏合金, 但巴氏合金市场小, 其牌号、数量受到了市场的制约[5]。由于没有经济合理的处理工艺, 含锑的锡中间物料返回锡冶炼系统中, 造成恶性循环, 导致锑在锡冶炼流程中逐渐积累。最终, 产出的锡阳极泥中含锑过高, 使得现有的盐酸浸出工艺[6]难度加大, 成本过高。为了使锡冶炼、焊锡电解及阳极泥等处理工艺正常化, 同时, 进一步回收有价金属, 锑的开路回收是当务之急。
据报道, 高锑锡阳极泥处理工艺主要有焙烧-酸浸法[7,8]、选-冶联合法[9]、真空分离法[10,11], 此外还有“因材施法”制备Sn-Sb功能材料[12,13,14], 这些方法不同程度地存在流程长、污染重、能耗高、难度大等缺点, 在生产上难以应用。本文提出了硫化焙烧处理锡阳极泥分离锑的新工艺, 对硫化过程进行了系统的热力学计算和趋势分析, 以期为此新工艺的研究提供理论支撑。
1 热力学分析
锡阳极泥呈灰黑色疏松的块状物, 因长期露天堆放, 大部分金属已氧化, 锡和锑主要分别以二氧化锡和四氧化二锑形态存在[15,16]。
查阅热力学数据手册[17]及参考相关资料[18]对锡阳极泥硫化焙烧过程进行热力学研究, 重点分析了硫化剂的选择、Sn-S-O系与Sb-S-O系优势区相图的对比、碳对硫化反应的影响。由于缺少相应的热力学数据, 在作Sn-S-O系与Sb-S-O系优势区相图的对比时, 本文仅对700和900 K两个温度进行研究。
1.1 离解压
锡、锑、铁的硫化物在高温下会发生离解, 其离解反应及标准吉布斯自由能与温度的关系式如下所示:
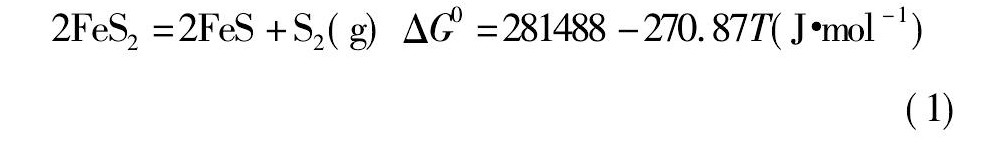
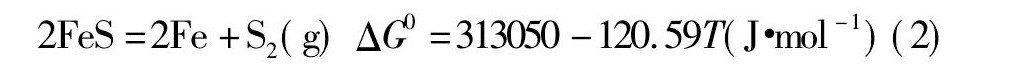
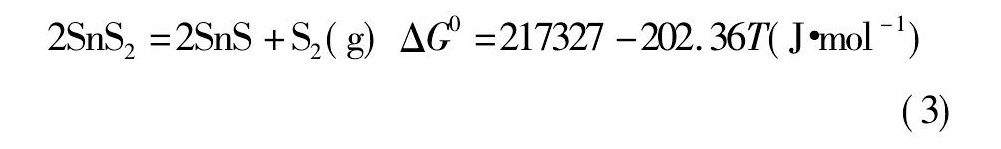
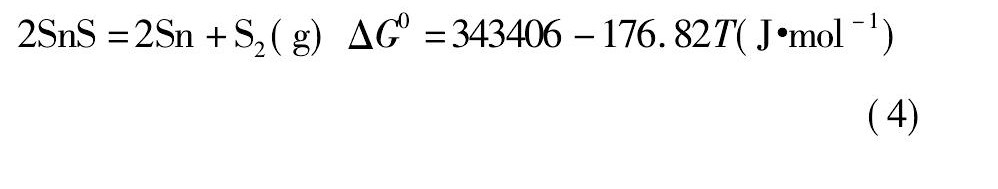
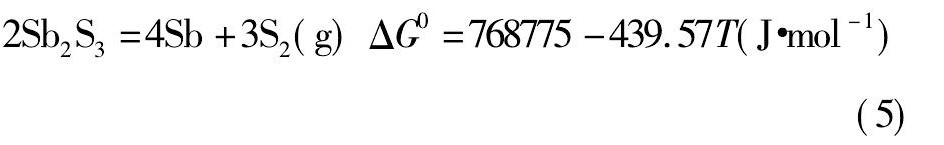
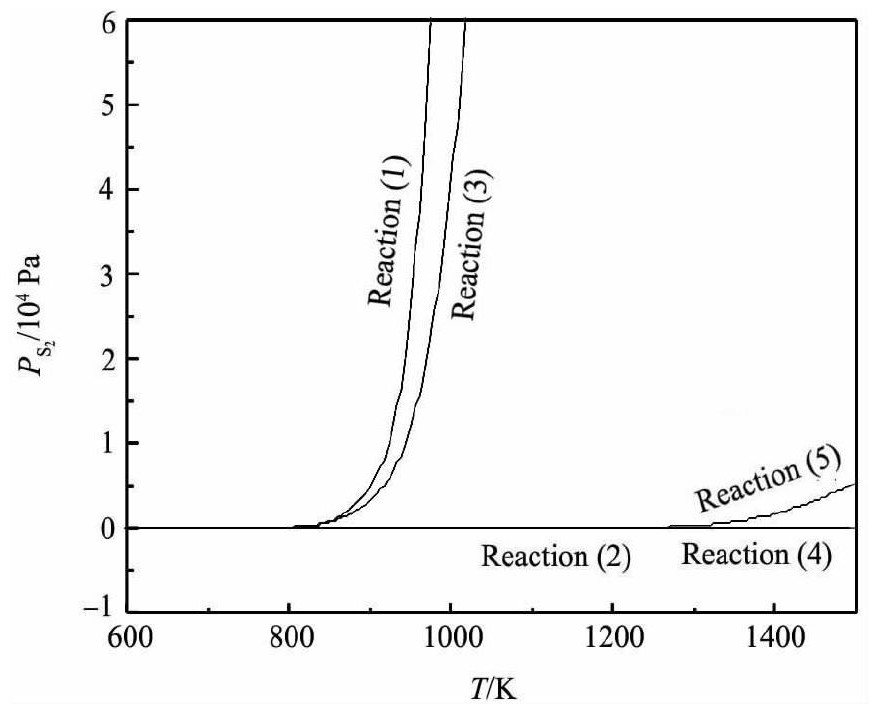
经计算, 以上物质的离解压曲线如图1所示。
由图1可知, 锡、锑、铁硫化物的离解压均随着温度的升高而逐渐增大。Fe S2和Sn S2的离解压在800 K左右开始增大, 而后增大的趋势较迅猛;而Sb2S3的离解压大约在1300 K时才开始有所增加, 且较为平缓;Sn S和Fe S的离解压随温度升高增加的趋势不明显。故Sn S和Fe S的离解压小, 较难分解, 只能被氧化;Sn S2的离解压较大, 高温下易分解, 温度高于793 K即分解[19];Fe S2的离解压比Sn S和Sb2S3的离解压大得多, 所以Fe S2可以将锡、锑硫化。黄铁矿是分布最广的硫化矿, 主要成分为Fe S2[20], 且价格便宜, 非常适合作为锡阳极泥焙烧分离锑工艺所需的硫化剂。
1.2 优势区相图
根据体系内各物质的热力学性质, 按照相律、同时平衡的原理及逐级转变原则的要求, 经过热力学计算, 可以绘制出各体系的优势区相图。700 K时, Sn-S-O系和Sb-S-O系中各反应的二氧化硫分压和氧分压的关系如表1所示。
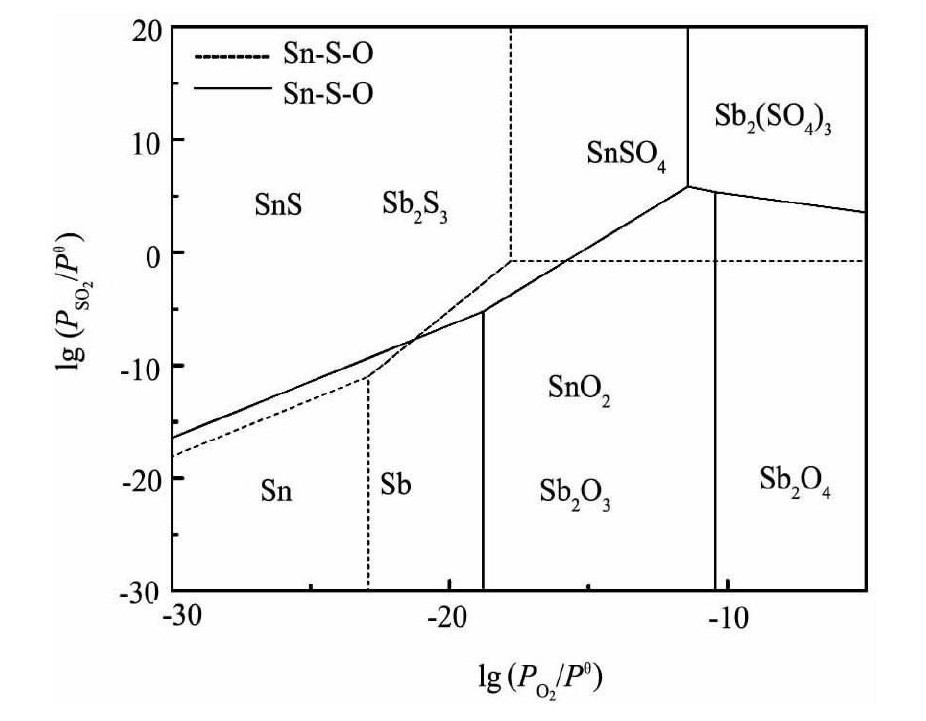
由表1中各反应的平衡关系可以分别作出SnS-O和Sb-S-O三元系的优势区相图, 然后再将其叠加, 得到700 K时Sn-S-O系和Sb-S-O系的优势区相图叠加图, 如图2所示。同理, 可以作出900K时Sn-S-O系和Sb-S-O系的优势区相图叠加图, 如图3所示。
图1 相关硫化物的离解压曲线Fig.1Dissociation pressure of relative sulfide
表1 700 K下平衡体系SO2分压与O2分压的关系Table 1Partial pressure relations of equilibrium systems of SO2and O2at 700 K 下载原图
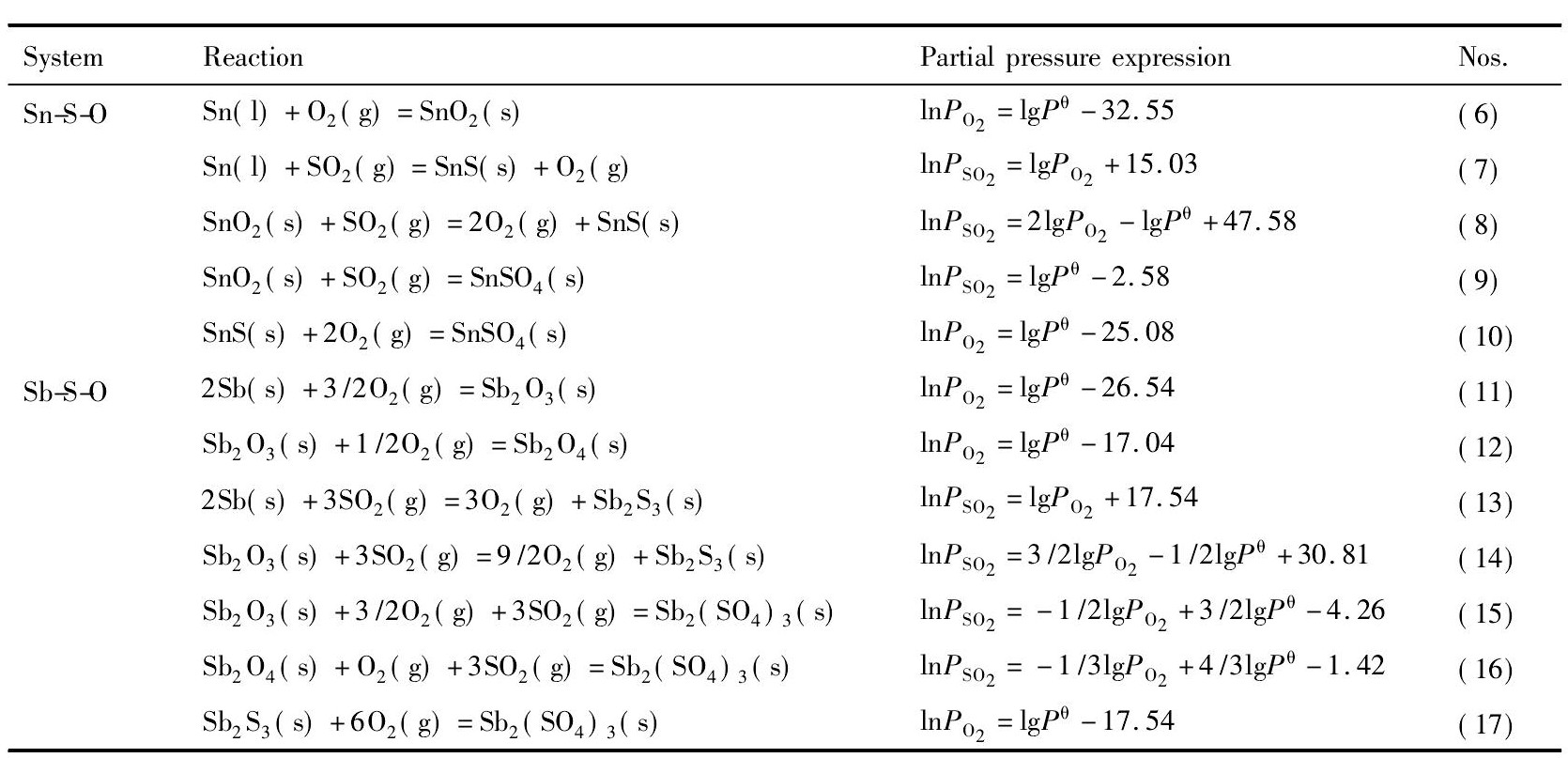
表1 700 K下平衡体系SO2分压与O2分压的关系Table 1Partial pressure relations of equilibrium systems of SO2and O2at 700 K
结合图2和3可知, 在700 K时, 要得到Sb2S3, 需控制lg (PO2/Pθ) <-17.54, 而要得到Sn S, 需控制lg (PO2/Pθ) <-25.08;在900 K时, 要得到Sb2S3, 需控制lg (PO2/Pθ) <-11.42, 而要得到Sn S, 需控制lg (PO2/Pθ) <-17.81。通过对比可以看出, 在同一体系中, 随着温度的升高, 锡、锑硫化物的稳定区域扩大, 即高温有利于锡、锑硫化物的生成;而在同一温度下, Sb2S3的稳定区比Sn S的稳定区大, 锑比锡更容易硫化。
在Sn-S-O系和Sb-S-O系的优势区相图中可以看出, 要得到相应的硫化物, 对SO2分压和O2分压均有要求, 也即是只有当体系中的氧势和硫势控制得当时, 硫化物才能生成并且稳定存在;若体系中氧势和硫势超过相应的数值时, 硫化物会氧化成氧化物或硫酸盐。
图2 700 K时Sn-S-O系和Sb-S-O系的优势区相图叠加图Fig.2Superposition of predominance area phase diagram in Sb-S-O system and Sn-S-O system at 700 K
图3 900 K时Sn-S-O系和Sb-S-O系的优势区相图叠加图Fig.3Superposition of predominance area phase diagram in Sb-S-O system and Sn-S-O system at 900 K
1.3 碳对硫化反应的影响
黄铁矿在高温下会发生离解反应生成S2和Fe S[21,22], 而S2, Fe S易与氧反应生成二氧化硫。在无还原剂碳存在的情况下, 锡阳极泥与黄铁矿的混合料焙烧过程中可能会发生反应 (18) ~ (21) ;而有还原剂碳存在的情况下, 则可能会发生反应 (22) ~ (25) 。
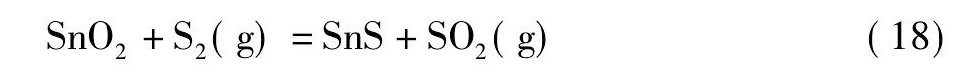
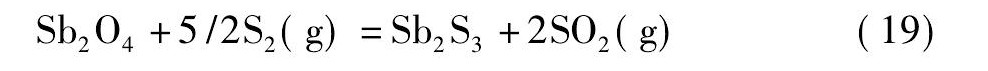
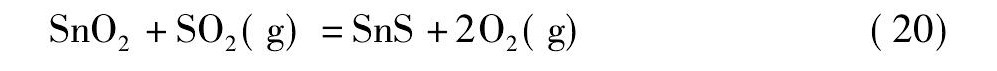
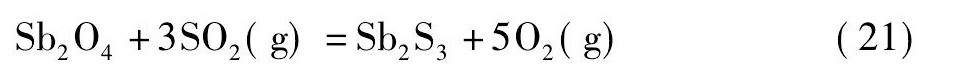


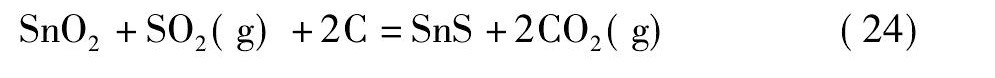
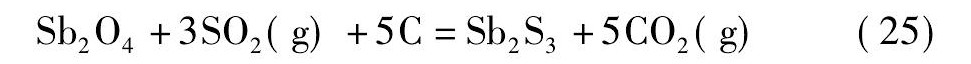
以上反应的吉布斯自由能与温度的关系如图4所示。
由图4可知, 从热力学角度考虑, 在计算的温度范围内, 当没有还原剂碳存在的情况下, 只有反应式 (19) 的标准自由能为负值, 说明反应式 (19) 可以自发进行;反应式 (18) 的标准自由能在1130 K时由正值变为负值, 热力学趋势逐渐变强, 表明仅当温度升高至1130 K后, 反应式 (18) 才自发进行;反应式 (20) 和反应式 (21) 的标准自由能为正值, 不能自发进行。然而, 在有还原剂碳存在的情况下, 反应式 (22) ~ (25) 的标准自由能均为负值, 可以自发进行, 随着温度的升高, 热力学趋势增强, 且呈反应式 (25) >反应式 (23) >反应式 (24) >反应式 (22) 的顺序。综上, 还原剂碳能促进Sb2O4和Sn O2硫化反应的进行, 且Sb2O4比Sn O2硫化的趋势更强。
基于以上原理, 结合锡、锑硫化物不同温度下的饱和蒸汽压[16]可知, 同一温度下, Sb2S3比Sn S的饱和蒸汽压大很多, 故锡阳极泥可以经硫化焙烧使锑以三硫化二锑的形式挥发[23,24], 达到锑开路的目的。
图4 反应式 (18) ~ (25) 的吉布斯自由能与温度关系Fig.4 Relationships betweenΔGTand T of reactions (18) to (25)
2 结论
1.Fe S2的离解压比Sn S和Sb2S3的离解压大得多, 可以将锡和锑硫化;再者黄铁矿主要成分为Fe S2, 且价格便宜, 可作为锡阳极泥焙烧分离锑工艺所需的硫化剂。
2.随着温度的升高, 锡、锑硫化物的稳定区域扩大, 即高温有利于锡、锑硫化物的生成;而在同一温度下, Sb2S3的稳定区比Sn S的稳定区大, 锑比锡更容易硫化。
3.还原剂碳能促进Sb2O4和Sn O2硫化反应的进行, 且Sb2O4比Sn O2硫化的趋势更强。
4.同一温度下, Sb2S3比Sn S的饱和蒸汽压大很多, 故锡阳极泥可以经硫化焙烧使锑以三硫化二锑的形式挥发, 达到锑开路的目的。
参考文献
[19] Huang W S.Tin[M].Beijing:Mechanical Industry Press, 2000.7. (黄位森.锡[M].北京:冶金工业出版社, 2000.7.)