- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 SEM images of as-cast alloys together with typical EDS spectra of sections A, B and C in Fig.1(c): (a) Mn0 alloy; (b) Mn0.2 alloy; (c) Mn0.4 alloy
- Fig.2 HRTEM micrographs and ED patterns of as-spun alloys (30 m/s): (a) Mn0 alloy; (b) Mn0.1 alloy; (c) Mn0.3 alloy
- Fig.3 XRD profiles of as-cast and spun Mn0 and Mn0.3 alloys: (a) Mn0 alloy; (b) Mn0.3 alloy
- Fig.4 HRTEM observation of Mg and MnNi phases in as-spun (30 m/s) Mn0.3 alloy together with typical EDS patterns of sections A and B: (a) MnNi phase; (b) Mg phase
- Fig.5 Hydrogen absorption kinetic curves of as-cast and spun alloys: (a) Mn0 alloy; (b) Mn0.3 alloy
- Fig.6 Evolutions of hydrogen absorption saturation ratio of alloys with spinning rate
- Fig.7 Semilogarithmic curves of anodic current vs time responses of Mn0 alloy electrodes in fully charged state
- Fig.8 Hydrogen desorption kinetic curves of as-cast and spun alloys: (a) Mn0 alloy; (b) Mn0.3 alloy
- Fig.9 Evolutions of values of alloys with spinning rate
J. Cent. South Univ. Technol. (2011) 18: 985-992
DOI: 10.1007/s11771-011-0791-6![]()
Hydriding and dehydriding kinetics of nanocrystalline and amorphous Mg2Ni1-xMnx (x=0-0.4) alloys prepared by melt spinning
ZHANG Yang-huan(张羊换)1, 2, QI Yan(祁焱)1, REN Hui-ping(任慧平)2,
MA Zhi-hong(马志鸿)1, 3, GUO Shi-hai(郭世海)1, ZHAO Dong-liang(赵栋梁)1
1. Department of Functional Material Research, Central Iron and Steel Research Institute, Beijing 100081, China;
2. Elected State Key Laboratory, Inner Mongolia University of Science and Technology, Baotou 014010, China;
3. Baotou Research Institute of Rare Earths, Baotou 014010, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract:
A partial substitution of Ni by Mn was implemented in order to improve the hydriding and dehydriding kinetics of the Mg2Ni-type alloys. The nanocrystalline and amorphous Mg2Ni-type Mg2Ni1-xMnx (x=0, 0.1, 0.2, 0.3, 0.4) alloys were synthesized by the melt-spinning technique. The structures of the as-cast and spun alloys were studied by X-ray diffractometry (XRD), scanning electron microscopy (SEM) and high resolution transmission electron microscopy (HRTEM). The hydrogen absorption and desorption kinetics of the alloys were measured with an automatically controlled Sieverts apparatus. The results show that the as-spun Mn-free alloy holds a typical nanocrystalline structure, whereas the as-spun alloys containing Mn display a nanocrystalline and amorphous structure, confirming that the substitution of Mn for Ni intensifies the glass forming ability of the Mg2Ni-type alloy. The hydrogen absorption and desorption capacities and kinetics of the alloys increase with increasing the spinning rate, for which the nanocrystalline and amorphous structure produced by the melt spinning is mainly responsible. The substitution of Mn for Ni evidently improves the hydrogen desorption performance. The hydrogen desorption capacities of the as-cast and spun alloys rise with the increase in the percentage of Mn substitution.
Key words:
Mg2Ni-type alloy; melt-spinning; structure; hydriding kinetics; dehydriding kinetics;
1 Introduction
The transportation applications based on hydrogen fuel cell technologies are being developed rapidly. Hydrogen is considered one of the potential fuels for the future because it can be directly mixed with air and supplied to the engine or it can also be introduced into the fuel cell to produce electric power [1]. The storage of hydrogen in the form of metal hydrides has attracted great attention in the last three decades. Especially, some systems based on Mg and Mg-based hydrides have been extensively studied because of great abundance, low density of Mg, and high hydrogen capacity of the hydrides, e.g. 7.6% (mass fraction) for MgH2, 3.6% for Mg2NiH4, 4.5% for Mg2CoH5 and 5.4% for Mg2FeH6. However, these kinds of the hydrides suffer from high thermodynamic stability, resulting in sluggish hydriding/ dehydriding kinetics. Therefore, a variety of attempts have been utilized to reduce their thermodynamic stability, including mechanical alloying (MA) [2], GPa hydrogen pressure method [3], melt spinning [4], gravity casting [5] , hydriding combustion synthesis [6], surface modification [7], alloying with other elements [8], adding catalysts [9] and others. Although a great prominent progress has been achieved in overcoming the above-mentioned drawbacks, the practical applications of the Mg and Mg-based hydrides are largely frustrated by their extremely poor hydriding and dehydriding kinetics at a temperature condition relevant to the practical operation of polymer electrolyte membrane fuel cell. The key challenge faced by researchers in this area still remains intact, reducing the thermodynamic stability of the hydrides.
High energy ball-milling, a very effective method for the fabrication of nanocrystalline and amorphous Mg and Mg-based alloys, is quite appropriate to solubilize the particular elements into MgH2 or Mg2NiH4 above the thermodynamic equilibrium limit, facilitating the destabilization of MgH2 or Mg2NiH4 [10]. However, the milled Mg and Mg-based alloys exhibit very poor hydrogen absorbing and desorbing stability on account of the evanishment of the metastable structures formed by ball milling during the multiple hydrogen absorbing and desorbing cycles [11]. The melt-spinning technique, contrarily, not only overcomes the aforementioned shortcoming but also prohibits the sharp degradation of the hydrogen absorbing and desorbing cyclic characteristics of Mg and Mg-based compounds [12]. Furthermore, the melt-spinning technique is an impactful method to yield a nanocrystalline structure and has been regarded to be the most appropriate for the mass-production of the nanocrystalline Mg-based alloys. It has also been testified that the nanocrystalline alloys produced by the melt-spinning method can exhibit excellent hydriding characteristics even at ambient temperature, which is similar to that of the alloys fabricated by the MA process. SPASSOV et al [13] have prepared Mg2(Ni,Y) hydrogen storage alloy with the composition of Mg63Ni30Y7 by rapid solidification, obtaining a maximum hydrogen absorption capacity of about 3.0% (mass fraction). HUANG et al [14] reported that the amorphous and the nanocrystalline Mg-based alloy (Mg60Ni25)90Nd10 prepared by the melt-spinning technique displays the highest hydrogen absorption capacity of 4.2% (mass fraction) H.
The objective of the present work is to synthesize the nanocrystalline and amorphous Mg-Ni-based Mg2Ni1-xMnx (x=0-0.4) alloys by the melt spinning technology and to examine their structures and hydriding and dehydriding kinetics.
2 Experimental
The selection of the compositions of the experimental alloys completely complies our group’s systemic research on the Mg2Ni-type alloy [15]. The nominal compositions of the experimental alloys were Mg2Ni1-xMnx (x=0, 0.1, 0.2, 0.3, 0.4). For convenience, the alloys were denoted with Mn content as Mn0, Mn0.1, Mn0.2, Mn0.3 and Mn0.4, respectively. The alloy ingots were prepared using a vacuum induction furnace in a helium atmosphere at a pressure of 0.04 MPa. A part of the as-cast alloys was re-melted and spun by melt-spinning with a rotating copper roller. The spinning rate was approximately expressed by the linear velocity of the copper roller because it was too difficult to measure the real spinning rate i.e. the cooling rate of the sample during spinning. The spinning rates used in the experiment were 15, 20, 25 and 30 m/s.
The phase structures of the as-cast and spun alloys were determined by XRD (D/max/2400). The diffraction, with the experimental parameters of 160 mA, 40 kV and 10 (°)/min was performed with Cu Kα1 radiation filtered by graphite. The morphologies of the as-cast alloys were examined by SEM (Philips QUANTA 400). The thin film samples of the as-spun alloys were prepared by the ion etching method for observing the morphology with HRTEM (JEM-2100F, operated at 200 kV), and for determining the crystalline state of the samples with electron diffraction (ED).
The hydrogen absorption and desorption kinetics of the alloys were monitored by an automatically controlled Sieverts apparatus. Prior to measuring the hydriding and dehydriding kinetics of the alloys, several hydrogen absorbing and desorbing cycles were carried out in order that the alloy can be completely activated. The hydrogen absorption was conducted at 1.5 MPa and 200 °C, and the hydrogen desorption at a pressure of 1×10-4 MPa and 200 °C.
The hydrogen diffusion coefficients in the alloys are calculated by the virtue of potential-step method. For the potentiostatic discharge, the test electrodes in the fully charged state were discharged at 500 mV potential steps for 3 500 s on an electrochemical workstation (PARSTAT 2273), using the CorrWare electrochemistry corrosion software.
3 Results and discussion
3.1 Microstructure characteristics
The SEM images of the as-cast alloys are demonstrated in Fig.1. It indicates that the substitution of Mn for Ni clearly changes the morphologies of the major phase Mg2Ni in the as-cast alloys from a typical dendritic structure to a feather-like one. The morphologies of the substituted alloys visibly exhibit the presence of some secondary phases. A rough conclusion can be obtained by the EDS spectra that the major phase of all the as-cast alloys retains invariably in the Mg2Ni phase (denoted as A) and Mn substitution leads to the formation of the secondary phases Mg (denoted as B) and MnNi (denoted as C).
The HRTEM micrographs and electron diffraction (ED) patterns of the as-spun (30 m/s) Mn0, Mn0.1 and Mn0.3 alloys are illustrated in Fig.2, showing a nanocrystalline microstructure for Mn0 alloy, and its ED pattern appears to be sharp multi-haloes, corresponding to a crystal structure. The morphologies of the as-spun Mn0.1 and Mn0.3 alloys exhibit a distinct feature of the nanocrystalline embedded in the amorphous matrix, and their ED rings consist of broad and dull halo, confirming the presence of an amorphous structure.
The XRD profiles of the as-cast and spun Mn0 and Mn0.3 alloys are shown in Fig.3. It is evident that no amorphous phase is detected in the as-spun Mn0 alloy, but an amorphous phase is clearly visible in the as-spun Mn0.3 alloy. Therefore, it can be concluded that the substitution of Mn for Ni intensifies the glass forming ability of the Mg2Ni-type alloy, which can be attributed to two reasons. First, the addition of the third element to Mg-Ni or Mg-Cu alloys can highly facilitate the glass-formation [16-17]. Second, the atomic radius of Mn is larger than that of Ni. The glass forming ability of the alloy is closely related to the difference of the atom radius of the alloy. A bigger difference of the atom radius predicates a higher glass forming ability of the alloy [18]. Figure 3 indicates that the substitution of Mn for Ni leads to the formation of secondary phases Mg and MnNi instead of modifying the major phase of Mg2Ni in the as-cast alloy, which further confirms the conclusion obtained by the EDS analysis. Listed in Table 1 are the lattice parameters, cell volume and full width at half maximum (FWHM) values of the main diffraction peaks of the as-cast and spun Mn0 and Mn0.3 alloys which were calculated by the software of Jade 6.0 based on the data in Fig.3. It demonstrates a notable enhancement in the FWHM values of the main diffraction peaks of the alloys caused by the melt spinning, which is undoubtedly attributed to the refined grains and the accumulated stress in grains conduced by the melt spinning. It is visible in Table 1 that the substitution of Mn for Ni causes not only the increase in the FWHM values of the main diffraction peaks but also an obvious enlargement in the lattice parameter and cell volume of the alloys owing to the larger atomic radius of Mn than that of Ni. The crystallite size, Dhkl, of the as-spun alloy has been calculated with the FWHM values of the broad diffraction peak (203) in Fig.1(b) by Scherer’s equation, to be in the range of 20-40 nm, consistent with the results reported by FRIEDLMEIER et al [19].
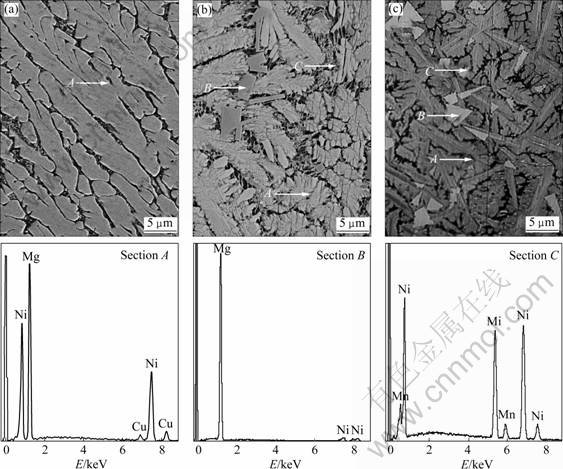
Fig.1 SEM images of as-cast alloys together with typical EDS spectra of sections A, B and C in Fig.1(c): (a) Mn0 alloy; (b) Mn0.2 alloy; (c) Mn0.4 alloy
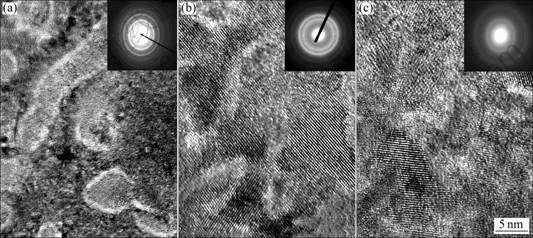
Fig.2 HRTEM micrographs and ED patterns of as-spun alloys (30 m/s): (a) Mn0 alloy; (b) Mn0.1 alloy; (c) Mn0.3 alloy
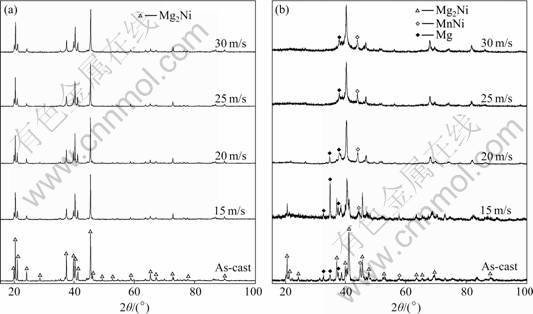
Fig.3 XRD profiles of as-cast and spun Mn0 and Mn0.3 alloys: (a) Mn0 alloy; (b) Mn0.3 alloy
Table 1 Lattice parameters, cell volumes and FWHM values of diffraction peaks of alloys

As analyzed by XRD, a multiphase structure in the as-spun alloy is detected by HRTEM. A grey block (denoted as A) and a white block with a regular polygon morphology (denoted as B) can been seen in Fig.4, which are confirmed to be MnNi and Mg phases analyzed by EDS.
3.2 Hydriding and dehydriding kinetics
The hydriding process was carried out under 1.5 MPa hydrogen pressure (in fact, this is initial pressure of hydriding process) at 200 °C, and the dehydriding process was carried out in a pressure of 1×10-4 MPa at 200 °C, too.
The hydrogen absorption kinetic curves of the as-cast and spun Mn0 and Mn0.3 alloys are depicted in Fig.5. It is evident that the hydrogen absorption kinetics is extremely fast so that the alloys absorbed more than 90% of their hydrogen capacities within the first 5 min. Furthermore, it is derived by comparing Figs.5 (a) with (b) that the hydrogen absorption capacity and kinetics of all the as-spun nanocrystalline Mg2Ni-type alloys studied are superior to those of the conventional polycrystalline materials with similar compositions.
The hydrogen absorption kinetics of the alloy is signified by the hydrogen absorption saturation ratio ![]() defined as
defined as ![]() where
where ![]() is the hydrogen absorption capacity in 100 min and
is the hydrogen absorption capacity in 100 min and ![]() is the hydrogen absorption capacity in time t, respectively. Apparently, for a fixed time t, a larger saturation ratio
is the hydrogen absorption capacity in time t, respectively. Apparently, for a fixed time t, a larger saturation ratio ![]() means better hydrogen absorption kinetics. The hydrogen absorption saturation ratio (
means better hydrogen absorption kinetics. The hydrogen absorption saturation ratio (![]() ) (t=5 min) of the alloys as a function of the spinning rate is presented in Fig.6. The figure indicates that
) (t=5 min) of the alloys as a function of the spinning rate is presented in Fig.6. The figure indicates that ![]() values of the alloys clearly mount up with increasing the spinning rate. As the spinning rate grows from 0 (as-cast was defined as the spinning rate of 0 m/s) to 30 m/s, the
values of the alloys clearly mount up with increasing the spinning rate. As the spinning rate grows from 0 (as-cast was defined as the spinning rate of 0 m/s) to 30 m/s, the ![]() value increases from 49.01% to 95.02% for the Mn0 alloy, and from 78.14% to 97.9% for the Mn0.3 alloy. The observed essential differences in the hydrogen absorption saturation ratio (
value increases from 49.01% to 95.02% for the Mn0 alloy, and from 78.14% to 97.9% for the Mn0.3 alloy. The observed essential differences in the hydrogen absorption saturation ratio (![]() ) of the as-spun nanocrystalline and amorphous alloys studied most probably have to be associated with the composition of the alloys as well as with the differences in their microstructure due to the different spinning rates. It has been documented by HONG et al [20] that the hydrogen diffusivity in Mg2NiH4 depends on its preparation process in virtue of the fact that the structures of the alloys are chiefly predominated by their fabrication technology. The improved hydrogenation characteristics can be explained by the enhanced hydrogen diffusivity in the amorphous and nanocrystalline microstructures as the amorphous phase around the nanocrystalline leads to an easier access of hydrogen to the nanograins, avoiding the long-range diffusion of hydrogen through an already formed hydride, which is often the slowest stage of absorption. Upon refining the microstructure, a lot of new crystallites and grain boundaries evolve, which may act as fast diffusion paths for hydrogen absorption. In order to determine the influence of the spinning rate on H diffusion ability in the alloy electrode, the H diffusion coefficients in selected as-cast and spun Mn0 alloy were measured using the potential step technique. A potential step of +500 mV versus the stabilized open circuit potential of the fully charged electrode was applied and the decrease in discharge current was monitored as a function of time. Figure 7 shows the semilograrithmic curves of anodic current versus working duration of the as-cast and spun Mn0 alloy electrode. The diffusion coefficient D of the hydrogen atoms in the bulk of the alloy can be calculated through the slope of the linear region of the corresponding plots according to the following formula [21]:
) of the as-spun nanocrystalline and amorphous alloys studied most probably have to be associated with the composition of the alloys as well as with the differences in their microstructure due to the different spinning rates. It has been documented by HONG et al [20] that the hydrogen diffusivity in Mg2NiH4 depends on its preparation process in virtue of the fact that the structures of the alloys are chiefly predominated by their fabrication technology. The improved hydrogenation characteristics can be explained by the enhanced hydrogen diffusivity in the amorphous and nanocrystalline microstructures as the amorphous phase around the nanocrystalline leads to an easier access of hydrogen to the nanograins, avoiding the long-range diffusion of hydrogen through an already formed hydride, which is often the slowest stage of absorption. Upon refining the microstructure, a lot of new crystallites and grain boundaries evolve, which may act as fast diffusion paths for hydrogen absorption. In order to determine the influence of the spinning rate on H diffusion ability in the alloy electrode, the H diffusion coefficients in selected as-cast and spun Mn0 alloy were measured using the potential step technique. A potential step of +500 mV versus the stabilized open circuit potential of the fully charged electrode was applied and the decrease in discharge current was monitored as a function of time. Figure 7 shows the semilograrithmic curves of anodic current versus working duration of the as-cast and spun Mn0 alloy electrode. The diffusion coefficient D of the hydrogen atoms in the bulk of the alloy can be calculated through the slope of the linear region of the corresponding plots according to the following formula [21]:
![]() (1)
(1)
![]() (2)
(2)
where J is the diffusion current density (A/g), D is the hydrogen diffusion coefficient (cm2/s), C0 is the initial hydrogen concentration in the bulk of the alloy (mol/cm3), Cs is the hydrogen concentration on the surface of the alloy particles (mol/cm3), a is the alloy particle radius (cm), d is the density of the hydrogen storage alloy (g/cm3), and t is the discharge time (s). The D values calculated by Eq.(2) are also presented in Fig.7. It can be seen that the melt spinning inflicts a visible effect on H diffusion in the alloy. As the spinning rate grows from 0 to 30 m/s, the hydrogen diffusion D of the Mn0 alloy always increases from 1.350×10-11 cm2/s to 2.367×10-11 cm2/s. SPASSOV and K?STER [22] also confirmed that the melt spinning could significantly improve the hydrogen absorption performance of the Mg-based alloy, and obtains the maximum hydrogen capacity of 4.0% H (mass fraction) for the as-spun Mg75Ni20Mm5 (Mm=Ce, La-rich mischmetal) alloy. The benefaction of Mn substitution on the hydrogen absorption capacity and kinetics of the alloy are attributed to the increased cell volume and the refined grain caused by Mn substitution. The enlargement in the cell volume is highly beneficial to the hydrogen absorption capacity, since the grain boundary possesses the capability of the largest hydrogen absorption [23].
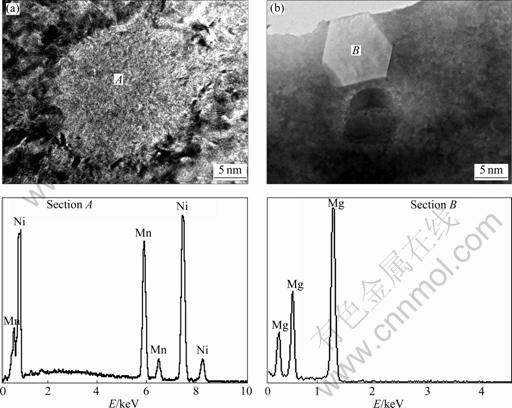
Fig.4 HRTEM observation of Mg and MnNi phases in as-spun (30 m/s) Mn0.3 alloy together with typical EDS patterns of sections A and B: (a) MnNi phase; (b) Mg phase
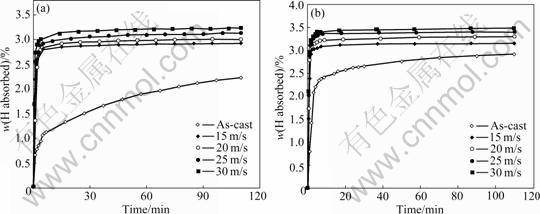
Fig.5 Hydrogen absorption kinetic curves of as-cast and spun alloys: (a) Mn0 alloy; (b) Mn0.3 alloy
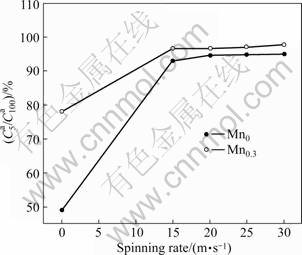
Fig.6 Evolutions of hydrogen absorption saturation ratio ![]() of alloys with spinning rate
of alloys with spinning rate
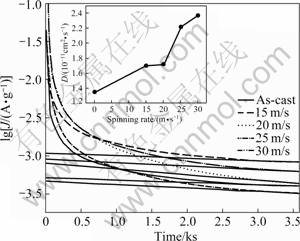
Fig.7 Semilogarithmic curves of anodic current vs time responses of Mn0 alloy electrodes in fully charged state
The hydrogen desorption kinetic curves of the as-cast and spun Mn0 and Mn0.3 alloys are plotted in Fig.8. An important feature of the dehydrogenation process in the alloys is very fast initial hydrogen desorption, followed by a slack increase in the amount of hydrogen absorbed.
Similarly, the hydrogen desorption kinetics of the alloy is signified by the hydrogen desorption ratio (![]() ), defined as
), defined as ![]() where
where ![]() is the hydrogen absorption capacity in 100 min and
is the hydrogen absorption capacity in 100 min and ![]() is the hydrogen absorption capacity in time t, respectively. Figure 9 displays the relationship between the hydrogen desorption ratio (
is the hydrogen absorption capacity in time t, respectively. Figure 9 displays the relationship between the hydrogen desorption ratio (![]() ) (t = 10 min) of the alloys with the spinning rate. It can be seen in Fig.9 that the substitution of Mn for Ni remarkably enhances the
) (t = 10 min) of the alloys with the spinning rate. It can be seen in Fig.9 that the substitution of Mn for Ni remarkably enhances the ![]() values of the alloys, suggesting that such substitution facilitates the hydrogen desorption of Mg2Ni-type alloys. Figure 9 exhibits that the
values of the alloys, suggesting that such substitution facilitates the hydrogen desorption of Mg2Ni-type alloys. Figure 9 exhibits that the ![]() values of the alloys evidently increase with increasing the spinning rate. With the increase in the spinning rate from 0 to 30 m/s, the
values of the alloys evidently increase with increasing the spinning rate. With the increase in the spinning rate from 0 to 30 m/s, the ![]() value increases from 4.47% to 18.82% for the Mn0 alloy, and from 17.93% to 41.58% for the Mn0.3 alloy. The improved dehydrogenation kinetics by Mn substitution is ascribed to two reasons. On one hand, the substitution of Mn for Ni notably intensifies the glass forming ability of the Mg2Ni-type alloys because amorphous Mg2Ni shows an excellent hydrogen desorption capability. On the other hand, such substitution decreases the stability of the hydride and makes the desorption reaction easier [24]. The as-spun alloys exhibit higher H-absorption capacity and faster hydriding/dehydriding kinetics than the as-cast ones. The viewed essential differences in the hydriding/ dehydriding kinetics of the as-cast and spun alloys studied most probably have to be associated with their microstructure due to the different spinning rates. It was reported that the high surface to volume ratios, i.e. high specific surface area, and the presence of large number of grain boundaries in nanocrystalline and amorphous alloys enhance the kinetics of hydrogen absorption/ desorption [13].
value increases from 4.47% to 18.82% for the Mn0 alloy, and from 17.93% to 41.58% for the Mn0.3 alloy. The improved dehydrogenation kinetics by Mn substitution is ascribed to two reasons. On one hand, the substitution of Mn for Ni notably intensifies the glass forming ability of the Mg2Ni-type alloys because amorphous Mg2Ni shows an excellent hydrogen desorption capability. On the other hand, such substitution decreases the stability of the hydride and makes the desorption reaction easier [24]. The as-spun alloys exhibit higher H-absorption capacity and faster hydriding/dehydriding kinetics than the as-cast ones. The viewed essential differences in the hydriding/ dehydriding kinetics of the as-cast and spun alloys studied most probably have to be associated with their microstructure due to the different spinning rates. It was reported that the high surface to volume ratios, i.e. high specific surface area, and the presence of large number of grain boundaries in nanocrystalline and amorphous alloys enhance the kinetics of hydrogen absorption/ desorption [13].
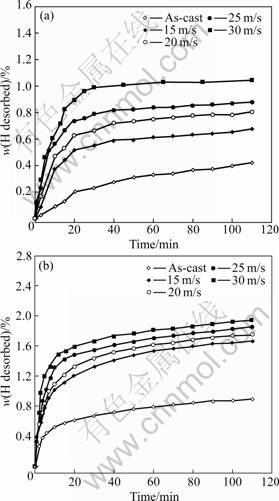
Fig.8 Hydrogen desorption kinetic curves of as-cast and spun alloys: (a) Mn0 alloy; (b) Mn0.3 alloy
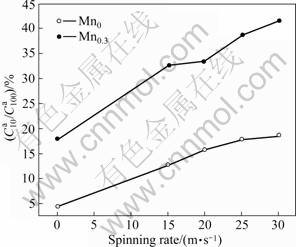
Fig.9 Evolutions of ![]() values of alloys with spinning rate
values of alloys with spinning rate
4 Conclusions
1) The investigation on the structures of the as-cast and spun Mg2Ni1-xMnx (x=0, 0.1, 0.2, 0.3, 0.4) alloys shows that no amorphous phase is detected in the as-spun Mn-free alloy, but the structure of the as-spun alloys substituted by Mn displays a feature of the nanocrystalline embedded in the amorphous matrix, confirming that the substitution of Mn for Ni enhances the glass forming ability of the Mg2Ni-type alloys. The Mn substitution changes the morphology of major phase Mg2Ni in the as-cast alloy from typical dendritic structure to feather-like one, and it leads to the formation of Mg and MnNi phases.
2) The melt spinning significantly improves the hydriding and dehydriding properties of the alloys. The hydriding and dehydriding capacities and rates of the alloy markedly rise with increasing the spinning rate, which is mainly attributed to the nanocrystalline and amorphous structure produced by the melt spinning.
3) The substitution of Mn for Ni significantly improves the hydrogen desorption capacity and kinetics of the as-cast and spun alloys, for which the decreased stability of the hydride and the intensified glass forming ability produced by Mn substitution is mainly responsible.
References
[1] ROSS D K. Hydrogen storage: The major technological barrier to the development of hydrogen fuel cell cars [J]. Vacuum, 2006, 80: 1084-1089.
[2] WOO J H, LEE K S. Electrode characteristics of nanostructured Mg2Ni-type alloys prepared by mechanical alloying [J]. J Electrochem Soc, 1999, 146(3): 819-823.
[3] KYOI D, SAKAI T, KITMURA N, UEDA A, TANASE S. Synthesis of FCC Mg-Ta hydrides using GPa hydrogen pressure method and their hydrogen-desorption properties [J]. Journal of Alloys and Compounds, 2008, 463: 306-310.
[4] PALADE P, SARTORI S, MADDANENA A, PRINCIPI G, LO RUSSO S, LAZARESCU M, SCHINTEIE G, KUNCSER V, FILOTI G. Hydrogen storage in Mg-Ni-Fe compounds prepared by melt spinning and ball milling [J]. Journal of Alloys and Compounds, 2006, 415: 170-176.
[5] SONG M Y, YIM C D, BAE J S, MUMMD D R, HONG S H. Preparation by gravity casting and hydrogen-storage properties of Mg-23.5 wt.%Ni-(5, 10 and 15 wt.%)La [J]. Journal of Alloys and Compounds, 2008, 463: 143-147.
[6] LIU X F, ZHU Y F, LI L Q. Structure and hydrogenation properties of nanocrystalline Mg2Ni prepared by hydriding combustion synthesis and mechanical milling [J]. Journal of Alloys and Compounds, 2008, 455: 197-202.
[7] LIU F J, SUDA S. A method for improving the long-term storability of hydriding alloys by air water exposure [J]. Journal of Alloys and Compounds, 1995, 231: 742-750.
[8] CZUJKO T, VARIN R A, CHIU C, WRONSKI Z. Investigation of the hydrogen desorption properties of Mg+10wt.%X (X=V, Y, Zr) submicrocrystalline composites [J]. Journal of Alloys and Compounds, 2006, 414: 240-247.
[9] SAKINTUNA B, LAMARI-DARKRIM F, HIRSCHER M. Metal hydride materials for solid hydrogen storage: A review [J]. International Journal of Hydrogen Energy, 2007, 32: 1121-1140.
[10] LIANG G Y. Synthesis and hydrogen storage properties of Mg-based alloys [J]. Journal of Alloys and Compounds, 2004, 370: 123-128.
[11] SONG M Y, KWON S N, BAE J S, HONG S H. Hydrogen-storage properties of Mg-23.5Ni-(0 and 5)Cu prepared by melt spinning and crystallization heat treatment [J]. International Journal of Hydrogen Energy, 2008, 33: 1711-1718.
[12] SAVYAK M, HIRNYJ S, BAUER H D, UHLEMANN M, ECKERT J, SCHULTZ L, GEBERT A. Electrochemical hydrogenation of Mg65Cu25Y10 metallic glass [J]. Journal of Alloys and Compounds, 2004, 64: 229-237.
[13] SPASSOV T, K?STR U. Thermal stability and hydriding properties of nanocrystalline melt-spun Mg63Ni30Y7 alloy [J]. Journal of Alloys and Compounds, 1998, 279: 279-286.
[14] HUANG L J, LIANG G Y, SUN Z B, WU D C. Electrode properties of melt-spun Mg-Ni-Nd amorphous alloys [J]. Journal of Power Sources, 2006, 160: 684-687.
[15] ZHANG Y H, LIU Z C, LI B W, MA Z H, GUO S H, WANG X L. Structure and electrochemical performances of Mg2Ni1-xMnx (x=0-0.4) electrode alloys prepared by melt spinning [J]. Electrochimica Acta, 2010, 56: 427-434.
[16] YAMAURA S I, KIM H Y, KIMURA H, INOUE A, ARATA Y. Thermal stabilities and discharge capacities of melt-spun Mg-Ni-based amorphous alloys [J]. Journal of Alloys and Compounds, 2002, 339: 230-235.
[17] INOUE A, MASUMOTO T. Mg-based amorphous alloys [J]. Material Science and Engineering A, 1993, 173: 1-8.
[18] CHEN H S. Thermodynamic considerations on the formation and stability of metallic glasses [J]. Acta Materialia, 1974, 22(12): 1505-1511.
[19] FRIEDLMEIER G, ARAKAWA M, HIRAIA T, AKIBA E. Preparation and structural, thermal and hydriding characteristics of melt-spun Mg-Ni alloys [J]. Journal of Alloys and Compounds, 1999, 292: 107-117.
[20] HONG S H, NA Y S, KWON S N, BAE J S, SONG M Y. Preparation and hydrogen-storage properties of 90(Mg-23.5Ni)- 10Ta2O5 alloy by melt spinning and oxide addition [J]. Journal of Alloys and Compounds, 2008, 465: 512-516.
[21] ZHONG G, POPOV B N, WHITE R E. Electrochemical determination of the diffusion coefficient of hydrogen through an LaNi4.25Al0.75 electrode in alkaline aqueous solution [J]. Journal of the Electrochemical Society, 1995, 142: 2695-2698.
[22] SPASSOV T, K?STER U. Hydrogenation of amorphous and nanocrystalline Mg-based alloys [J]. Journal of Alloys and Compounds, 1999, 287: 243-250.
[23] ORIMO S, FUJII H. Materials science of Mg-Ni-based new hydrides [J]. Applied Physics A, 2001, 72: 167-186.
[24] TAKAHASHI Y, YUKAWA H, MORINAGA M. Alloying effects on the electronic structure of Mg2Ni intermetallic hydride [J]. Journal of Alloys and Compounds, 1996, 242: 98-107.
(Edited by PENG Chao-qun)
Foundation item: Projects(50871050, 50961001) supported by the National Natural Science Foundation of China; Project(2010ZD05) supported by the Natural Science Foundation of Inner Mongolia, China; Project(NJzy08071) supported by the High Education Science Research Program of Inner Mongolia, China
Received date: 2010-06-29; Accepted date: 2010-12-28
Corresponding author: ZHANG Yang-huan, Professor, PhD; Tel: +86-10-62187570; E-mail: zyh59@yahoo.com.cn
- Hydriding and dehydriding kinetics of nanocrystalline and amorphous Mg2Ni1-xMnx (x=0-0.4) alloys prepared by melt spinning


