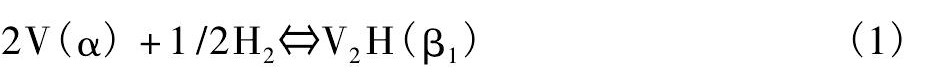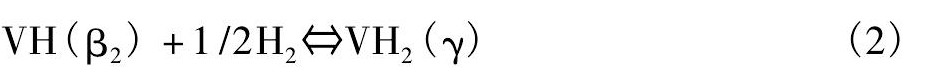网络首发时间: 2017-03-20 15:17
稀有金属2017年第11期
钒基固溶体贮氢合金的研究进展
罗龙 吴文远 边雪 齐健博
东北大学冶金学院
内蒙古科技大学分析测试中心
摘 要:
基于目前钒基固溶体贮氢合金研究现状, 介绍了钒氢反应原理, 对钒基贮氢合金理论计算、合金制备及性能改善等方面的研究进行了总结, 并对合金成本等问题进行了讨论。钒基体心立方结构固溶体贮氢合金具有高容量、氢化反应条件温和、抗粉化性能好, 动力学性能优越等特点。尤其作为燃料电池用贮氢罐的候选材料, 具有很好的开发应用前景。钒从吸氢至完全饱和的整个过程, 结构在发生变化:BCC (α) →BCT (β) →FCC (γ) 。利用材料计算方法对合金热力学、晶格参数、电子原子比等方面进行理论计算, 能够为实验研究提供理论指导。通过一种或多种元素添加或替代和热处理能够显著改善钒基固溶体贮氢合金的贮氢性能, 但在高吸氢容量的前提下保证合金能够在适中条件下释放大部分氢仍是该系合金开发的关键之一。高纯钒价格昂贵, 在不显著减小最大容量的基础上有效降低钒基贮氢合金的成本是该合金研究的另外一个关键点。
关键词:
钒基贮氢合金;氢化反应;贮氢容量;晶体结构;
中图分类号: TG139.7
作者简介:罗龙 (1983-) , 男, 内蒙古乌兰察布人, 博士研究生, 讲师, 研究方向:贮氢合金;E-mail:ljd0928@aliyun.com;;吴文远, 教授;电话:024-83680527;E-mail:neuwuwenyuan@126.com;
收稿日期:2016-12-05
基金:国家自然科学基金项目 (51274060);内蒙古自然科学基金项目 (2014MS0529);中央高校基本科研业务费项目 (N150204019);内蒙古科技大学创新基金项目 (2014QDL048) 资助;
Research Progress of Vanadium-Based Solid Solution Hydrogen Storage Alloys
Luo Long Wu Wenyuan Bian Xuan Qi Jianbo
School of Metallurgy, Northeastern University
Analytical and Testing Center, Inner Mongolia of Science and Technology
Abstract:
Based on the current research status of vanadium-based hydrogen storage alloys, the principle of vanadium hydrogen reaction was introduced. The research on the theoretical computation, the preparation and performance improvement of vanadium based hydrogen storage alloys was summarized. And the problem concerning the cost of alloy was discussed. Vanadium-based solid solution hydrogen storage alloys had characteristic of high capacity, moderate conditions for hydrogenation reaction, good resistance to powder and excellent dynamic performance, etc. In particular, as a candidate material for hydrogen storage tanks for fuel cells, it had a good prospect for development and application. The whole process of hydrogen absorption of vanadium based alloys to fully saturated, the structure of the alloy was changed as follows: BCC ( α) →BCT ( β) →FCC ( γ) . The materials computation methods were used to calculate the thermodynamic, lattice parameter, electron atomic ratio and so on, which could provide theoretical guidance for the experimental study. The use of one or more elements addition or substitution and heat treatment could significantly improve the hydrogen storage properties of vanadium based solid solution hydrogen storage alloys. However, under the premise of high hydrogen absorption capacity, it was essential to ensure that most of the hydrogen in the alloy could be released under moderate conditions. High pure vanadium was expensive, and it was a key point to reduce the cost of vanadium based hydrogen storage alloys on the basis of not reducing the maximum capacity significantly.
Keyword:
vanadium-based hydrogen storage alloys; hydrogenation reaction; hydrogen storage capacity; crystal structure;
Received: 2016-12-05
近年来, 化石能源消耗量逐年上升, 同时带来的温室效应、雾霾等环境污染问题也日趋严重。氢是清洁无污染, 可再生的能源[1], 被认为是解决逐渐枯竭的化石燃料和保护地球环境很好的替代能源, 从使用角度来说, 氢的存贮是“氢经济”的重点, 其中, 作为氢存贮材料的金属氢化物是人们研究的重点之一。由于V基合金具有较高的体积贮氢容量 (VH2为0.16 g·cm-3, 大约为液体氢的2.25倍[2], La Ni5H6为0.115 g·cm-3[3]) 和质量贮氢容量 (理论上为3.8%[4,5], La Ni5为1.4%[3]) , 氢化反应条件温和[6], 抗粉化性能好, 动力学性能优越[7]等优点, 所以近些年越来越多的研究人员投入到V基固溶体合金研究中。然而, V固溶体合金放氢量有限, 尤其是低温放氢, 近几年来, 许多研究人员努力去提高V基合金的贮氢性能, 经过研究发现, 合金熔炼方法、热处理、成分等都是影响合金贮氢性能的因素。本文对国内外近几年钒基固溶体合金的研究动态及发展现状进行了概述, 并对今后发展方向进行了展望。
1 钒氢化原理
在钒基体心立方结构固溶体合金中, 氢原子能稳定存在于四面体晶格 (配位数4) 间隙和八面体晶格 (配位数6) 间隙, 吸氢时氢原子大部分进入四面体间隙位置, 由于每个晶胞中存在12个四面体间隙, 这样为氢原子的进入提供了较多的间隙位置, 所以理论储氢量高[4]。钒氢反应的温度比较低, 室温就可吸收大量的氢。表1列出了不同温度下氢在钒中的溶解度[8], 随着温度升高, 溶解度逐渐降低。
钒氢化过程可分4个阶段: (1) 合金表面吸附并解离氢分子为氢原子; (2) 氢原子固溶于钒合金中形成固溶相, 表面吸附的氢原子向合金体内扩散; (3) 氢与饱和固溶相进一步反应生成氢化物相层; (4) 氢原子通过氢化物层进一步向合金体内扩散进行反应。
表1 不同温度下氢在钒中的溶解度Table 1 Solubility of hydrogen in vanadium at different temperatures 下载原图

表1 不同温度下氢在钒中的溶解度Table 1 Solubility of hydrogen in vanadium at different temperatures
钒吸氢首先形成β1相 (V2H低温相) 。随着吸氢的进行, β1相转变为β2相 (V2H高温相或VH) 。然后, 吸氢完全后转变为γ相 (VH2) [9]。结果, 在V-H系统的PCT曲线上存在两个平台, 如图1[10]。
在这里, 第一个平台对应着α相 (氢固溶相) 与β1相共存:
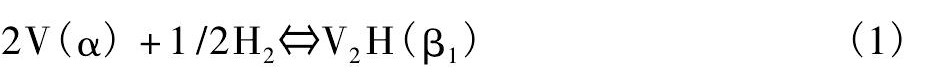
β1相非常稳定, 第一个平台对应的平衡氢压在353K下为0.1 Pa。因此在中等温度条件下从β1相的放氢反应很难发生。第二个平台对应着β2相 (VH) 与γ相共存:
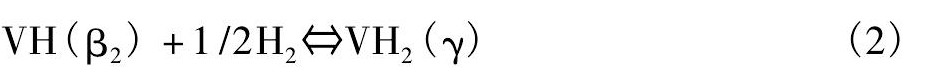
钒从吸氢至完全饱和的整个过程, 结构在发生变化, 如图2[11]。第一平台对应BCC (α) 和BCT (β) 共存, 第二平台对应BCT (β) 和FCC (γ) 共存, 其中β相BCT结构可以认为是略微变形的FCC。γ相并不稳定, 能够在适中的温度和压力下吸氢或放氢, 在13℃时候, 离解压达1.01×105Pa。因此, 吸入钒金属中的氢大约只有一半能够放出。
2 理论计算
材料的计算机设计, 是通过对理论模型的计算, 预测或设计材料结构与性能, 使材料的研究更具前瞻性、方向性, 有利于原始创新, 可大大提高研究效率。鉴于上述特点, 近些年, 研究人员对V固溶体贮氢合金做了一些富有成果的理论研究。Hiroshi[12]利用计算机模拟了晶格缺陷对钒氢化物贮氢性能的影响, DFT计算表明, 在β相钒氢化物中Mo替代V增强了氢原子从八面体间隙位点转移到临近的四面体间隙位点;对α相中的刃型位错经典分子动力学计算表明, 位错核附近的氢聚集减小了刃型位错之间的相互作用;对多表面纳米颗粒钒的经典分子动力学模拟结果表明, 氢化生成五重对称多晶结构, 结果产生多重孪晶界, 这一过程与BCC转变BCT有关。Zhang等[13]研究了Ti-V-Cr合金有效贮氢容量CH与外层电子数、原子直径差、电负性差之间的关系。推出了定量关系式, 表明外层电子数对合金的Ti-V-Cr有效贮氢容量CH影响最大, 电负性差对CH影响最小, 对应参数在适当范围时, 有效贮氢量大于2.0%。同样, 刘守平等[14]利用逐步回归法研究了钒基固溶体贮氢合金的焓变、组成和键参数之间关系上, 建立了氢化反应焓变的半经验数学模型。结果表明, 电负性差和电子浓度越小、原子尺寸因素越大, 合金的生成焓负值越大, 合金氢化物越稳定。
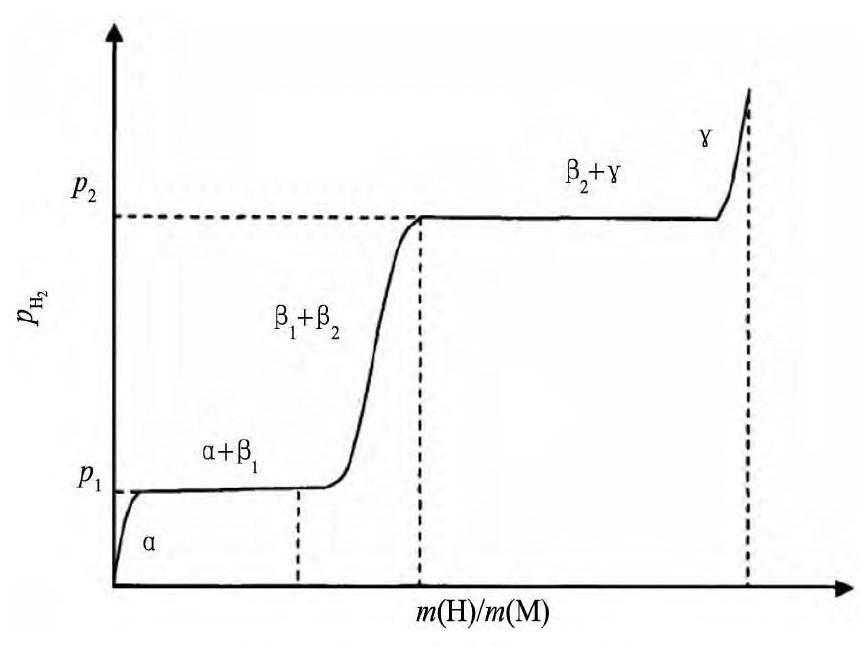
图1 钒氢系统的PCT图Fig.1 PCT diagram of vanadium hydrogen system
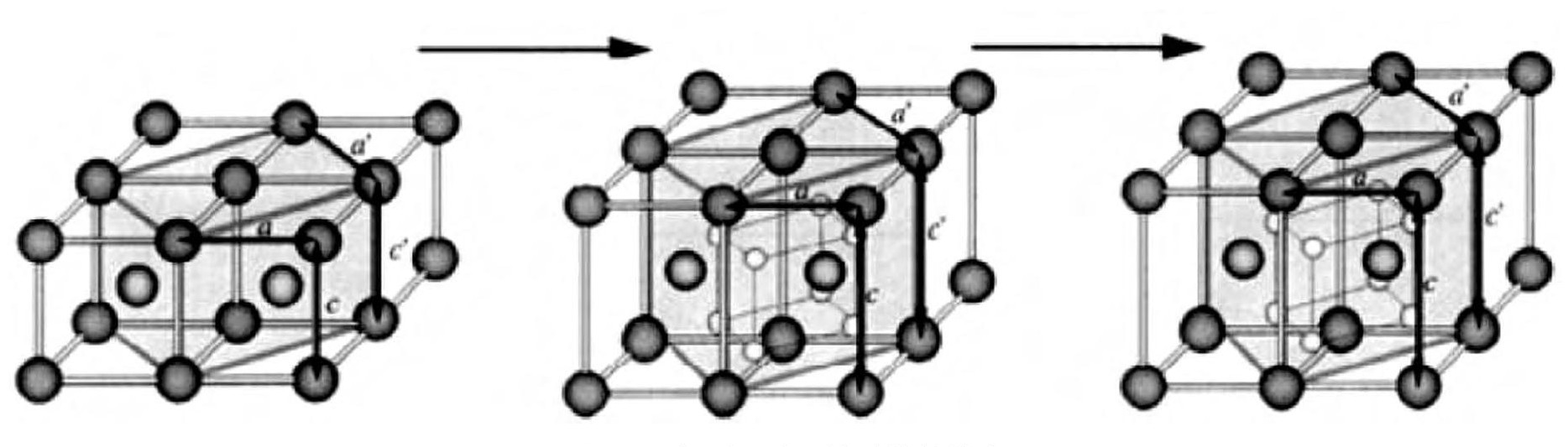
图2 吸氢过程中晶格结构的转变Fig.2 Change of crystal structure of metal lattice during hydrogenation
周晶晶等[15]为了研究V基合金力学性能对循环稳定性的影响, 采用基于密度泛函理论的第一性原理赝势平面波方法研究了 (59Cr-41Ti) 100-xVx (x=5, 15, 30, 60, 80, 100) 合金的弹性性质, 并从电子成键上分析相关特性的微观机制。分析认为, 弹性性质并不是影响钒基贮氢材料循环性能的最主要原因。娄珀瑜等[16]采用密度泛函方法计算了钒氢分子及离子 (VHn, n=0, +1, +2) 的势能函数与垂直电离势, 结果与实验值和文献值基本吻合。
以上研究表明, 计算机模拟计算对钒基固溶体贮氢合金性能的研究是有用的和成功的, 能够为实验研究提供理论指导。
3 制备方法
合金的制备过程决定了合金的微观结构及性能。研究人员在贮氢合金的制备中会根据合金的类型选择合适的制备方法, 如高频感应熔炼、电弧熔炼、熔体急冷法、机械合金化法、气体雾化法、还原扩散法、粉末烧结法等。Singh等[17]采用机械球磨制备了Ti0.32Cr0.43V0.25, 研究了高能球磨对相稳定性、晶格应变、晶粒尺寸、晶格参数的影响, 结果表明增加球磨时间导致晶格应变的增加和晶粒尺寸减少, 导致贮氢容量减少。Yan等[18,19]采用电弧熔炼制备了30V-Ti-Cr-Fe合金, 室温下吸氢容量大于3.6%, 有效放氢容量大于2.0%。Suwarno等[20]分别采用电弧熔炼和熔体急冷两种方法制备了铸态和快凝Ti-V合金, 对比研究发现, 熔体急冷方法细化了合金晶粒, 增加了合金中的吸氢相, 晶粒细化可以加快放氢动力学过程。Pei等[21]研究了铸态及快速凝固V35Ti25Cr40合金的微观结构及贮氢性能, 结构表明, 通过真空铸造熔体急冷方法明显细化了合金树枝状晶, 同时, 引起树枝区的元素非平衡分布。熔体急冷增加了合金的空位浓度, 增加了晶格参数, 吸氢速率大幅提高, 吸氢容量从铸态的2.86%提高到2.99%;吸氢动力学机制从铸态的三维扩散控制转变为快速冷凝的化学反应控制, 快速凝固降低了合金的活化性能。Planté等[22]采用高频感应熔炼制备了富钒Ti-V-Cr合金, 有效吸氢量为1.7%。Mi等[23]采用悬浮熔炼方法制备了Ti27.25Cr28.05V37.25Fe7.45Ce1.0, 常温下吸氢容量达到3.56%。
其他研究人员也采用以上的方法制备了合金[24,25,26,27]。Hong等[28]利用脉冲电流烧结成功制备了Ti-Cr-V合金。Suwarno等[29]利用熔体纺丝法制备了Ti0.8V0.2合金, 合金的吸氢动力学有所提高, 但贮氢容量有所下降。总之, 制备方法不同得到的合金性能差别较大, 详见表2[8,30]。
表2 合金制备方法及特征Table 2 Preparation methods and characteristics 下载原图
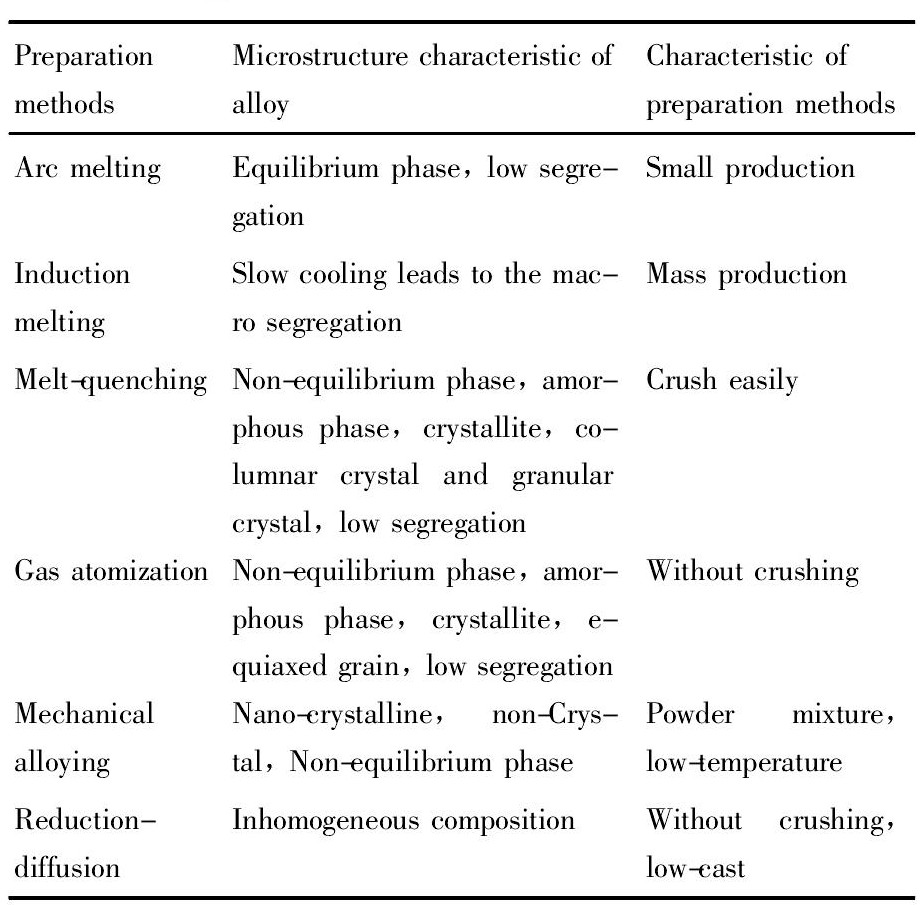
表2 合金制备方法及特征Table 2 Preparation methods and characteristics
4 合金改性
尽管V基贮氢合金具有许多优点, 但是还存在如活化性能差、吸放氢平台倾斜、有效贮氢容量低、吸放氢平台滞后效应明显、循环稳定性差等问题, 达不到实际应用要求[31]。为了改善这些问题, 研究人员采用了多种手段来改善其吸放氢性能, 例如元素添加及替代、热处理、制备工艺等。其中制备工艺方法在上一节已经做了讨论, 此节不再阐述。
4.1 元素添加及替代
采用一种或多种元素添加或替代来改善贮氢合金材料性能, 已经有大量报道, 不同类型贮氢合金添加或替代同种元素, 所起的作用也是不同的。总结不同元素在V基贮氢合金中起的作用对后续研究有重要意义。Yukawa等[32]研究了合金元素M (M=Ti, Fe, Ni, Al, Si等) 对VHx稳定性的影响, 发现元素周期表中第八族元素的添加使γ相 (VH2) 的稳定性最低。Asano等[33]对Cr添加对氢在V-H系统中的扩散及氢化物相的稳定性进行了研究, 结果表明随着Cr元素含量的增加, 抑制了间隙位置氢的扩散, 降低了氢化物相的稳定性。随后, Asano等[34,35]又研究了Al, Fe添加对V-H系统相变及氢的扩散, 结果表明Al部分替代V增强了氢原子在氢化物中的扩散, Fe影响氢化物相的稳定性, 抑制氢的扩散。
以上研究表明, 合金元素的添加对钒基贮氢性能的改善具有重要作用。Mn为放氢型元素, Ti为吸氢型元素, 因此, 常把两种元素组合添加到基体合金中去调节贮氢性能。由于V与Ti可以以任何比例固溶, 同时Ti是在不显著减小初始最大容量的前提下有效降低钒基BCC合金吸放氢压力必需元素[36], 所以目前开发的V基贮氢合金主要有V-Ti-Mn系、V-Ti-Cr系、V-Ti-Fe系、V-Ti-Ni系及多元合金。Bibienne等[37]研究了Mn含量对V2-x Ti Mnx合金性能的影响, 结果表明随着Mn含量的增加, 吸氢量下降。Towata等[38,39]分别研究了Nb和Fe部分替代Cr对Ti25Cr50V25及Ti16Cr34V50吸放氢循环性能及贮氢量的影响, 结果表明, Fe部分替代提高了循环稳定性, 但降低了贮氢容量;Nb提高了循环稳定性, 但是贮氢容量并没有降低。Yan等[40]研究了添加Al对V30Ti35Cr25Fe10贮氢性能的影响, 结果显示, 随着Al含量从0.6% (原子分数) 逐渐增加到5%, 晶格常数变大, 合金的吸放氢容量减小, 放氢平台压提高, 这与V, Ti, Cr规律正好相反, 分析认为是Al与过度元素之间较大的原子特性差异造成的。除此之外, 还有一些研究人员分别尝试了添加Pd[41], Ni[42]及稀土元素[23,43,44,45]等, 取得了一些有益的结论。
除了上面添加金属元素外, 一些研究发现非金属元素的添加对V基合金的贮氢性能也有影响。例如, Tong等[46]为了提高V2Ti0.5Cr0.5Ni合金电极充放电循环稳定性, 添加了不同含量的O元素, 随着O含量的增加, 合金电极的最大放电容量从366.8 m Ah·g-1降低到225.3 m Ah·g-1, 循环稳定性先增强后减弱。Shen等[47]研究了添加C元素对退火态Ti25V35Cr40合金循环吸氢稳定性的影响, 结果显示, 掺杂微量的C可有效减少吸氢的退化, 分析认为, BCC晶格中的C元素抑制了合金与氢的歧化反应。Cho等[48]研究发现在Ti0.32Cr0.43V0.25合金中添加B, 平台压发生了明显变化, 有效吸氢容量也明显降低。Yan等[49]研究了添加Si对V30Ti35Cr25Fe10合金性能的影响, 结果发现添加Si元素, 合金中出现了Laves相, 合金的活化性能改善, 吸放氢容量降低, 平台压提高, 原因是添加Si导致晶格常数减小和Laves相的出现。经过上述讨论发现, 元素的合理添加可以很好地改善V基合金贮氢性能, 另外, 由于金属元素与非金属元素电负性差异, 组合添加可能会取得不错的效果。
4.2 热处理
除了元素添加或者替代外, 热处理也是人们普遍认为改性的有效方法之一。在表2中看到, 合金熔炼后缓冷会形成偏析, 对性能不利。为了有效降低这些影响, 经常采用热处理的方法。Yan等[7]将制备的合金V30Ti32Cr32Fe6在1673 K保温30min, 发现贮氢容量提高了, 平台更加平坦, 原因是热处理使成分更加均匀, 同时消除了Laves相。Zhou等[50]对铸态V35Ti20Cr45进行退火热处理 (973K保温72 h) 后, 活化性能与动力学性能得到极大改善, 分析认为是退火后合金中出现具有催化活性的C14-Laves第二相。葛红卫等[51]采用热处理 (1473 K保温2 h) 对 (Ti-Cr) 40V55Zr5改性后, 合金动力学性能及活化性能均得到改善, 室温下最大吸氢量略有降低, 放氢平台倾斜度降低, 平台宽度稍有增加。
淬火也常被应用于贮氢合金热处理。Cho等[52]对Ti0.32Cr0.43V0.25合金进行研究, 认为淬火能够提高合金贮氢容量和改善平台倾斜度。然而, 郑欣等[53]通过对V40Ti26Cr26Fe8淬火研究发现, 淬火后合金放氢量和平台压均出现下降, 放氢平台倾斜, 滞后增大, 这与文献[52]结论恰好相反, 可能是淬火过程中减少合金Laves相和产生缺陷这两方面在不同合金中所起的主导作用不同, 所以单纯的淬火不一定是很好的方法, 多种热处理手段结合可能会得到最佳效果。
通过上面研究可以看出, 恰当的热处理工艺可以改变材料表面或内部的化学成分与组织, 从而改善合金性能。同时要考虑到, 热处理会造成某种元素一定程度的挥发损耗, 一般来讲, 时间越长温度越高损耗越大, 对合金性能影响越大。
5 降低成本
我国的钒资源比较丰富, 可冶炼比较困难, 所以纯钒 (>99%) 的价格非常昂贵 (大约390美元/kg[54]) , 限制了钒基贮氢合金的实际应用。最近人们对如何降低成本做了大量研究。Yan等[7]采用Fe V80制备了V30Ti32Cr32Fe6, 合金性能较好, 室温下吸氢量为3.76%, 放氢量为2.35%。在此基础上, Mi等[23]也采用商业化钒铁合金制备了Ti27.25Cr28.05V37.25Fe7.45, 合金298 K温度下吸氢容量为3.56%, 453 K温度下放氢容量为2.54%。有研究表明[55]钒铁合金中的杂质会降低合金贮氢容量, 增加平台倾斜度, 添加Ce元素会有效抑制杂质的副作用。Ulmer等[54]用商业Fe V80制备了V (40-40·x) Fe (8-8·x) Ti26Cr26 (Fe V) (48·x) (0≤x≤0.9) 合金, 研究表明, 纯钒制备的合金 (x=0) 可逆存贮100 kg氢需要合金成本为978130美元, 钒铁部分替代后 (x=0.9) , 可逆存贮100 kg氢需要合金成本为325000美元, 在折算钒铁替代降低部分放氢量的因素后, 成本降低到原来的1/3。除了钒铁替代纯钒外, 人们还通过V2O5制备钒基固溶体贮氢合金。刘守平等[56]用工业V2O5采用金属铝热反应直接制备了钒基固溶体贮氢合金V3Ti Ni0.56Al0.2, 贮氢量为1.62%。虽然容量相对较低, 但是这种替代对降低成本是一个不错选择。
6 结束语
钒基固溶体贮氢合金存在诸如活化性能差、吸放氢平台倾斜等缺点, 但是, 高容量、氢在氢化物中扩散速度快等优点使其仍然是车载贮氢最佳的候选材料之一。虽然近十多年的研究取得了丰富成果, 但是离实际应用还有一段距离, 需要从以下几个方面做进一步研究:
1.更多的利用材料计算方法对合金热力学、晶格参数、电子原子比等方面进行理论计算, 为实验提供理论指导。
2.探索新的合金制备方法。
3.改进改性工艺, 同时利用廉价原料 (如V2O5, NH4VO3, Fe V等) 降低合金成本。
4.将V基贮氢合金与动力学性能优越的稀土基合金进行复合。
参考文献
[1] Lin H C, Lin K M, Wu K C, Hsiung H H, Tsai H K.Cyclic hydrogen absorption-desorption characteristics ofTi Cr V and Ti0.8Cr1.2V alloys[J].International Journal of Hydrogen Energy, 2007, 32 (18) :4966.
[2] Lototsky M V, Yartys V A, Zavaliy I Y.Vanadiumbased BCC alloys:phase-structural characteristics and hydrogen sorption properties[J].Journal of Alloys and Compounds, 2005, 404-406 (12) :421.
[3] Chen P, Zhu M.Recent progress in hydrogen storage[J].Mater.Today, 2008, 11 (12) :36.
[4] Pei P, Zhang P L, Zhang B, Song X P.V based hydrogen storage alloys and alloying research[J].Materials Review, 2006, 20 (10) :123. (裴沛, 张沛龙, 张蓓, 宋西平.V系储氢合金及其合金化[J].材料导报, 2006, 20 (10) :123.)
[5] Wang W, Luo Y C, Qiu J P, Kang L.Microstructure and hydrogen storage properties of Sc-based laves phase alloys Sc0.8Zr0.1Y0.1Mn2-xNix (x=0~2.0) [J].Chinese Journal of Rare Metals, 2015, 39 (8) :696. (王稳, 罗永春, 邱建平, 康龙.钪基Laves相合金Sc0.8Zr0.1Y0.1Mn2-xNix (x=0~2.0) 的微观结构和储氢性能[J].稀有金属, 2015, 39 (8) :696.)
[6] Song X P, Pei P, Zhang P L, Chen G L.The influence of alloy elements on the hydrogen storage properties in vanadium-based solid solution alloys[J].Journal of Alloys and Compounds, 2008, 455 (1-2) :392.
[7] Yan Y G, Chen Y H, Wu C L, Tao M D, Liang H.A low-cost BCC alloy prepared from a Fe V80 alloy with a high hydrogen storage capacity[J].Journal of Power Sources, 2007, 164 (2) :799.
[8] Hu Z L.Hydrogen Storage Materials[M].Beijing:Chemical Industry Press, 2000.186. (胡子龙.贮氢材料[M].北京:化学工业出版社, 2000.186.)
[9] Hiroshi Y, Akira T, Daisuke Y, Shigeyuki I, Shu Y, Masahiko M.Alloying effects on the hydriding properties of vanadium at low hydrogen pressures[J].Journal of Alloys and Compounds, 2002, 337 (1-2) :264.
[10] Yan Y G.Research on Struetures and Hydrogen Absorption/Desorption Performances of V-Ti-Cr-Fe Alloys[D].Chengdu:Sichuan University, 2007.12. (严义刚.V-Ti-Cr-Fe贮氢合金的结构与吸放氢行为研究[D].成都:四川大学, 2007.12.)
[11] Fei Y, Kong X C, Wu Z, Li H H, Peterson V K.In situ neutron-diffraction study of the Ti38V30Cr14Mn18structure during hydrogenation[J].Journal of Power Sources, 2013, 241:355.
[12] Hiroshi O.Computational study of lattice defects in metal-hydrogen systems[J].Journal of Alloys and Compounds, 2013, 580 (18) :131.
[13] Zhang J L, Jin H J, Meng X H, Fang S S, Zhou Z Q.Effects of bond parameters on hydrogen storage capacity of Ti-V-Cr BCC phase alloys[J].Rare Metal Materials and Engineering, 2011, 40 (7) :1152.
[14] Liu S P, Liu Z H, Liu X J, Liu J H.A mathematical model of enthalpy of formation for V-based hydrogen storage alloys[J].Materials Review, 2008, 22 (11) :120. (刘守平, 刘志红, 刘小军, 刘杰慧.钒基贮氢合金氢化反应生成焓的计算模型[J].材料导报, 2008, 22 (11) :120.)
[15] Zhou J J, Chen Y G, Wu C L, Pang L J, Zheng X, Gao T.The first-principles study on the elasticity of Vbased solid solution hydrogen storage materials[J].Acta Physica Sinica, 2009, 58 (10) :7044. (周晶晶, 陈云贵, 吴朝玲, 庞立娟, 郑欣, 高涛.钒基固溶体储氢材料弹性性质第一性原理研究[J].物理学报, 2009, 58 (10) :7044.)
[16] Lou P Y, Huang P, Ran M.Potentional energy function and vertical ionization potential of VHn (n=0, +1, +2) [J].Journal of Atomic and Molecular Physics, 2008, 25 (4) :861. (娄珀瑜, 黄萍, 冉鸣.钒氢分子及离子的势能函数与垂直电离势[J].原子与分子物理学报, 2008, 25 (4) :861.)
[17] Singh B K, Shim G, Cho S W.Effects of mechanical milling on hydrogen storage properties of Ti0.32Cr0.43V0.25alloy[J].International Journal of Hydrogen Energy, 2007, 32 (18) :4961.
[18] Yan Y G, Chen Y G, Zhou X X, Liang H, Wu C L, Tao M D.Some factors influencing the hydrogen storage properties of 30V-Ti-Cr-Fe alloys[J].Journal of Alloys and Compounds, 2008, 453 (1) :428.
[19] Yan Y G, Chen Y G, Liang H, Wu C L, Tao M D.Hydrogen storage properties of V30-Ti-Cr-Fe alloys[J].Journal of Alloys and Compounds, 2007, 427 (1-2) :110.
[20] Suwarno S, Jan K S, Jan P M, Bente K, Brre T B, Esther O, Erling R, Mario W, Roman D, Volodymyr A Y.Microstructure and hydrogen storage properties of as-cast and rapidly solidified Ti-rich Ti-V alloys[J].Trans.Nonferrous Met.Soc.China, 2012, 22 (8) :1831.
[21] Pei P, Song X P, Liu J, Chen G L, Qin X B, Wang BY.The effect of rapid solidification on the microstructure and hydrogen storage properties of V35Ti25Cr40hydrogen storage alloy[J].International Journal of Hydrogen Energy, 2009, 34 (19) :8094.
[22] PlantéD, Andrieux J, Laversenne L, Miraglia S.In situ X-ray diffraction study of hydrogen sorption in V-rich Ti-V-Cr bcc solid solutions[J].Journal of Alloys and Compounds, 2015, 648:79.
[23] Mi J, LüF, Liu X P, Jiang L J, Li Z N, Wang S M.Enhancement of cerium and hydrogen storage property of a low-cost Ti-V based BCC alloy prepared by commercial ferrovanadium[J].Journal of Rare Earths, 2010, 28 (5) :781.
[24] Massicot B, Latroche M, Joubert J M.Hydrogenation properties of Fe-Ti-V bcc alloys[J].Journal of Alloys and Compounds, 2011, 509 (2) :372.
[25] Miao H, Gao M X, Liu Y F, Lin Y, Wang J H, Pan H G.Microstructure and electrochemical properties of TiV-based multiphase hydrogen storage electrode alloys Ti0.8Zr0.2V2.7Mn0.5Cr0.8-xNi1.25Fex (x=0.0~0.8) [J].International Journal of Hydrogen Energy, 2007, 32 (16) :3947.
[26] Wang J H, Pan H G, Li R, Zhong K, and Gao M X.The effect of particle size on the electrode performance of Ti-V-based hydrogen storage alloys[J].International Journal of Hydrogen Energy, 2007, 32 (15) :3381.
[27] Mi J, Guo X M, Liu X P, Jiang L J, Li Z N, Hao L, Wang S M.Effect of Al on microstructures and hydrogen storage properties of Ti26.5Cr20 (V0.45Fe0.085) 100-x AlxCe0.5alloy[J].Journal of Alloys and Compounds, 2009, 485 (1-2) :324.
[28] Hong S H, Song M Y.Synthesis of a Ti-Cr-V alloy by pulsed current assisted reaction[J].Journal of Industrial and Engineering Chemistry, 2013, 19 (4) :1267.
[29] Suwarno S, Solberg J K, Yartys V A, Krogh B.Hydrogenation and microstructural study of melt-spun Ti0.8V0.2[J].Journal of Alloys and Compounds, 2011, 509 (S2) :S775.
[30] Li D, Lou Y W, Du J L, Pu C H, Huang T S, Li Z L, Wu Z, Li C H.Research progress of vanadium-based hydrogen storage alloy[J].Materials Review, 2015, 29 (12) :92. (李朵, 娄豫皖, 杜俊霖, 蒲朝辉, 黄铁生, 李志林, 吴铸, 李重河.钒基储氢合金的研究进展[J].材料导报, 2015, 29 (12) :92.)
[31] Pukazhselvan D, Kumar V, Singh S K.High capacity hydrogen storage:basic aspects, new developments and milestones[J].Nano Energy, 2012, 1 (4) :566.
[32] Yukawa H, Takagi M, Teshima A, Morinaga M.Alloying effects on the stability of vanadium hydrides[J].Journal of Alloys and Compounds, 2002, s330-332 (1) :105.
[33] Asano K, Hayashi S, Nakamura Y, Akiba E.Effect of substitutional Cr on hydrogen diffusion and thermal stability for the BCT monohydride phase of the V-H system studied by1H NMR[J].Journal of Alloys and Compounds, 2012, 524:63.
[34] Asano K, Hayashi S, Nakamura Y.Enhancement of hydrogen diffusion in the body-centered tetragonal monohydride phase of the V-H system by substitutional Al studied by proton nuclear magnetic resonance[J].Acta Materialia, 2015, 83:479.
[35] Asano K, Hayashi S, Nakamura Y.Formation of hydride phase and diffusion of hydrogen in the V-H system varied by substitutional Fe[J].International Journal of Hydrogen Energy, 2016, 41 (15) :6369.
[36] Kim H, Sakaki K, Saita I, Enoki H, Noguchi K, Machid A, Watanuki T, Nakamura Y.Reduction and unusual recovery in the reversible hydrogen storage capacity of V1-xTixduring hydrogen cycling[J].International Journal of Hydrogen Energy, 2014, 39 (20) :10546.
[37] Bibienne T, Tousignant M, Bobet J L, Huot J.Synthesis and hydrogen sorption properties of Ti V (2-x) Mnx BCC alloys[J].Journal of Alloys and Compounds, 2015, 624:247.
[38] Towata S H, Noritake T, Itoh A, Aoki M, Miwa K.Cycle durability of Ti-Cr-V alloys partially substituted by Nb or Fe[J].Journal of Alloys and Compounds, 2013, 580 (24) :S226.
[39] Towata S, Noritake T, Itoh A, Aoki M, Miwa K.Effect of partial niobium and iron substitution on shortterm cycle durability of hydrogen storage Ti-Cr-V alloys[J].International Journal of Hydrogen Energy, 2013, 38 (7) :3024.
[40] Yan Y G, Chen Y G, Liang H, Wu C L, Tao M D, Tu M J.Effect of Al on hydrogen storage properties of V30Ti35Cr25Fe10alloy[J].Journal of Alloys and Compounds, 2006, 426 (1-2) :253.
[41] Liu Y F, Zhang S S, Li R, Gao M X, Zhong K, Miao H, Pan H G.Electrochemical performances of the Pd-added Ti-V-based hydrogen storage alloys[J].International Journal of Hydrogen Energy, 2008, 33 (2) :728.
[42] Pan H G, Li R, Gao M X, Liu Y F, Lei Y Q, Wang Q D.Effects of Ni on the structural and electrochemical properties of Ti-V-based hydrogen storage alloys[J].International Journal of Hydrogen Energy, 2006, 31 (9) :1188.
[43] Wu C L, Yan Y G, Chen Y G, Tao M D, Zheng X.Effect of rare earth (RE) elements on V-based hydrogen storage alloys[J].International Journal of Hydrogen Energy, 2008, 33 (1) :93.
[44] Li S C, Wen B Q, Zhai J, Guo X.Effect of rare earth elements substitution for vanadium on microstructures and electrochemical properties of Ti0.26Zr0.07V0.24Mn0.1Ni0.33alloy[J].Powder Technology, 2016, 303 (1) :1.
[45] Liu X P, Cuevas F, Jiang L J, Latroche M, Li Z N, Wang S M.Improvement of the hydrogen storage properties of Ti-Cr-V-Fe BCC alloy by Ce addition[J].Journal of Alloys and Compounds, 2009, 476 (1-2) :403.
[46] Tong Y W, Gao J C, Deng G, Zhang X F, Wang N W.Influence of oxygen content on microstructure and electrochemical properties of V2-xTi0.5Cr0.5Ni Ox (x=0~0.35) hydrogen storage alloys[J].Rare Metal Materials and Engineering, 2015, 44 (5) :1052.
[47] Shen C C, Li H C.Cyclic hydrogenation stability ofγ-hydrides for Ti25V35Cr40alloys doped with carbon[J].Journal of Alloys and Compounds, 2015, 648:534.
[48] Cho S W, Yoo J H, Shim G, Park C N, Choi J.Effects of B addition on the hydrogen absorption-desorption property of Ti0.32Cr0.43V0.25alloy[J].International Journal of Hydrogen Energy, 2008, 33 (6) :1700.
[49] Yan Y G, Chen Y G, Liang H, Wu C L, Tao M D.The effect of Si on V30Ti35Cr25Fe10BCC hydrogen storage alloy[J].Journal of Alloys and Compounds, 2007, 441 (s1-2) :297.
[50] Zhou H Y, Wang F, Wang J, Wang Z M, Yao Q R, Deng J Q, Tang C Y, Rao G H.Hydrogen storage properties and thermal stability of V35Ti20Cr45alloy by heat treatment[J].International Journal of Hydrogen Energy, 2014, 39 (27) :14887.
[51] Ge H W, Liu J, Fan X L, Hang Z M, Chen C P, Chen L X.Modification by heat treatment for (Ti-Cr) 40V55Zr5hydrogen storage alloy[J].Journal of Materials Science&Engineering, 2008, 26 (4) :567. (葛红卫, 刘剑, 范修林, 杭州明, 陈长聘, 陈立新. (Ti-Cr) 40V55Zr5贮氢合金的热处理改性[J].材料科学与工程学报, 2008, 26 (4) :567.)
[52] Cho S W, Shim G, Choi G S, Park C N, Yoob J H, Choi J.Hydrogen absorption-desorption properties of Ti0.32Cr0.43V0.25alloy[J].Journal of Alloys and Compounds, 2007, 430 (1-2) :136.
[53] Zheng X, Chen Y G, Wu C L, Tong G R, Zhou J J.Effect of water quench on V40Ti26Cr25Fe8hydrogen storage alloy[J].Journal of Functional Materials, 2009, 40 (8) :1319. (郑欣, 陈云贵, 吴朝玲, 童桂蓉, 周晶晶.淬火对V40Ti26Cr25Fe8贮氢合金的影响[J].功能材料, 2009, 40 (8) :1319.)
[54] Ulmer U, Asano K, Patyk A, Enoki H, Nakamur Y, Pohl A, Dittmeyer R, Fichtner M.Cost reduction possibilities of vanadium-based solid solutions——Microstructural, thermodynamic, cyclic and environmental effects of ferrovanadium substitution[J].Journal of Alloys and Compounds, 2015, 648:1024.
[55] Luo L S, Wu C L, Yang S, Zhou J J, Chen Y G, Yang F, Xu Y M, Liu P P.Decaying behaviors of V40 (Ti Cr) 51Fe8Mn hydrogen storage alloys with different particle sizes[J].Journal of Alloys and Compounds, 2015, 645:S179.
[56] Liu S P, Xu A L, Zhou S Q, Ren Q, Tian W G.Direct synthesis of the vanadium-based solid solution hydrogen storage alloy from the industrial V2O5[J].Materials Review, 2006, 20 (7) :144. (刘守平, 徐安莲, 周上祺, 任勤, 田卫国.用工业V2O5直接制备钒基固溶体贮氢合金[J].材料导报, 2006, 20 (7) :144.)