
Preparation of layered Li(Ni1/3Co1/3Mn1/3)O2 as positive material for lithium-ion secondary battery
HU Chuan-yue(胡传跃), WU Hong-tu(吴宏图), GUO Jun(郭 军),
WANG Xing-yan(汪形艳), YI Tao(易 涛)
Department of Chemistry and Material Science, Hunan Institute of Humanities Science and Technology, Loudi 417000, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The uniform layered Li(Ni1/3Co1/3Mn1/3)O2 cathode material for lithium-ion secondary batteries were synthesized by using (Ni1/3Co1/3Mn1/3)(OH)2 synthesized by a liquid phase co-precipitated method as precursors and with NiSO4, CoSO4, MnSO4 and NH3·H2O as raw materials. The influence of the preparation conditions such as precursors preparation, calcinations temperature and calcinations time on the structural and electrochemical properties of the Li(Ni1/3Co1/3Mn1/3)O2 were systematicelly studied. The result of XRD shows the I003/I104 value of the Li(Ni1/3Co1/3Mn1/3)O2 powder synthesized at 950 ℃ for 10 h is 1.26, which illustrates the well-ordered layer-structure. The average particle size of uniform Li(Ni1/3Co1/3Mn1/3)O2 powder is about 400 nm in diameter as observed by scanning electron microscopy. The first discharge capacity of Li(Ni1/3Co1/3Mn1/3)O2 electrode is 174.6 mA?h/g at 16 mA/g between 2.8 V and 4.5 V versus Li at room temperature, and the capacity retention is 95.2% of the initial discharge capacity after 50 cycles at 32 mA/g.
Key words:
Co-precipitation method; Li(Ni1/3Co1/3Mn1/3)O2; positive materials; lithium ion batteries;
1 Introduction
LiCoO2 is the mostly wide used positive material in commercial secondary batteries, as it is easy to prepare and has good recharge ability even at high rate performances. However, the toxicity and high cost of cobalt represent some of the problems of this material. Therefore, extensive research has been carried out to find alternative positive electrode materials such as LiFePO4[1-2] and LiMn2O4[3-4].
Recently, the layer-structural LiNi1/3Co1/3Mn1/3O2 compound has been considered as a promising candidate of next-generation cathode materials to replace LiCoO2 for rechargeable lithium ion batteries due to its large capacity and stable structure[5-10]. The layered Li(Ni1/3Co1/3Mn1/3)O2 material could be synthesized with various methods such as hydroxide co-precipitation route[5, 7, 11], carbonate co-precipitation route [12-13], oxalate co-precipitation route[14-15] and all solid state method[16]. The structure stability and the cycling performance of LiNi1/3Co1/3Mn1/3O2 could be improved by doping or substituting various ions such as Cr[17], Al[18-19], and F[15-18]. However, the preparation of the homogeneous Li(Ni1/3Co1/3Mn1/3)O2 is not easy. Selecting a suitable preparation route is important for obtaining phase-pure final products. In the present work, Li(Ni1/3Co1/3Mn1/3)O2 is synthesized by mixing the co- precipitation metal hydroxide (Ni1/3Co1/3Mn1/3)(OH)2 with 8% excess LiOH·H2O followed by heat-treatment; the influence of the preparation conditions of (Ni1/3Co1/3Mn1/3)(OH)2, the calcinations temperature and time on the structure and electrochemical properties of the (Ni1/3Co1/3Mn1/3)(OH)2 are studied in detail.
2 Experimental
In order to prepare a homogeneous precursor (Ni1/3Co1/3Mn1/3)(OH)2, we applied the hydroxide co-precipitation method as LEE et al reported[6]. An aqueous solution consisting of the NiSO4·6H2O, CoSO4·7H2O and MnSO4·H2O (cationic ratio of Ni?Co?Mn=1?1?1) with a concentration of 2 mol/L was precipitated by adding NaOH solution (aq.) of 2 mol/L and a desired amount of NH3·H2O solution (aq.) separately under argon atmosphere along with continued stirring. The solution was maintained at 60 ℃ for 12 h and the pH was controlled to 11-12. Then, the spherical precursor was filtered, washed and dried in a vacuum at 100 ℃ for 12 h. The obtained precursor powder was mixed with 8% excess LiOH·H2O (excess amount of Li salts was used to compensate for possible Li loss during the calcinations) thoroughly using a ball mill. The mixture was initially heated to 480 ℃ for 5 h and then calcined at 850-950 ℃ for 8-20 h in air to obtain Li(Ni1/3Co1/3Mn1/3)O2.
Powder X-ray diffraction was carried out using a Cu Kα radiation of Rigaku D/max 2550 diffractometer. Scanning electron micrographs(SEM) was used to characterize the images of the prepared powder.
The cathode was prepared by mixing the active material with carbon black and PVDF in a mass ratio of 90.5?3.5?6.0. The R2025 cells consisting of the cathode, the lithium foil using as the anode and the electrolyte of 1 mol/L LiPF6 ethylene carbonate(EC)-dimethylene carbonate(DMC)-ethylmethyl carbonate(EMC) (1?1?1 in mass) were assembled in an argon-filled glove box. Cycle tests were performed on the cells between 2.8 V and 4.5 V at 16 mA/g and 25 ℃ with a BK6061 Testing System (160 mA/g was assumed to be 1 C rate).
3 Results and discussion3.1 Phase structure of precursors
The obtained brown colored powder from the hydroxide co-precipitation process was used as precursors. Three precursors of (Ni1/3Co1/3Mn1/3)(OH)2 were prepared with n(NH3·H2O)?n(transition metal cation, TM=Ni, Co, Mn)=1.0?1, 1.5?1 and 2.0?1 (molar fraction), and were referred as S10, S15 and S20. Fig.1 shows the XRD patterns of the precursor (Ni1/3Co1/3Mn1/3)(OH)2. As being seen in Fig.1, the X-ray diffraction pattern of the precursor prepared with n(NH3·H2O)?n(TM cation)=
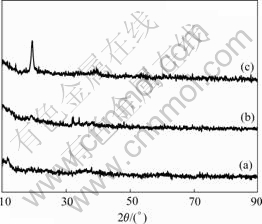
Fig.1 X-ray diffraction patterns of (Ni1/3Co1/3Mn1/3)(OH)2 prepared with different ratios of n(NH3·H2O)?n(Ni2+-Co2+- Mn2+): (a) 1.0?1; (b) 2.0; (c) 1.5
1.5?1 shows broad integrated lines and a high peaks at around 2θ=19?, which can be attributed to TM hydroxides. Fig.2 shows the scanning electron micrographs(SEM) images for the precursor S15. The results show an uniform hydroxide co-precipitation, (Ni1/3Co1/3Mn1/3)(OH)2, was successfully prepared.
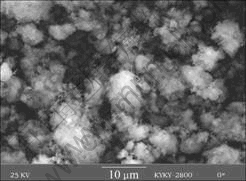
Fig.2 SEM image of precursor (Ni1/3Co1/3Mn1/3)(OH)2 synthesized with n(NH3·H2O)?n(Ni2+-Co2+-Mn2+)=1.5?1
3.2 Phase structure and electrochemical properties of Li(Ni1/3Co1/3Mn1/3)O2
Fig.3 illustrates the powder’s X-ray diffraction patterns of the Li(Ni1/3Co1/3Mn1/3)O2 samples prepared with different precursors at 900 ℃ for 10 h. All the samples can be indexed to the hexagonal α-NaFeO2 structure (space group: R![]() m). As seen in Fig.3, the splits in the (006)/(102) and (018)/(110) doublets can be observed in all the XRD patterns, respectively in the X-ray curve of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with precursor S15. The results show that the layered Li(Ni1/3Co1/3Mn1/3)O2 cathode materials have been successfully synthesized with all the precursors though the samples synthesized with precursors S14 and S20 show the bad (006)/(102) doublets. Some researchers[20]
m). As seen in Fig.3, the splits in the (006)/(102) and (018)/(110) doublets can be observed in all the XRD patterns, respectively in the X-ray curve of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with precursor S15. The results show that the layered Li(Ni1/3Co1/3Mn1/3)O2 cathode materials have been successfully synthesized with all the precursors though the samples synthesized with precursors S14 and S20 show the bad (006)/(102) doublets. Some researchers[20]
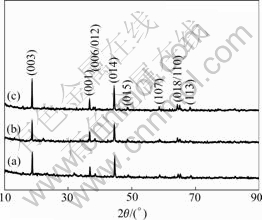
Fig.3 X-ray diffraction patterns of Li(Ni1/3Co1/3Mn1/3)O2 powder synthesized with different precursors at 900 ℃ for 10 h (Precursors (Ni1/3Co1/3Mn1/3)(OH)2 prepared with n(NH3·H2O)? n(Ni2+-Co2+-Mn2+)=1.0?1 (a), 2.0?1 (b) and 1.5?1 (c))
used the high integrated intensity ratio of the I003/I104 to indicate the cation mixing of the layered structure. Generally, when I003/I104>1.2, the cathode materials has a good layered structure due to the small cation mixing. The I003/I104 values of the three samples synthesized with precursor S10, S20 and S15 are 1.17, 1.09 and 1.2 in this work, which shows only the Li(Ni1/3Co1/3Mn1/3)O2 synthesized with precursors S15 at 900℃ for 10 h formed good layer structure.
In order to further study the influence of the preparation conditions of the precursors on the electrochemical performance of Li(Ni1/3Co1/3Mn1/3)O2, the tests cells were operated at a constant current density of 16 mA/g between 2.8 V and 4.5 V versus Li at room temperature. Fig.4 shows the initial charge/discharge curves of the Li(Ni1/3Co1/3Mn1/3)O2 powders synthesized with the three precursors at 900 ℃ for 10 h. As seen from Fig.4, the Li(Ni1/3Co1/3Mn1/3)O2 prepared with precursor S15 shows quite smooth and monotonous charge/discharge curves. On charging at 16 mA/g, voltage suddenly increases to about 3.75 V and then hold on 3.75-3.9 V until the charge capacity reaches about 115 mA?h/g, which can be attributed to the Ni2+/Ni4+ redox reaction occurred in this region[17]. On the further charging, the voltage curves monotonously increases to 4.5 V. Charge-discharge capacities and capacity retention ratios of all the samples are listed in Table 1. The Li(Ni1/3Co1/3Mn1/3)O2 prepared with precursor S15 shows the highest charge capacity of 190 mA?h/g and discharge capacity of 160.9 mA?h/g in the first cycle, for which the efficiency corresponds to 84.75%. The discharge capacities of 71.7 mA?h/g and 107.4 mA?h/g are obtained at the first cycle for Li(Ni1/3Co1/3Mn1/3)O2 prepared with the precursor S10 and precursor S20.
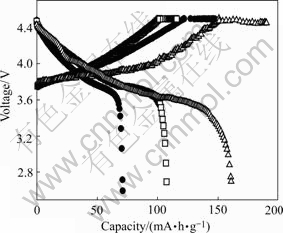
Fig.4 Initial charge-discharge profiles of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with different precursors at 900 ℃ for 10 h and 16 mA/g at 30 ℃ (Precursors prepared with n(NH3·H2O)? n(Ni2+-Co2+-Mn2+)=1.0?1 (a), 2.0?1 (b) and 1.5?1 (c))
Table 1 Charge-discharge capacity of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with various precursors at 900 ℃ for 10 h
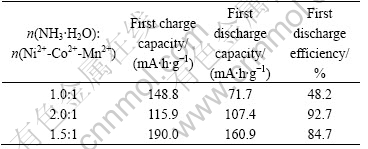
Fig.5 demonstrates the X-ray diffraction pattern of the Li(Ni1/3Co1/3Mn1/3)O2 powder synthesized with precursors S15 at various temperatures for 10 h. As seen in Fig.5, the splits in the (006)/(102) and (018)/(110) doublets are observed in all the XRD patterns. The I003/I104 values of the samples calcined at 850, 900 and 950 ℃ are calculated as 1.59, 1.2 and 1.26. The I003/I104 values of all samples synthesized with precursors S15 are larger than 1.2. The results show that the desirable good layer structure of Li(Ni1/3Co1/3Mn1/3)O2 prepared are obtained.
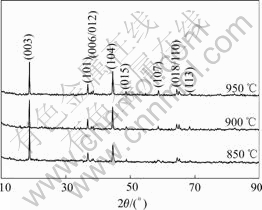
Fig.5 X-ray diffraction pattern of Li(Ni1/3Co1/3Mn1/3)O2 powder synthesized with n(NH3·H2O)?n(Ni2+-Co2+-Mn2+)=1.5?1 at various temperatures for 10 h
Fig.6 shows the initial charge/discharge curves of the Li(Ni1/3Co1/3Mn1/3)O2 powders synthesized with precursor S15 at various temperature for 10 h. The charge-discharge capacities of all the samples are listed in Table 2. The results show that the sample synthesized at 950 ℃ for 10 h delivers the highest discharge capacity of 174.6 mA?h/g and the largest discharge efficiency of 90.3% in the first charge- discharge cycle.
Fig.7 illustrates the X-ray diffraction pattern of the Li(Ni1/3Co1/3Mn1/3)O2 powder synthesized with the precursors S15 at 950 ℃ for various calcinations time. The I003/I104 values of the samples synthesized at 950 ℃ for 8, 10, 15 and 20 h are 1.23, 1.26, 1.16 and 1.24, which reveal that the powder of Li(Ni1/3Co1/3Mn1/3)O2 synthesized at 950 ℃ for 10 h has the better layer structure.
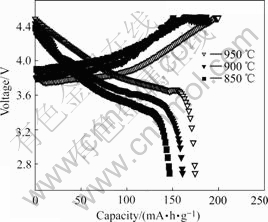
Fig.6 Initial charge-discharge profiles of Li(Ni1/3Co1/3Mn1/3)O2 (2.8-4.5 V) synthesized with n(NH3·H2O)? n(Ni2+-Co2+-Mn2+) =1.5?1 at various temperatures for 10 h and operated in 16 mA/g at 30 ℃
Table 2 Charge-discharge capacity of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with n(NH3·H2O)?n(Ni2+-Co2+-Mn2+)=1.5?1 at various temperatures for 10 h
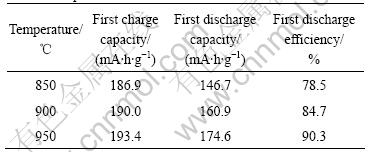
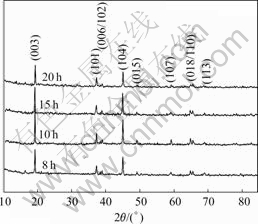
Fig.7 X-ray diffraction patterns of Li(Ni1/3Co1/3Mn1/3)O2 powder synthesized with n(NH3·H2O):n(Ni2+-Co2+-Mn2+)=1.5:1 at 950 ℃ for various time
The initial charge-discharge curves of the Li(Ni1/3Co1/3Mn1/3)O2 powder prepared with precursor S15 at 950 ℃ for various calcination time are shown in Fig.8. The charge-discharge capacities and retention ratios of all of the samples are tabulated in Table 3. The results show the sample synthesized at 950 ℃ for 10 h delivers the higher discharge capacity in the first cycle at 16 mA/g at room temperature. The discharge capacity of the Li(Ni1/3Co1/3Mn1/3)O2 in the first cycle decreases with the increase of calcination time at 950 ℃.
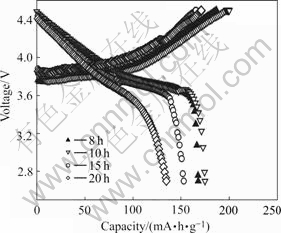
Fig.8 Initial charge-discharge profiles of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with n(NH3·H2O):n(Ni2+-Co2+-Mn2+)=1.5:1 at 950 ℃ for various time at 16 mA/g and 30 ℃
Table 3 First Charge-discharge capacity of Li(Ni1/3Co1/3- Mn1/3)O2 synthesized with n(NH3·H2O):n(Ni2+-Co2+-Mn2+)= 1.5:1 at 950 ℃ for different time
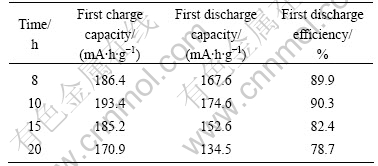
Fig.9 shows the discharge capacities as a function of cycle number of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with precursors S15 at 950 ℃ for various time at 32 mA/g. The results show that the reversible capacities of Li(Ni1/3Co1/3Mn1/3)O2 material after 50 cycles at 32 mA/g are 149.8 mA?h/g (89.4% of the initial discharge capacity), 166.6 mA·h/g (95.4% of the initial discharge capacity), 116.7 mA·h/g (76.5% of the initial discharge capacity)and 103 mA·h/g (76.6% of the initial discharge capacity) when calcinations time at 950 ℃ are 8, 10, 15 and 20 h, respectively. The results show that the Li(Ni1/3Co1/3Mn1/3)O2 powder synthesized with precursors S15 at 950 ℃ for 10 h delivers the better layered structure and the better electrochemical properties, such as the higher discharge capacity of 174.6 mA·h/g at the first cycle, the larger capacity retention of 95.4% after 50 cycles at 32 mA/g.

Fig.9 Discharge capacities as function of cycle number of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with n(NH3·H2O):n(Ni2+- Co2+-Mn2+)=1.5:1 at 950 ℃ for various time
Fig.10 shows the scanning electron micrographs (SEM) images for the Li(Ni1/3Co1/3Mn1/3)O2 synthesized with precursors S15 at 950 ℃ for 10 h. It can be seen the average particle size of Li(Ni1/3Co1/3Mn1/3)O2 is nearly 400 nm and homogenous Li(Ni1/3Co1/3Mn1/3)O2 are successfully synthesized.

Fig.10 SEM image of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with n(NH3·H2O)?n(Ni2+-Co2+-Mn2+)=1.5?1 at 950 ℃ for 10 h
4 ConclusionsA uniform co-precipitation melted hydroxide (Ni1/3Co1/3Mn1/3)(OH)2 is successfully prepared with n(NH3·H2O)?n(transition metal cation Ni2+-Co2+-Mn2+)= 1.5?1 of molar ratio. And a well-ordered layered Li(Ni1/3Co1/3Mn1/3)O2 powder is successfully synthesized by mixing uniform co-precipitation metal hydroxide (Ni1/3Co1/3Mn1/3)(OH)2 with 8% excess LiOH·H2O under different calcinations temperatures. The XRD results show that the I003/I104 values of all samples are larger than 1.2, which illustrates no undesirable cation mixing to be occurred. The results of electrochemical properties experiments show that the ideal synthesizing conditions are 950 ℃ for 10 h. The layered Li(Ni1/3Co1/3Mn1/3)O2 material prepared under the optimal conditions has the I003/I104 ratio of 1.26 and delivers the highest initial discharge capacity of 174.6 mA?h/g (2.8-4.5 V, 16 mA/g), the smallest irreversible capacity loss of 9.7%, and the highest capacity retention of 95.4% after 50 cycles at 32 mA/g.
References
[1] WANG Sai, QIU Wei-hua, GUAN Yun-long, YU Bi-tao, ZHAO Hai-lei, LIU Wei. Electrochemical characteristics of LiMxFe1-xPO4 cathode with LIBOB based electrolytes [J]. Electrochimica Acta, 2007, 52(15): 4907-4910.
[2] TENG T H, YANG Mu-rong, WU She-hung, CHIANG Yi-ping. Electrochemcial properties of LiFe0.9Mg0.1PO4/carbon cathode materials prepared by ultrasonic spray pyrolysis [J]. Solid State Communications, 2007, 142(7): 389-392.
[3] LI Chi-lin, FU Zheng-wen. All-solid-state rechargeable thin film lithium batteries with LixMn2O4 and LixMn2O4-0.5ZrO2 cathodes [J]. Electrochimica Acta, 2007, 52(20): 6155-6164.
[4] BAI Ying, WU Chuan, WU Feng, WANG Guo-qing. Cyclic voltammetry studies on 4 V and 5 V plateaus of non-stoichiometric spinel Li1+xMn2-yO4 [J]. Trans nonferrous Met Soc China, 2006, 16(2): 402-408.
[5] LUO Xu-fang, WANG Xian-you, LIAO Li, WANG Xi-min, GAMBOA S, SEBASTIAN P J. Effects of synthesis conditions on the structural and electrochemical properties of layered Li[Ni1/3Co1/3Mn1/3]O2 cathode material via the hydroxide co-precipitation method LIB SCITECH [J]. J Power Sources, 2006, 161(1): 601-605.
[6] LEE M H, KANG Y J, MYUNG S T, SUN Y K. Synthetic optimization of Li[Ni1/3Co1/3Mn1/3]O2 via co-precipitation [J]. Eelectrochimica Acta, 2004, 50(4): 939-948.
[7] HE Yu-shi, MA Zi-feng, LIAO Xiao-zhen, JIANG Yi. Synthesis and characterization of submicron-sized Li[Ni1/3Co1/3Mn1/3]O2 by a simple self-propagating solid-state metathesis method [J]. J Power Sources, 2007, 163(2): 1053-1058.
[8] LIU Zhao-lin, YU Ai-shui, LEE J Y. Synthesis and characterization of Li[Ni1-x-yCoxMny]O2 as the cathode materials of secondary lithium batteries [J]. J Power Sources, 1999, 81/82: 416-419.
[9] LIU Zhi-min, HU Guo-rong, PENG Zhong-dong, DENG Xin-rong, Liu Ye-xiang. Synthesis and characterization of layered Li(Ni1/3Mn1/3Co1/3)O2 cathode materials by spray-drying method [J]. Trans Nonferrous Met Soc China, 2007, 17(2): 291-295.
[10] YU Xiao-yuan, HU Guo-rong, PENG Zhong-dong, XEAO Jin, LIU Ye-xiang. Synthesis and electrochemical characterization of layered Li[Ni1/3Co1/3Mn1/3]O2 cathode material for Li-ion batteries [J]. Trans Nonferrous Met Soc China, 2005, 15(6): 1425-1428.
[11] MYUN S T, LEE M H, KOMABA S, KUMAGAI N, SUN Y K. Hydrothermal synthesis of layered Li[Ni1/3Co1/3Mn1/3]O2 as positive electrode material for lithium secondary battery [J]. Eelectrochimica Acta, 2005, 50(24): 4800-4806.
[12] LEE D K, PARK S H, AMINE K, BANG H J, PARAKASH J, SUN Y M. High capacity Li[Li0.2Ni0.2Mn0.6]O2 cathode materials via a carbonate co-precipitation method [J]. J Power Sources, 2006, 162(2): 1346-1350.
[13] ZHANG Yao, CAO Hui, ZHANG Jian, XIA Bao-jia. Synthesis of LiNi0.6Co0.2Mn0.2O2 cathode material by a carbonate co-precipitation method and its electrochemical characterization [J]. Solid State Ionics, 2006, 177: 3303-3307.
[14] CHO T H, SHIOSAKI Y, NOGUCHI H. Preparation and characterization of layered Li[Ni1/3Co1/3Mn1/3]O2 as cathode material by an oxalate co-precipitation method [J]. J Power Sources, 2006, 159(2): 1322-1327.
[15] HE Yu-shi, PEI Li, LIAO Xiao-zhen, MA Zi-feng. Synthesis of Li[Ni1/3Co1/3Mn1/3]O2-zFz cathode material from oxalate precursors for lithium ion battery [J]. J Fluorine Chemistry, 2007, 128(1): 139-143.
[16] YABUUCHI N, OHZUKU T. Electrochemical behaviors of Li[Ni1/3Co1/3Mn1/3]O2 in lithium batteries at elevated temperature [J]. J Power Sources, 2005, 146(1/2): 636-639.
[17] SUN Yu-cheng, XIA Yong-gao, NOGUCHI H. The improved physical and electrochemical performance of LiNi0.35Co0.3-xMn0.35O2 cathode materials by the Cr doping for lithium ion batteries [J]. J Power Sources, 2006, 159(2): 1377-1382.
[18] LIAO Li, WANG Xiang-you, LUO Xu-fang, WANG Xi-ming, GAMBOA S, SEBASTIAN P J. Synthesis and electrochemical properties of layered Li[Ni0.333Co0.333Mn0.293Al0.04]O2-zFz cathode materials prepared by the sol-gel method [J]. J Power Sources, 2006, 160(1): 657-661.
[19] LIU Dao-tan, WANG Zhao-xiang, CHEN Li-quan. Comparison of structure and electrochemistry of Al- and Fe-doped LiNi1/3Co1/3Mn1/3O2 [J]. Electrochimica Acta, 2006, 51(20): 4199-4203.
[20] LI De-cheng, MUTA T, ZHANG Lian-qi, YOSHIO M, NOGUCHI H. Effect of synthesis method on the electrochemical performance of LiNi1/3Co1/3Mn1/3O2 [J]. J Power Sources, 2004, 132: 150-155.
(Edited by LAI Hai-hui)
Corresponding author: HU Chuan-yue; Tel: +86-738-8325065; E-mail: huchuanyue@vip.sina.com


