Trans. Nonferrous Met. Soc. China 23(2013) 824-831
Depression effect of pseudo glycolythiourea acid in flotation separation of copper-molybdenum
Jian-hua CHEN1, Li-hong LAN2,3, Xing-jin LIAO1
1. College of Resources and Metallurgy, Guangxi University, Nanning 530004, China;
2. College of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China;
3. Key Laboratory of Chemical and Biological Transformation Process of Guangxi Higher Education Institutes, School of Chemistry and Chemical Engineering, Guangxi University for Nationalities, Nanning 530006, China
Received 20 December 2011; accepted 26 November 2012
Abstract:
Pseudo glycolythiourea acid (PGA) was synthesized and used as depressant for flotation separation of Cu and Mo. The results indicate that a low amount of PGA has strong depression effect on chalcopyrite. Mo grade of 26.17% and recovery of 89.83% were achieved with rougher and scavenger one time and cleaners twice, while the recovery of Mo was deceased by 2% when Na2S was used as depressant. Measurement on adsorption of PGA on the mineral surface indicates that PGA and xanthate were adsorbed on mineral surface by competition, and PGA was adsorbed on chalcopyrite surface much stronger than on molybdenite surface. FTIR results indicate a chemical absorption process for PGA on chalcopyrite surface and a physical adsorption process on molybdenite surface. The frontier orbital calculation shows that the S atom is an active center, and the depression of PGA can be explained with the Fermi level of energy based on the electrochemical mechanism.
Key words:
pseudo glycolythiourea acid (PGA); chalcopyrite; molybdenite; depressant; Cu-Mo separation; competitive adsorption;
1 Introduction
Sodium sulfide, sodium cyanide, iron/ferrous cyanide, sodium thiocyanate, Knox reagent (phosphorus and arsenic Knox reagent) and oxidants (hypochlorite, peroxide, permanganate and potassium dichromate, etc) are major conventional inorganic depressants for flotation separation of Cu-Mo [1,2]. In the separation process, a large amount of depressants will be required and toxic substances such as S-2, CN-1 and H2S were produced. Nowadays organic depressants have attracted much attention for flotation separation of sulfide minerals. For example, polysaccharide was used for the adsorption of mineral at mineral/aqueous solution interface [3] and sodium humate for separating Cu-S bulk concentrate in alkaline medium [4]. Tanning extract was reported to improve the flotation of jamesonite [5]. CHEN et al [6,7] investigated the relationship between the molecular structures of small organic molecule and azo depressants, and their depression effect on the flotation of sulphide minerals. Thioglycolic acid was found to be the most effective depressant for Cu-Mo separation [8,9], and satisfactory separation can be obtained in industrial process. However, the cost of thioglycolic acid restricts its further application [10]. Development of new depressants with low cost, good stability and low toxicity is most important for the flotation separation of Cu-Mo. In 1937, BROWN [11] used starch as molybdenite depressant. JORJANI et al [12] used the mixture of dextrin, sodium silicate, and sodium hexametaphosphate (20%, 40%, and 40% by mass fraction, respectively) for flotation separation of copper–molybdenum from Sarcheshmeh porphyry ores. The copper recovery was improved by about 3.2% with dextrin at 200 g/t. LING [13] studied the flotation process of certain molybdenum ore with a new depressant BK510. The grade and recovery of molybdenum in molybdenum concentrate were 51.07% and 89.09% with the depressant at 30 g/t. ANSARI and PAWLIK [14] investigated the floatability of chalcopyrite and molybdenite in the presence of lignosulfonates, and showed that it is possible to selectively float chalcopyrite from molybdenite using lignosulfonates to depress molybdenite. JIANG et al [15] used small organic molecule DPS as depressant to successfully separate Cu-Mo concentrate. The results indicated that DPS has strong depression effect on chalcopyrite. Compared with sodium thioglycolate and Na2S, the same inhibitory effect was achieved with the amount of DSP used less than that of 1/5 sodium thioglycolate or 1/10 Na2S.
Dexing copper mine is one with huge quantity of porphyry copper associated with molybdenum in China. Na2S has been used as a copper depressant for decades. According to statistics reports from 2009 to 2010, 45-50 kg/t Na2S was required for the flotation process. Since Na2S can be oxidized easily in pulp, leading to depression effect failure, excessive Na2S is required.
Pseudo glycolythiourea acid (PGA) is an odorless and white powder. It is a promising substitute for Na2S in flotation separation of Cu-Mo. Although PGA had been used as depressant to separate Cu-Mo concentrate from Jinping, Yunnan, China in early 1990s, there were few investigations on the mechanism of PGA used in the separation process. In this work, PGA was prepared by a simple way and used as depressant to separate Cu-Mo concentrate from Dexing porphyry copper mine, and the mechanism of separation was discussed.
2 Experimental
2.1 Minerals and reagents
Cu-Mo samples were obtained from Dexing copper mine, Jiangxi Province of China, and were treated by the thickeners and hydrocyclones. The content of Cu was about 17% and the content of Mo was 0.359%. The mineralogical analysis showed that the minerals consist mainly of chalcopyrite and molybdenite, with minor pyrite, chalcocite, tetrahedrite bornite, etrahedrite and other impurities; and the gangue minerals are mainly quartz and mica. The screen analysis of the tested samples showed that more than 85% of the particles were less than 74 μm, indicating that the mineral samples were fine-grained. Samples less than 44 μm were used for adsorption tests. All the samples were stored in capped plastic bottles, which were filled with nitrogen, sealed with gasket cement and stored in refrigerator at -4 °C. The purity of minerals was confirmed by the X-ray analysis. The mineral contents of chalcopyrite and molybdenite were 93.88% and 95.36%, respectively. Carbon disulfide, alcohol, acid, thiourea, sulfuric acid and sodium hydroxide in analytical grade were used as main reagents in the experiments. Sodium silicate and kerosene were in industrial grade. PGA and BX were prepared.
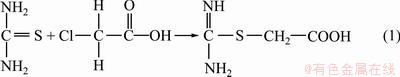
Pseudo glycolythiourea acid (PGA) was prepared as Eq. (1). Thiourea and chloroacetic acid were dissolved in deionized water separately to obtain the saturated solution. Both solutions were then mixed and stirred for 30 min at room temperature, stood still for 24 h. The crystals were collected by filtration and washed with water, then dried, to give product PGA as white crystals.
2.2 Flotation process
Samples obtained from Mo concentrator were put into the container and the mass concentration was measured after completely stirring, then the pulp was divided into several samples and they were sealed. Kerosene was used as a collector, and Na2SiO3 was used as a flocculant. In addition, the dosage of kerosene was fixed, as the same as that in the concentrator of Dexing copper mine. The experimental flowsheet is shown in Fig. 1. Single-factor experiments were employed to confirm the system of reagents, such as the consumption of depressant and flocculant as well as the range of pH value.
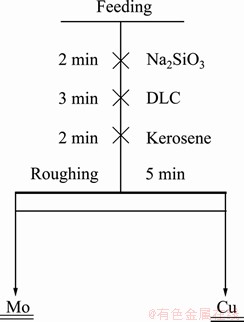
Fig. 1 Experimental flow sheet
2.3 Adsorption test
At room temperature, the appropriate concentrations of PGA and XB were scanned between 200 nm to 900 nm by UV spectrophotometer (TU-1810PC, Beijing, PERSEE General Instrument Co., Ltd., China), and their characteristic absorption peaks were obtained at 241 nm and 300 nm, respectively. Then the standard curves between agent concentration and absorbance were drawn at these peaks. 1.0 g mineral sample was transferred into a 50 mL flask, then 30 mL distilled water was added, and the mineral surface was cleaned for 5 min in the ultrasonic purifier. The upper layer liquid was decanted and suspended in a known concentration of starch solution. The flask was then placed in a mechanical shaker at 25 °C for 2 h which is long enough for a complete adsorption, and stood for a few minutes until the liquid was divided into two layers. Supernatant was used for absorbance measurements at agent characteristic absorption peaks after centrifugal separation. The amount of PGA or XB adsorption was calculated by the following formula [16]:
Q=(C0-C1)×V/W (2)
where Q is the amount of agent adsorption on the mineral surface; C1 is the agent concentration in supernatant after adsorption equilibrium (mg/L), which is calculated by the standard curve equation of agent; C0 is the initial concentration of agent (mg/L); V is the solution volume (L); W is the quality of mineral sample (g).
2.4 IR spectrum test
1.0 g mineral sample was dissolved in 30 mL distilled water in a flask, and the mineral surface was cleaned for 5 min in the ultrasonic purifier. The upper layer liquid was decanted, and 40 mL 1 g/L PGA or XB solution was added and then stirred for 1 h by magnetic stirrer at room temperature, which can make agent full adsorption on the mineral surface. The subsequent solid- liquid was separated by the centrifuge. Mineral samples were washed fully by using NaOH solution at pH=11 and distilled water successively, then dried in vacuum at room temperature. Samples were prepared in tablets with potassium bromide (the mass ratio of mineral sample and KBr is about 1:250), and their diffusion reflectance FTIR spectroscopy testing was carried out by using the Nicolet MAGNA-IR550 FT-IR spectrometer (Nicolet Instrument Corporation, America) with a wave length range of 4000 and 500 cm-1.
2.5 Calculation of frontier orbital
The frontier orbitals of chalcopyrite and molybdenite were calculated by Material Studio calculation software DMol3 module with a single-point energy method after optimization using CASTEP, while both the structure optimization and frontier orbital calculations on PGA and BX were performed using DMol3, with GGA-PW91 [17,18] method, DNP basis set, effective core potentials, a fine quality, and SCF convergence threshold of 1.0×10-6 eV/atom.
3 Results and discussion
3.1 Best process parameters of actual ore flotation
The PGA depressant ability tests were conducted in a 0.75 L laboratory flotation cell with a rotation rate of 1100 r/min. The pulp density was adjusted as 50% solid. 200 g/t of the collector kerosene and different dosages of PGA were applied. It can be seen from Fig. 2 that the grade and recovery of Mo were a function of the amount of PGA, and were enhanced with the increase of PGA dosage. In addition, the flotation recovery of Mo rose rapidly when the PGA consumption increased to 3 kg/t, but no obvious change occurred when the consumption was more than 3 kg/t. The experimental results showed that PGA has good selectivity to the Cu-Mo separation and the optimal consumption of PGA is 3 kg/t.
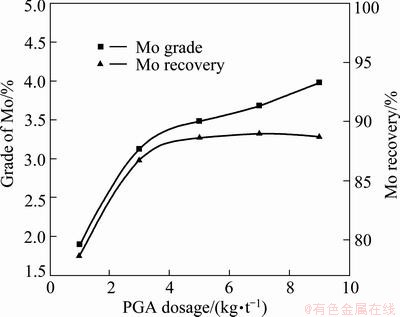
Fig. 2 Effect of PGA on Cu-Mo separation
In order to investigate the effect of pH on the depressing ability of PGA, the effect of pH value regulated by H2SO4 or NaOH was tested in the presence of 200 g/t of the kerosene and 3 kg/t of PGA. Mo flotation indexes are shown in the Fig. 3. The results show that the grade and recovery of Mo had no significant change in the range of pH 5-12, which indicates that pH has little effect on the Cu-Mo separation index and Cu-Mo separation can be carried out in a wide pH range.
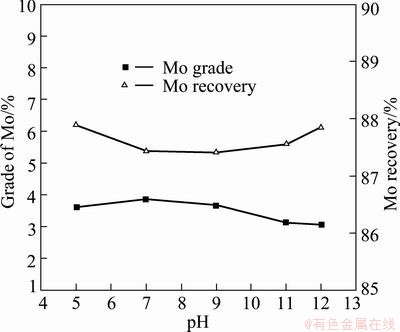
Fig. 3 Effect of pH value on Mo floatation
The influence of Na2SiO3 on the Cu-Mo separation in the presence of 200 g/t kerosene and 3 kg/t PGA was tested. Figure 4 shows that the Mo flotation recovery was not enhanced obviously when the Na2SiO3 dosage was below 500 g/t, but increased very fast when the dosage was greater than 500 g/t. When the dosage was greater than 800 g/t, the grade of Mo presented no obvious change.
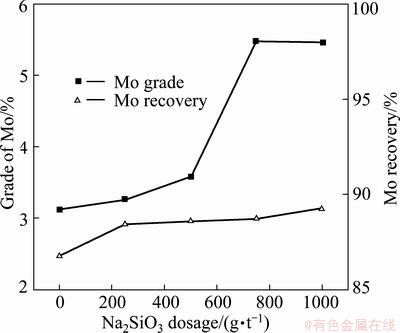
Fig. 4 Effect of Na2SiO3 dosage on Mo flotation in presence of PGA
According to the single-factor experiments, the reagent system was determined for close circuit tests. The experimental flow sheets and reagent dosage are shown in Figs. 5 and 6. It can be seen from Table 1 that the Mo recovery of 89.83% and the concentrate containing 26.17% Mo can be obtained by one rougher, one scavenger and two cleaners, while the Mo recovery decreased by 2% when Na2S was used as a depressant. Therefore, compared with Na2S, PGA has a stronger depression effect on chalcopyrite with a low consumption.
Table 1 Close circuit test results
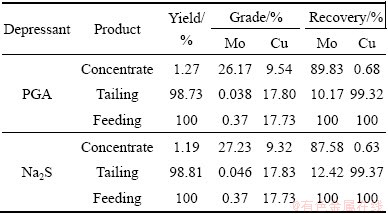
3.2 Adsorption of PGA on two minerals
Figure 7 shows the adsorption of PGA on the chalcopyrite and molybdenite surfaces with different PGA concentrations at pH=11. Obviously, the adsorption amount of PGA on the two different mineral surfaces increased steadily with the increase of the PGA concentration, but the adsorption capacity on the chalcopyrite surface was much higher than that on the molybdenite surface (twice that on molybdenite when the PGA concentration is 10-30 mg/L). Hence, the hydrophilicity of chalcopyrite would be increased in the presence of PGA.
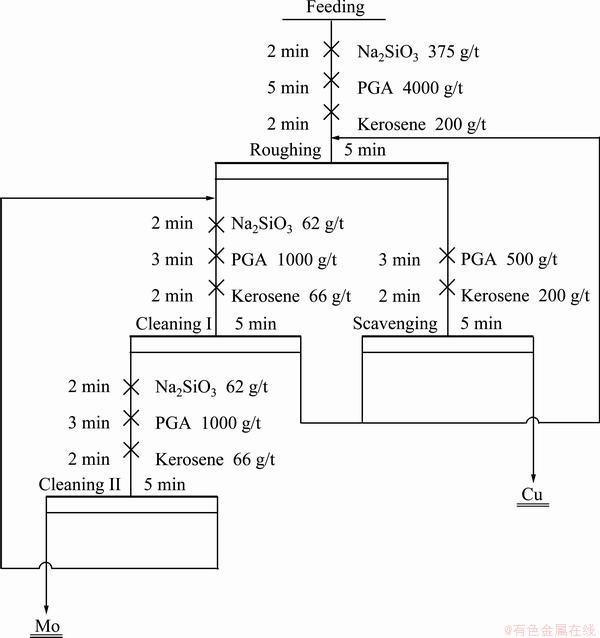
Fig. 5 Close circuit experimental flowsheet in presence of PGA
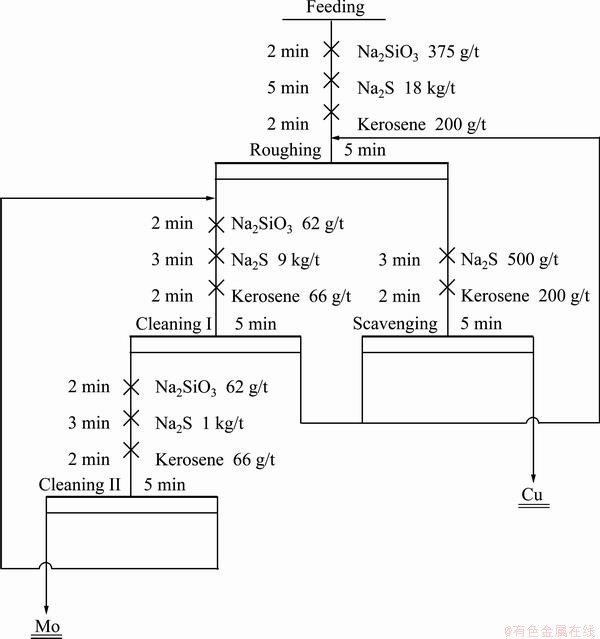
Fig. 6 Close circuit experimental flowsheet in presence of Na2S
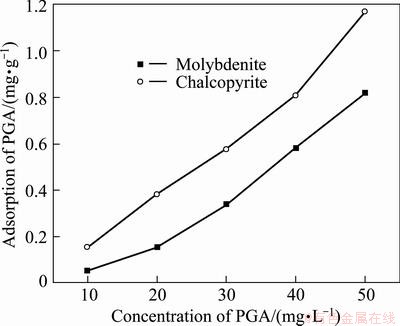
Fig. 7 Adsorption of PGA on chalcopyrite and molybdenite as function of PGA concentration
Butyl xanthate (BX) is the most common collector for sulphide ore flotation. Coadsorption or competitive adsorption would take place between collector and depressant on the mineral surface when the reagents coexist in the pulp. The adsorption capacities of PGA on the chalcopyrite and molybdenite surface were measured in the presence of BX at the concentration of 5×10-5 mol/L, and the results are shown in Figs. 8 and 9. Before the measurement, the mineral surface was cleaned according to the method in section 2.3. The cleaned sample was then added into the PGA solution with stirring, followed by the slow addition of BX.
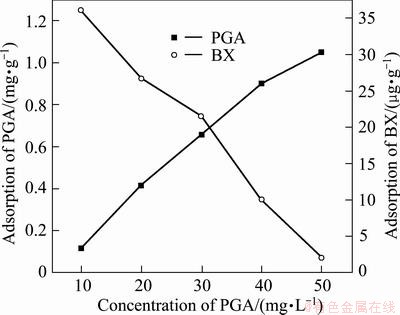
Fig. 8 Adsorption of PGA on chalcopyrite in presence of BX of 1×10-5mol/L
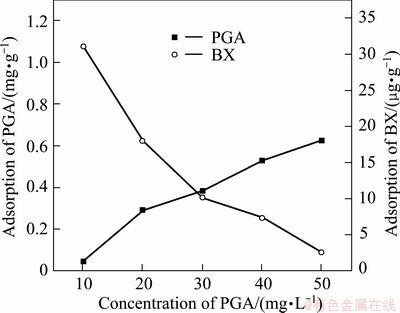
Fig. 9 Adsorption of PGA on molybdenite in presence of BX of 1×10-5mol/L
As shown in Figs. 8 and 9, with the increase of concentration of PGA, the adsorption amount of PGA on the two mineral surfaces increased steadily whereas the adsorption amount of BX decreased, and the adsorption amount of PGA on chalcopyrite surface was more than that on molybdenite. Therefore, it can be concluded that competitive adsorption would take place between PGA and BX on the chalcopyrite and molybdenite surfaces.
3.3 Infrared spectrum analysis
IR spectra of PGA adsorption before and after adsorption on the minerals were studied, as shown in Figs. 10-12. Figure 10 shows the transmission IR spectroscopy of the synthetic PGA, and the adsorption arrangements are listed in Table 2. The characteristic adsorption peaks of the main groups (—NH2, —COOH, —CH2—, —NH—, —CS— and —C=N—) in PGA molecular structure were observed.
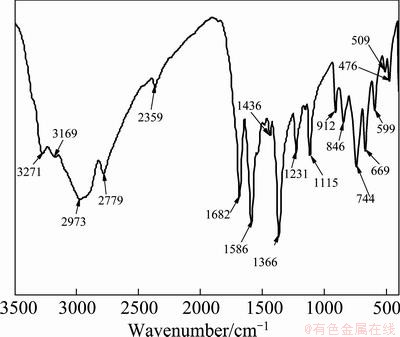
Fig. 10 Transmission IR spectrum of PGA
Table 2 Group ownership of peaks corresponding to PGA infrared spectra
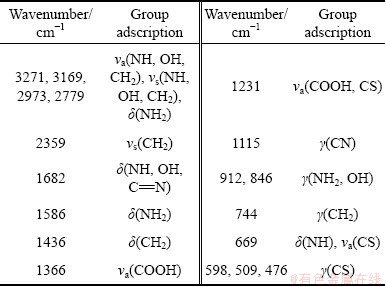
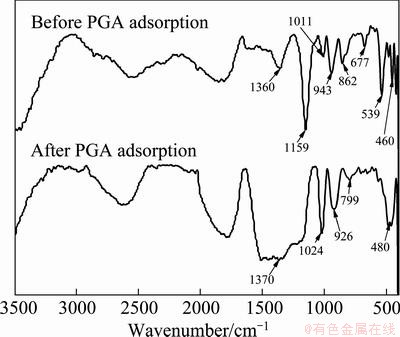
Fig. 11 Diffusion reflectance FTIR spectra of chalcopyrite before and after PGA adsorption
Figure 11 shows the diffusion reflectance FTIR spectra of chalcopyrite before and after PGA adsorption. There are three major differences after the adsorption of PGA: 1) the appearance of new peaks: the strong peaks located at 1370 cm-1 and 480 cm-1 are generated by —COOH and —CS— of PGA, respectively; 2) the disappearance of the original peaks: the peaks located at 1360, 1159, 1011 , 539 and 460 cm-1 disappear after the addition of PGA; 3) the great shift of the 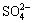 peaks: the peaks at 943, 862 and 677 cm-1 are related to
peaks: the peaks at 943, 862 and 677 cm-1 are related to 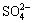 . After the adsorption of PGA, they shift to 1024, 926 and 799 cm-1, respectively. This is caused by the electronic inductive effect [19]. After PGA adsorption, a covalent bond is formed between the S atom in PGA and the Cu atom on chalcopyrite surface. It causes copper ion with less charge, weaker force between copper ion and
. After the adsorption of PGA, they shift to 1024, 926 and 799 cm-1, respectively. This is caused by the electronic inductive effect [19]. After PGA adsorption, a covalent bond is formed between the S atom in PGA and the Cu atom on chalcopyrite surface. It causes copper ion with less charge, weaker force between copper ion and 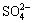 . Therefore, the adsorption of
. Therefore, the adsorption of 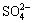 moves to higher wavelength (blue shift).
moves to higher wavelength (blue shift).
Based on the above discussion, we can conclude that PGA would be chemically adsorbed on the chalcopyrite surface.
As shown in Fig. 12, the diffusion reflectance FTIR spectra of molybdenite before and after the PGA adsorption are similar. Only small shifts of absorption peaks (<3 cm-1) are observed after PGA adsorption. The characteristic absorption peak of PAG cannot be found on molybdenite surface. This suggests that the adsorption of PGA on the molybdenite surface can be ascribed to simple physical adsorption [20].
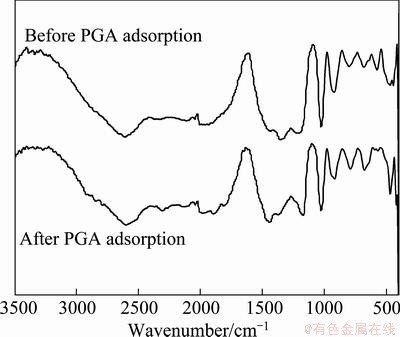
Fig. 12 Diffusion reflectance FTIR spectra of molybdenite before and after PGA adsorption
3.4 Calculation results of frontier orbital and atomic orbital coefficients
According to the front molecular orbital theory, the electrons belonging to the HOMO (highest occupied molecular orbital) of the molecule have the greatest activity. A larger electron density indicates a greater reactivity of the HOMO. The atom with the largest electron density is most likely to participate in the reaction between molecules [21]. In addition, the contribution of atoms to the frontier orbital can be well assessed by the atomic orbital coefficients. A greater value of coefficient (absolute value) indicates a greater contribution of the atom to the frontier orbital. The molecular structure and the calculated HOMO of PGA are shown in Fig. 13. The shadow surrounding the atoms in Fig. 13(b) indicates the magnitude of the electron density. It is shown that the electron density of the HOMO of PGA molecule is mainly located on S atom and C=N bond. It can be known from calculation result that S atom has the greatest orbital coefficient (0.901035), while the coefficients of the other atoms are very small. This suggests that S atom is the reactive site between PGA and mineral. The composition of PGA HOMO can be expressed as follows:
+0.901035S(2p) - 0.000792C4(2p)-0.000604H10(1s) +0.000555C2(2p) + 0.000491N1(2s) + 0.000485H9(1s) - 0.000364N12(2s) + 0.000318C5(2p) + 0.000071O6(2p) + 0.000050 O7(2p)
It has been confirmed that the interaction of BX with chalcopyrite and molybdenite involves the electrochemical process, and dixanthogen will produce on the mineral surface. PGA has strong reducibility similar to Na2S, and the depression of PGA should be explained based on the electrochemical mechanism. Fermi level represents the system average electro- chemical orbital of the electron, and the direction of electron transition is always from high energy level to low energy level. From Table 3, EF values of PGA, chalcopyrite and molybdenite are -3.936, -5.433 and -4.138 eV, respectively. This indicates that chalcopyrite surface can obtain electrons from PGA more easily than from molybdenite, suggesting that dixanthogen could be easily reduced to xanthate molecule and desorbed from chalcopyrite surface. While the EF values of PGA and molybdenite are very close, suggesting that dixanthogen can not be easily desorbed from molybdenite surface due to the electrochemical effect.
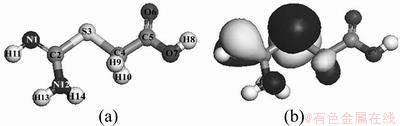
Fig. 13 Molecular structure (a) and HOMO (b) of PGA
Table 3 Fermi level energy of reagents and minerals

4 Conclusions
1) The results of flotation indicated that PGA has strong depression effect with a small consumption. Mo grade of 26.17% and recovery of 89.83% were achieved after rougher and scavenger one time and cleaners twice, while the recovery of Mo was deceased by 2% when Na2S was used as depressant.
2) The adsorption measurements of PGA on the mineral surface indicated that PGA and xanthate were adsorbed on mineral surface by competition, and PGA adsorption on chalcopyrite surface was much larger than that on molybdenite surface.
3) FTIR results indicated a chemical absorption process for PGA on chalcopyrite surface and a physical adsorption process on molybdenite surface.
4) The frontier orbital calculation showed that S atom is an active center. PGA has a strong reducibility similar to Na2S, and the depression of PGA can be explained with the Fermi level of energy based on the electrochemical mechanism.
References
[1] QIU Li-na, DAI Hui-xin. Flotation process and recent reagents status of molybdenum ore [J]. Modern Mining, 2009(7): 22-23. (in Chinese)
[2] LIU Guang-yi, LU Yi-ping, ZHONG Hong, CAO Zhan-fang, XU Zheng-he. A novel approach for preferential flotation recovery of molybdenite from a porphyry copper–molybdenum ore [J]. Minerals Engineering, 2012, 36-38: 37-44.
[3] LASKOWSKI J S, LIU Q, O'CONNOR C T. Current understanding of the mechanism of polysaccharide adsorption at the mineral/ aqueous solution interface [J]. International Journal of Mineral Processing, 2007, 84(1-4): 59-68.
[4] CHEN Jian-hua, LI Yu-qiong, CHEN Ye. Cu-S flotation separation via combination of sodium humate and lime in a low pH medium [J]. Minerals Engineering, 2011, 24(1): 58-63.
[5] CHEN Jian-hua, LI Yu-qiong, LONG Qiu-rong. Improving the selective flotation of jamesonite using tanning extract [J]. International Journal of Mineral Processing, 2011, 100(1-2): 54-56.
[6] CHEN Jian-hua, LI Yu-qiong, LONG Qiu-rong. Molecular structures and activity of organic depressant for marmatite,jamesonite and pyrite flotation [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 1993-1999.
[7] CHEN Jian-hua, LIANG Mei-lian, LAN Li-hong. Depression effect of azo organic depressants on sulphide minerals [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(11): 2239-2247. (in Chinese)
[8] LIU Qi-ying. Synthetical technological research of Cu-Mo separation inhibitor sodium thioglycollate [D]. Xi,an: Northwest University, 2010: 3-5. (in Chinese)
[9] FU Jian-gang, ZHONG Hong, OU Yue-ming. Application of thioglycolic acid in molybdenite-copper sulphide separation [J]. Mining and Metallurgical Engineering, 2002, 22(4): 36-38. (in Chinese)
[10] NIE Qi. Discussions on research status and separation methods for molybdenum ore beneficiation [J]. Yunnan Metallurgy, 2010, 39(2): 35-36. (in Chinese)
[11] BROWN E H. Separation of molybdenite from copper sulfides: US, 2070076 [P]. 1937.
[12] JORJANI E, BARKHORDARI H R, TAYEBI KHORAMI M, FAZELI A. Effects of aluminosilicate minerals on copper– molybdenum flotation from Sarcheshmeh porphyry ores [J]. Minerals Engineering, 2011, 24(8): 754-759.
[13] LING Shi-sheng. Application of new depressant BK510 in certain molybdenum ore [J]. Nonferrous Metals: Mineral Processing Section, 2010(1): 46-48. (in Chinese)
[14] ANSARI A, PAWLIK M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation [J]. Minerals Engineering, 2007, 20(6): 609-616.
[15] JIANG Yu-ren, ZHOU Li-hui, XUE Yu-lan, ZHU Jian-guang. Separation of molybdenite from chalcopyrite using new depressant DPS [J]. Mining and Metallurgical Engineering, 2001, 21(1): 33-36. (in Chinese)
[16] LI Zhen-xing, LI Wan-xin, ZHANG Gen-cheng, FEI Zheng-hao. Adsorption on behavior and mechanism of direct black on organobentonites [J]. Ion Exchange and Adsorption, 2010, 26(1): 33-39. (in Chinese)
[17] PERDEW J P, BURKE K, ERNEZERHOF M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865-3868.
[18] PERDEW J P, CHEVARY J A, VOSKO S H, JACKSON K A, PEDERSON M R, SINGH D J, FIOLHAIS C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation [J]. Physical Review B, 1992, 46(11): 6671-6687.
[19] NING Yong-cheng. Organic spectroscopy spectra resolve [M]. Beijing: Science Press, 2010: 107-108. (in Chinese)
[20] LI Jian-hua, SUN Xiao-jun. Mechanism of collector-DLZ in chalcopyrite and pyrite flotation [J]. Nonferrous Metals: Mineral Processing Section, 2010(2): 45-48. (in Chinese)
[21] SUN Wei, YANG Fan, HU Yue-hua, HE Guo-yong, LIU Wen-li. Application of frontier orbital in developing new collectors of chalcopyrite [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(8): 1524-1532. (in Chinese).
假乙内酰硫脲酸在铜-钼浮选分离中的抑制性能
陈建华1,蓝丽红2,3,廖幸锦 1
1. 广西大学 资源与冶金学院,南宁 530004;
2. 广西大学 化学化工学院,南宁 530004;
3. 广西民族大学 化学化工学院,广西高校化学与生物转化过程新技术重点实验室,南宁 530006
摘 要:将合成的假乙内酰硫脲酸(PGA)用作铜-钼分离抑制剂。该药剂闭路实验结果表明:假乙内酰硫脲酸在较小的用量下对黄铜矿有较强的抑制作用,经一次粗选、一次扫选、两次精选,可获得Mo品位大于26%、回收率大于89%的浮选指标,而用Na2S做抑制剂时钼的回收率下降了2%。药剂吸附量测试结果表明,PGA与丁基黄药在矿物表面发生竞争吸附,PGA在黄铜矿表面上的吸附量远大于在辉钼矿表面的。红外光谱分析表明,PGA在黄铜矿表面是化学吸附,而在辉钼矿表面属于物理吸附。前线轨道计算结果表明,在PAG分子中,硫原子是反应活性的中心。利用矿物、丁黄药及PGA的费米能级能量大小可以从电化学作用角来度解释PGA的抑制机理。
关键词:假乙内酰硫脲酸;黄铜矿;辉钼矿;抑制剂;铜钼分离;竞争吸附
(Edited by Hua YANG)
Foundation item: Project (51164001) supported by the National Natural Science Foundation of China; Project (0991082) supported by the Science and Technology Department of Guangxi, China; Project (GJR201147-12) supported by Guangxi Higher Education Institutes Talent Highland Innovation Team Scheme, China
Corresponding author: Li-hong LAN; Tel: +86-771-3262683; E-mail: lanlihong2004@163.com
DOI: 10.1016/S1003-6326(13)62535-2
Abstract: Pseudo glycolythiourea acid (PGA) was synthesized and used as depressant for flotation separation of Cu and Mo. The results indicate that a low amount of PGA has strong depression effect on chalcopyrite. Mo grade of 26.17% and recovery of 89.83% were achieved with rougher and scavenger one time and cleaners twice, while the recovery of Mo was deceased by 2% when Na2S was used as depressant. Measurement on adsorption of PGA on the mineral surface indicates that PGA and xanthate were adsorbed on mineral surface by competition, and PGA was adsorbed on chalcopyrite surface much stronger than on molybdenite surface. FTIR results indicate a chemical absorption process for PGA on chalcopyrite surface and a physical adsorption process on molybdenite surface. The frontier orbital calculation shows that the S atom is an active center, and the depression of PGA can be explained with the Fermi level of energy based on the electrochemical mechanism.


