
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 97-103
Composition and corrosion resistance of palladium film on
316L stainless steel by brush plating
TANG Jun-lei, ZUO Yu, TANG Yu-ming, XIONG Jin-ping
School of Materials Science and Engineering,Beijing University of Chemical Technology, Beijing 100029, China
Received 20 December 2010; accepted 28 April 2011
Abstract:
Palladium films with good adhesive strength were deposited on 316L stainless steel by brush plating. Scanning electronic microscopy, energy dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy (XPS), mass loss tests and electrochemical methods were used to study the properties of the films. The brush plated palladium film mainly consisted of palladium. XPS analysis indicated that palladium was present in the films as metal state. The palladium plated stainless steel samples showed excellent corrosion resistance in boiling 20% H2SO4 solution and boiling acetic/formic acids with 0.005 mol/L Br- ions added. The corrosion rates of the palladium plated 316L stainless steel samples were about two orders of magnitude lower than those of the original 316L stainless steel samples. This method provides a possibility to prepare protective palladium films on stainless steel facilities with large areas in industrial sites.
Key words:
palladium film; brush plating; corrosion; stainless steel;
1 Introduction
Corrosion resistance of stainless steels is mainly due to the formation of passive films on the surface. Stainless steels show good corrosion resistance in oxidizing corrosion mediums where the passive films formed on the surface are stable. However, in many reducing corrosion mediums such as boiling dilute sulfuric acids or boiling acetic plus formic acids, passivity cannot be steadily established on the surface and active corrosion happens for stainless steels. On the other hand, corrosion of stainless steel may become much heavier when halogen ions are contained in the mediums, due to the detrimental effect of halogen ions on the passive films [1].
Both titanium alloys and stainless steels are passive alloys. For passive alloys, if their corrosion potential is raised from active potential into passive region by applied anodic current or by alloying with elements with higher oxidation/reduction potentials, corrosion resistance would be improved. It was reported [2] that palladium deposition on titanium surface by ion beam mixing may effectively improve corrosion resistance of titanium in reductive acidic solutions. There were also some reports on deposition of palladium on stainless steels [3]. However, most of the studies were aimed to prepare catalytic membrane reactors [4, 5], since palladium film is permeable for hydrogen and catalytically active to many hydrogen-involved reactions. The method to deposit a Pd composite membrane on porous stainless steel tube by electroless plating was studied in Refs. [6-7]. The membrane possessed high hydrogen selectivity and thermal stability at high temperature. Also Pd membrane on porous stainless steel tube was prepared by electroless plating [8-10]. SHI et al [11] studied the initial formation of palladium membrane on a porous stainless steel substrate by electroless method and the morphology of pores in the membrane was examined. GUAZZENE et al [12] reported the effects of surface activity, defects and mass transfer on hydrogen permeation in palladium-porous stainless steel membrane. In our previous studies [13-15], palladium films were deposited on stainless steels by electroless plating, electroplating and brush plating. However, both electroless plating and electroplating are not suitable for large facilities. Brush plating process may be applied in industrial sites to protecting stainless equipments with large areas. The effects of plating parameters on the properties of brush plated palladium films were reported in our previous work [15]. The effects of brush plating parameters on electrochemical behaviors of the films are mainly attributed to the effect of co-deposited hydrogen in the films. However, the composition of brush plated film and its corrosion resistance in typical reducing mediums have not been studied. In this work, corrosion behaviors of the Pd films/stainless steel in typical reducing mediums, including boiling 20% H2SO4 solution and boiling acetic/formic acids with different amounts of Br- ion, were studied. Scanning electronic microscopy, energy dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy were used to study the features of the films.
2 Experimental
2.1 Testing material
The testing material was rolled 316L stainless steel which was annealed and quenched, with a thickness of 2 mm. The steel composition was as follows: Cr 16.80%, Ni 13.50%, C 0.02%, Mn 1.40%, Si 0.32%, P 0.017%, S 0.014%, Mo 2.30%, Fe balance.
2.2 Brush plating conditions for palladium film
The stainless steel plates with dimensions of 50 mm×25 mm were finished on both sides with abrasive papers up to 1000#. The brush plating conditions are listed as follows.
2.2.1 Brush plating procedure
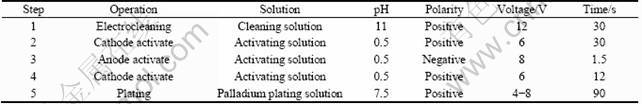
The brush plating process of palladium film on 316L stainless steel is listed in Table 1. The sample was rinsed with deionized water after every step.
2.2.2 Solutions
Cleaning solution consisted of Na2CO3 40-60 g/L, NaOH 25-40 g/L, Na3PO4·12H2O 30-50 g/L, NaCl 15-25 g/L and deionized water.
Activating solution consisted of H2SO4 150-200 g/L, (NH4)2SO4 60-90 g/L, NaCl 20-30 g/L and deionized water.
Palladium plating solution consisted of Pd(NH3)4Cl2 12-20 g/L, NH4Cl 50-75 g/L, (NH4)2HPO4 60-80 g/L, NH3·H2O 40-60 mL and deionized water.
The schematic diagram of the brush-plating apparatus is shown in Fig. 1. During brush plating the electrolyte was supplied automatically to the anode by a pump.

Fig. 1 Illustration of brush-plating apparatus: 1—Anode connection; 2—Electrode handle; 3—Carbon anode; 4—Absorbent membrane; 5—Stainless steel specimen; 6—Solution recycle; 7—Solution feed tube; 8—Cathode connection; 9—Solution reservoir; 10—Specimen holder
2.3 Testing methods
Energy dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS) were used to analyze the composition of the films, and the film surface was observed with scanning electronic microscope (SEM). The film thickness was measured according to the mass gain of the sample before and after brush plating. The adhesive strength of the film to the stainless steel was measured according to ASTM D 3359 -90 (ASTM Standard Test Method for Film Adhesion by Tape Test) Test Method B – Cross-Cut Tape Test.
Corrosion behavior of the palladium plated samples in 20% sulfuric acid boiling acetic/formic acids with different amounts of added Br- ion solutions was studied by polarization tests, electrochemical impedance tests and mass loss tests at boiling temperature. The samples were sealed with phenolic resin, leaving an area of 0.5 cm2 exposed to the solution. Polarization measurements were performed with an EG & G model 273A potentiostat. The sample was first polarized from the open circuit potential to a cathodic potential which was 100 mV negative to the open circuit potential, then was polarized in the positive direction with a potential scanning rate of 0.66 mV/s. A saturated calomel electrode (SCE) was used as the reference electrode, and the counter electrode was platinum. Electrochemical impedance spectroscopy (EIS) was measured with a Model 5210 lock in amplifier connected to the cell via a Model 273A potentiostat, at the open circuit potential with a perturbation of 10 mV and the frequency range was from 100 kHz to 10 mHz. All the reagents used were of analytic grade, and the solutions were open to the air.
Table 1 Brush plating process of palladium film
3 Results and discussion
3.1 Features of palladium film
The palladium film obtained on stainless steel showed smooth surface and black color. For the brush plating time of 1.5 min, the obtained palladium film was about 1.5 μm in thickness. Figure 2 shows surface morphology of the film under SEM. The film is continuous and dense but not quite smooth in micro-view. There were some micro-cracks on the surface, as shown in Fig. 2(b). In an enlarged photo (Fig. 2(c)) the deposited particles can be clearly seen to have the average size of 200-300 nm.
Figure 3 shows surface morphology of the samples before and after 3 min of brush plating. After brush plating, the sample surface looks more smooth, indicating the good coverage of the brush plated film.

Fig. 2 SEM images of brush plated Pd film: (a) Palladium film; (b) Micro-cracks on film surface; (c) Crystal of palladium film

Fig. 3 Optical microscope photograph before (a) and after (b) brush plating
The adhesive strength of the plated film was examined by cross-cut tape test according to ASTM-D3359-90 (Method B: Standard Test Method for Film Adhesion by Cross-Cut Tape Test). The adhesion of the film to stainless steel reached 4B level, which means the adhesion was quite good. According to the mass gains before and after brush plating, the film thickness was estimated to be 0.6-2 μm under the experimental conditions.
Figure 4 shows composition of the brush plated palladium film measured with EDX, and the quantitative results are given in Table 2. Mainly Pd was detected in the film, and the traces of Fe and Cr should come from the stainless steel matrix since the film was very thin. XPS analysis was carried out to further understand the states of the elements in the films. Figure 5(a) shows the wide scan XPS spectrum on surface of the palladium plated films. The binding energy values and the atomic percentages for the elements are shown in Table 3. It is seen that the brush plated film surface was mainly composed of palladium, oxygen, and carbon which was added for calibration of the spectrum. It was reported [16] that for metallic palladium, binding energy values of the spin-orbit couple peaks of Pd 3d are 335.30 eV and 340.65 eV respectively, which are very close to the measured values in Table 3. When Pd is oxidized, binding energy of Pd 3d5/2 may shift from 335.4 eV to 336.8 eV [17]. Hence the results in Table 3 suggest that palladium in the surface film was present in metallic state. In addition, binding energy of Pd 3p3/2 (532.4 eV) [18] is close to that of O 1s which was reported to be near 531.8 eV when O2 adsorbed on Pd-Ag film [19]. Thus the peak around 532 eV in Fig. 5(a) should be the overlapped result of Pd 3p3/2 peak and O 1s peak. Figure 5(b) shows the deconvolution of the peaks for the palladium film, where oxygen should come from the adsorbed oxygen on the surface because the binding energy values for O 1s in PdxOy were reported as 529.65 eV to 530.38 eV [20], obviously different from the measured values.
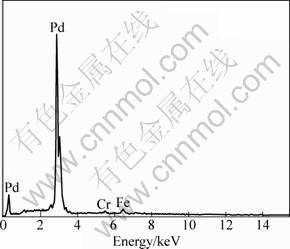
Fig. 4 EDX spectrum of palladium film
Table 2 Element contents in brush plated film by EDX

Table 3 Measured binding energy values and element contents in film by XPS

3.2 Corrosion resistance of 316L samples with brush plated palladium films
3.2.1 Mass loss tests
Table 4 shows mass loss testing results of the stainless steel samples with brush plated palladium film in boiling 20% H2SO4 solution and boiling 10% formic acid + 90% glacial acetic acid (mass fraction) mixture with 0.005 mol/L Br- ions. The results for original 316L stainless steel samples are also shown as comparison. In boiling 20% H2SO4 solution, 316L stainless steel samples experienced severe corrosion after only 2 h of immersion, while little mass losses were measured for palladium brush plated samples after 144 h of immersion. In boiling acetic/formic acids with 0.005 mol/L Br- ion, severe corrosion happened for 316L stainless steel samples after 48 h of immersion, while again little mass losses were measured for palladium brush plated samples after 144 h of immersion. For the palladium plated samples, the corrosion rates in both mediums were about 2 orders of magnitude lower than original 316L samples. Considering that the thickness of the brush plated palladium film is only about 1 μm, the protective ability of the film is quite good.
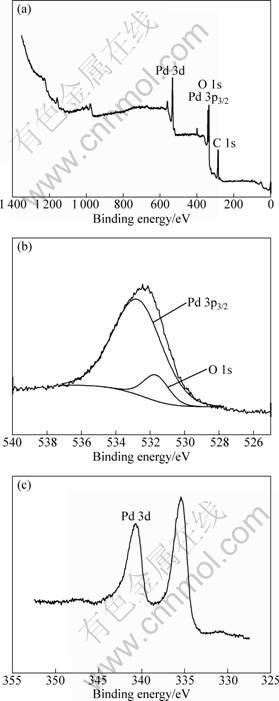
Fig. 5 XPS spectra for surface of palladium film: (a) Wide scan spectrum; (b) Deconvolution of Pd 3p3/2 peak and O 1s peak; (c) Pd 3d peak
Table 4 Mass loss testing results for brush plated samples

3.2.2 Polarization measurements
Figure 6 shows polarization curves of 316L stainless steel samples with and without Pd films in boiling 20% H2SO4 solutions (102 °C). For 316L stainless steel without palladium film, measured corrosion potential was about -0.22 V, at which the corresponding corrosion current density was quite high (10-2 A/cm2) and active dissolution happened. However, for Pd brush plated sample, corrosion potential was greatly raised to passive region, and stable passivation was obtained. Corrosion potential of the Pd brush plated sample was about 0.2 V. The corrosion current density for Pd plated sample (10-5 A/cm2) was almost three orders of magnitude lower than that of original stainless steel. For the sample with Pd film, a current step was observed at higher potential range, which may be explained by oxidation of palladium (Pd/Pd2+ equilibrium potential 0.743 V (vs SCE)).
Figure 7 shows polarization curves of 316 stainless steel (SS) samples with and without Pd films in boiling 90% glacial acetic acid + 10% formic acid mixture with a small amount of Br- added. With 0.005 mol/L Br- added, the Pd plated samples still showed more positive corrosion potential and lower anodic current density.
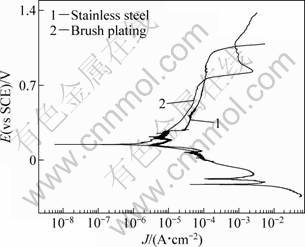
Fig. 6 Polarization curves of 316L stainless steel samples with and without Pd films in boiling 20% H2SO4 solutions (102 °C)
This result is consistent to the results by mass loss tests. Figure 8 shows the effect of Br- concentration on polarization behavior of 316L stainless steel samples with brush plated Pd film in boiling 90% glacial acetic acid + 10% formic acid mixture. With the increase of Br- addition in the medium, the anodic current density increased, and corrosion potential tended to decrease, implying that the corrosion resistance of the Pd plated samples decreased. The above results show that the palladium plated stainless steel is corrosion resistant when a small amount of halogen ions, such as 0.005 mol/L, present in the medium. But with the increase of halogen ion concentration, the corrosion resistance will be damaged.
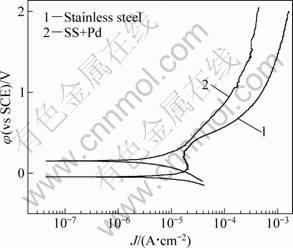
Fig. 7 Polarization curves of 316L stainless steel samples with and without Pd brush plated films in boiling glacial acetic containing 10% formic acids and 0.005 mol/L Br-

Fig. 8 Polarization curves of 316L stainless steel samples with Pd film in boiling glacial acetic containing 10% formic acids and with Br-
Figure 9 shows polarization curves of 316L stainless steel samples with and without Pd film in boiling 90% glacial acetic acid + 10% formic acid mixture + 0.005 mol/L Br- under the condition of stirring (625 r/min). Under such a condition, the anodic current densities of all the samples tended to increase by an order of magnitude. However, for palladium plated samples, the corrosion potentials were still higher and the anodic current densities were lower than original stainless steel samples. The results are consistent with those from immersion tests, indicating that although stirring increased corrosion rates of both 316L stainless steel and the palladium plated samples, the corrosion resistance was still obviously higher for palladium brush plated samples in contrast to the unprotected samples.

Fig. 9 Polarization curves of 316L stainless steel samples with and without Pd film in boiling glacial acetic containing 10% formic acids and 0.005 mol/L Br- + stirring (625 r/min)
3.2.3 Electrochemical impedance measurements
Figure 10 shows the measured electrochemical impedance spectra of 316L stainless steel samples with and without Pd films in boiling 90% glacial acetic acid + 10% formic acid mixture with different amounts of Br- at open circuit potentials. The palladium plated samples showed greater impedance than original 316L stainless steel samples, indicating that more stable passivation was established on the surface of the palladium plated samples. With the increase of Br- concentration, for the Pd plated sample the impedance tended to decrease. Palladium may react with halogen ions such as Cl- at relatively higher potentials [17]. However, the above experimental results showed that samples with Pd films were more resistant than original stainless steel in solutions with a small amount of halogen ions, e.g., less than 0.01 mol/L. The increased corrosion resistance to Br- ions should be attributed to the increase of passivity.
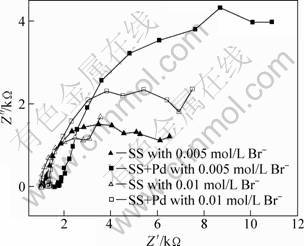
Fig. 10 Electrochemical impedance spectra of 316L stainless steel samples with and without Pd films in boiling glacial acetic containing 10% formic acids and Br-
In previous studies [16, 17] palladium films were deposited on stainless steels by electroless plating or electroplating and excellent corrosion resistance was obtained in reducing mediums. The mechanism of palladium films to increase corrosion resistance is that Pd on stainless steel surface raises the surface potential of stainless steel and promotes passivation of the steel. The results obtained in this study show that palladium film on stainless steels can also be prepared by brush plating. Similar to electroless or electroplated palladium films, the Pd film by brush plating on stainless steel also showed very good corrosion resistance in reducing mediums. The obvious advantage of brush plating technology is that the electrolyte tanks are not necessary during brush plating. Hence this method provides a possibility to prepare protective palladium films on stainless steel facilities with large areas in industrial sites.
4 Conclusions
1) Palladium films with good adhesive strength were deposited on 316L stainless steel by brush plating. The films were uniform with the thickness of 0.6-2 μm.
2) The brush plated palladium film was almost pure palladium. XPS analysis indicated that palladium was present in the films as metallic state.
3) The palladium plated stainless steel samples by brush plating showed excellent corrosion resistance in strong reducing corrosion mediums. In boiling 20% sulfuric acid solutions or boiling 90% glacial acetic acid + 10% formic acid mixture with 0.005 mol/L Br-, corrosion rates of the palladium plated 316L stainless steel samples were about 2 orders of magnitude lower than those of the original 316L stainless steel samples.
4) The brush plating method provides a possibility to prepare protective palladium films on stainless steel facilities with large areas in industrial sites.
References
[1] TURNBULL A, RYAN M, WILLETTS A, ZHOU Sheng-qi. Corrosion and electrochemical behaviour of 316L stainless steel in acetic acid solutions[J]. Corrosion Science, 2003, 45(5): 1051-1072.
[2] BARSON S D, SKELDON P, THOMPSON G E, KOLITSCH A, RICHTER E, WIESER X, PIEKOSZEWSKI J, CHMIELEWSKI A G, WERNER Z. Investigation of ion assisted palladium treatments for improved corrosion resistance of titanium foil in the electron beam dry scrubber process [J].Surface and Coating Technology, 2000, 127(2-3): 179-192.
[3] MENG Chun-ying. Study on preparing Pd/porous stainless-steel composite membranes by electroless plating [D]. Tianjin: Hebei University of Technology, 2006: 23. (in Chinese)
[4] UEMIYA S, SATO N, ANDO H, KUDA Y, MATSUDA T, KIKUCHI E. Separation of hydrogen through palladium thin film supported on a porous glass tube [J]. Journal of Membrane Science, 1991, 56(3): 303-313.
[5] SHU J, GRANDJEAN B P A, GHALI E, KALIAGUINE S. Simultaneous deposition of Pd and Ag on porous stainless steel by electroless plating[J]. Journal of Membrane Science, 1993, 77(2-3): 181-195.
[6] LI A, LIANG W, HUGHES R. The effect of carbon monoxide and steam on the hydrogen permeability of a Pd/stainless steel membrane[J]. Journal of Membrane Science, 2000, 165(1): 135-141.
[7] KANG Xin-ting, TAN Ping, GE Yuan, TANG Hui-ping, WANG Qiang-bing, ZHU Ji-lei, WANG Jian-yong. Progress of Pd composite membrane prepared by electroless plating [J]. Rare Metal Materials and Engineering, 2008, 137(S4): 580-585.
[8] WANG D, TONG J H, XU H, MATSUMURA Y. Preparation of palladium membrane over porous stainless steel tube modified with zirconium oxide [J]. Catalysis Today, 2004, 93-95: 689-693.
[9] TONG J H, SHIRAI R, KASHIMA Y, MATSUMURA Y. Preparation of a pinhole-free Pd-Ag membrane on a porous metal support for pure hydrogen separation [J]. Journal of Membrane Science, 2005, 260(1-2): 84-89.
[10] TONG J H, SUDA H, HARAYA K, MATSUMURA Y. A novel method for the preparation of thin dense Pd membrane on macroporous stainless steel tube filter [J]. Journal of Membrane Science, 2005, 260(1-2): 10-18.
[11] SHI Z L, WU S Q, SZPUNAR J A, ROSHD M. An observation of palladium membrane formation on a porous stainless steel substrate by electroless deposition [J]. Journal of Membrane Science, 2006, 280(1-2): 705-711.
[12] GUAZZENE F, ENGWALL E E, MA Y H. Effects of surface activity, defects and mass transfer on hydrogen permeance and n-value in composite palladium-porous stainless steel membranes [J].Catalysis Today, 2006, 118(1-2): 24-31.
[13] TANG J L, ZUO Y. Study on corrosion resistance of palladium films on 316L stainless steel by electroplating and electroless plating [J]. Corrosion Science, 2008, 50(10): 2873-2878.
[14] ZUO Y, TANG J L, FAN C Z, TANG Y M, XIONG J P. An electroless plating film of palladium on 304 stainless steel and its excellent corrosion resistance [J].Thin Solid Films, 2008, 516 (21): 7560-7570.
[15] TANG J L, ZUO Y, TANG T M, XIONG J P. The preparation of corrosion resistant palladium films on 316L stainless steel by brush plating [J]. Surface and Coating Technology, 2010, 204 (9-10): 1637-1645.
[16] MOULDER J, STICKLE W F, SOBOL P E, BOMBEN K D. Handbook of X-ray photoelectron spectroscopy [M]. Eden Prairie: Perkin Elmer Co., 1992.
[17] BRUM M, BERTHET A, BERTOLINI J C. XPS, AES and Auger parameter of Pd and PdO[J]. Journal of Electron Spectroscopy and Related Phenomena, 1999, 104(1-3): 55-60.
[18] SCH?N G. High resolution Auger electron spectroscopy of metallic copper[J]. Journal of Electron Spectroscopy and Related Phenomena, 1972, 1(4): 377-387.
[19] WANG Huai-ming, DENG Jing-fa, DONG Shu-zhong, QIU Yue-ming. Adsorption of Oxygen on a Ag-Pd alloy surface [J]. Chinese Journal of Chemical and Physics, 1991, 4(1): 59-64. (in Chinese)
[20] GABASCH H, UNTERGER W, HAYEK K, KL?TZER B, KLEIMENOV E, TESCHNER D, ZAFEIRATOS S, H?VECKER M, KNOP-GERICKE A, SCHL?GL R, HAN J Y, RIBEIRO F H, ASZALOS-KISS B, CURTIN T, ZEMLYANOV D. In situ XPS study of Pd(111) oxidation at elevated pressure, Part 2: Palladium oxidation in the 10-1mbar range [J]. Surface Science, 2006, 600(15): 2980-2989.
316L不锈钢上电刷镀钯膜的组成与耐蚀性
唐鋆磊,左 禹,唐聿明,熊金平
北京化工大学 材料科学与工程学院,北京 100029
摘 要:利用电刷镀工艺在316L不锈钢上制备了结合力良好的钯膜。使用扫描电子显微镜、X射线能谱、X射线光电子能谱(XPS)、质量损失实验和电化学测试研究了膜层的性能。结果表明,电刷镀钯膜主要是由钯元素构成的;XPS分析表明,膜层中的钯为金属态。电刷镀钯后的不锈钢试样在沸腾的20%硫酸溶液和含0.005 mol/L溴离子的甲酸和乙酸混合溶液中均显示了非常好的耐蚀性能。镀钯试样的腐蚀速率比不锈钢试样的下降了2个数量级。
关键词:钯膜;电刷镀;腐蚀;不锈钢
(Edited by YANG Hua)
Corresponding author: ZUO Yu; Tel: +86-10-64423795; E-mail: zuoy@mail.buct.edu.cn
DOI: 10.1016/S1003-6326(11)61146-1
Abstract: Palladium films with good adhesive strength were deposited on 316L stainless steel by brush plating. Scanning electronic microscopy, energy dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy (XPS), mass loss tests and electrochemical methods were used to study the properties of the films. The brush plated palladium film mainly consisted of palladium. XPS analysis indicated that palladium was present in the films as metal state. The palladium plated stainless steel samples showed excellent corrosion resistance in boiling 20% H2SO4 solution and boiling acetic/formic acids with 0.005 mol/L Br- ions added. The corrosion rates of the palladium plated 316L stainless steel samples were about two orders of magnitude lower than those of the original 316L stainless steel samples. This method provides a possibility to prepare protective palladium films on stainless steel facilities with large areas in industrial sites.


