
Synthesis of photocatalytic TiO2 nanoparticles at low cost
LIU Qing-ju(柳清菊)1, ZHOU Mi(周 密)2, LIU Qiang(刘 强)1,
ZHANG Jin(张 瑾)1, ZHU Zhong-qi(朱忠其)1
1. Department of Material Science and Engineering, Yunnan University, Kunming 650091,China;
2. Joinyou Fengyu Science and Technology Company Limited, Kunming 650101,China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Anatase TiO2 nanoparticles were synthesized by using HTiO3 as raw material and adopting TiOSO4 solution neutralizing process. The results show that the hydrolytic reaction temperature and time, molar ratio of urea to Ti precursors (R), volume ratio of distilled water to the TiOSO4 solution (F) determine the yield of TiO2; the average crystalline size of TiO2 nanoparticles can be controlled through the sintering temperature and the optimum temperature is about 600 ℃; the TiO2 particles have good photocatalytic activity for decolorization of methyl orange.
Key words:
synthesis; hydrolytic efficiency; particle size; photocatalytic activity; TiO2; TiOSO4;
1 Introduction
Anatase TiO2 has been widely used for different environmental applications, such as photocatalytic purification and treatment of water and air[1,2], sterilization, deodorization[3-7] and water photosplitting into H2 and O2[8]. The photocatalytic activity of TiO2 nanoparticles is strongly dependent on the size and the extensive application is up to the production cost. In order to get TiO2 nanoparticles, several preparation methods have been reported, such as sol-gel method, chemical vapour deposition, precipitation and mechanical alloying[9]. However, the preparation of TiO2 nanoparticles by using cheap HTiO3 as raw material and adopting TiOSO4 solution neutralizing process has not been reported yet. This work is devoted to study the influence of different parameters such as reaction and sintering temperature, quantity of urea and water, on the preparation of TiO2. The nanoparticles were characterized with an X-ray diffractometer(XRD), the photocatalytic activity of these materials was evaluated using aqueous methyl orange as pollutant.
2 Experimental
The preparation of TiO2 nanoparticles was described as follows: first, HTiO3 was dissolved in H2SO4 solution and TiOSO4 was generated, then distilled water was added to the solution at volume ratios of distilled water to TiOSO4 solution(F) from 10 to 100. The mixtures were stirred at room temperature for about 2 h, and then urea ((NH2)2CO) was directly added to the aqueous solution at molar ratios of urea to Ti precursors from 0.5 to 2.5, stirred vigorously at 70-90 ℃, and the hydrolytic reaction took place and the precipitation TiO(OH)2 was produced slowly. After a certain time, the precipitation TiO(OH)2 was separated and sintered at 500-700 ℃ for 2 h in air using an electric furnace.
The crystalline phase was determined with XRD (type D/maxⅢ, Japan) using Cu Kα radiation. The acce- lerating voltage was 35 kV and the applied current was 25 mA. The average crystallite size of TiO2 particles was determined according to the Scherrer equation using the full width of half maximum data.
The photocatalytic activities of TiO2 nanoparticles were examined by using methyl orange degradation decolorization described below. The same mass TiO2 particles and TiO2-P25 (purity 99.8%, Degussa Corporation) were added in aqueous methyl orange with an initial content of 25 mg/L in quartz cells, respectively. An ultra-violet lamp (30 W) was used as the light source. The average intensity of UV radiation was 78 μW/cm2 measured with an UV irradiance meter. Its wavelength range was 320-400 nm, and peak wavelength was 360 nm. The density of methyl orange was determined with a spectrometer (type UV-VIS8500, China).
The yield of TiO(OH)2 from TiOSO4 in the solution was determined by the hydrolytic efficiency η. The larger the η, the higher the TiO2 yield. Absorbance A can be measured according to the following formular:
![]() (1)
(1)
where Ainitial is the absorbance of TiOSO4 solution before adding urea, Aend is the absorbance of the solution after adding urea and finishing the reaction. Both the above solutions contain 50%(volume fraction) H2O2. The absorbance was determined with an UV-Vis spectrophotometer (UV-VIS8500, China) at 411 nm.
3 Results and discussionThe hydrolytic reaction in TiOSO4 aqueous solutions containing urea generated at 70, 80 and 90 ℃, respectively. The effect of the temperature on the hydrolytic efficiency η is shown in Fig.1. It is found that the hydrolytic efficiency is too low at 70 ℃, but η achieves 97% at 90 ℃. Therefore, the hydrolytic reaction temperature should be higher than 90 ℃. However, the temperature has little influence on the average crystallite size of TiO2 particles. At 70, 80 and 90 ℃, the corresponding crystallite size (sintered at 550 ℃ for 2 h) is 19.1, 14.9 and 16.5 nm, respectively.

Fig.1 Effect of temperature on hydrolytic efficiency
Fig.2 shows the hydrolytic efficiency and average crystalline size at different R. It can be seen that the hydrolytic efficiencies are all above 95% and the crystalline sizes are all less than 20 nm while R varies from 0.5 to 2.5. The minimal size of TiO2 crystalline can be obtained at R=1.5.
The change of the crystalline size with R can be explained in terms of two contrary factors: the precipitant’s increasing and decreasing. On the one hand, the precipitant (NH4OH) increases with the increase of urea ((NH2)2CO), resulting in TiO2 crystalline growing larger, on the other hand, however, if there is too much urea, the density of precipitant (NH4OH) generated by the urea hydrolytic reaction decreases, which causes the inhibition of TiO2 crystalline growing. In this work R is around 1.5, which makes the crystalline size turn from decrease to increase.
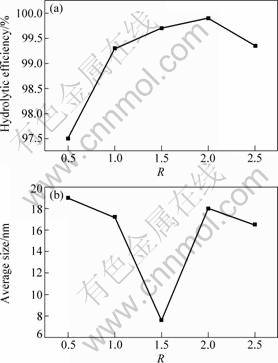
Fig.2 Hydrolytic efficiency (a) and average crystalline size (b) at different molar ratios of urea to Ti precursors (R)
Fig.3 shows the hydrolytic efficiency and average crystalline size at different F. It is observed that the hydrolytic efficiencies rise from below 10% to over 90%,
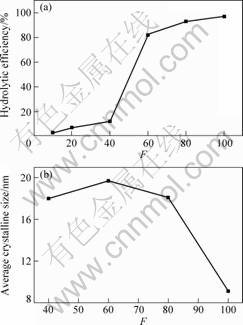
Fig.3 Hydrolytic efficiency (a) and average crystalline size (b) at different volume ratios of distilled water to TiOSO4 solution (F)
but the sizes reduce from about 20 nm to below 10 nm. It is due to that more NH4OH yield and TiO2 crystalline particles can not easily grow bigger in relatively dilute TiOSO4 aqueous solution containing urea.
Fig.4 shows the hydrolytic efficiency after different reaction time. It indicates that the hydrolytic efficiencies increase obviously in the first 2 h and up to 95% after 3 h. Therefore, the reaction time should be longer than 3 h to improve the production of TiO2 nanoparticles.
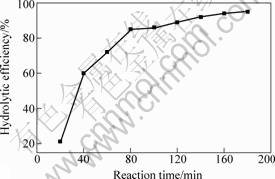
Fig.4 Effect of reaction time on hydrolytic efficiency
Fig.5 shows the average crystalline sizes of TiO2 sintered for 2 h at 500, 550, 600, 650 and 700 ℃, respectively. It can be seen that the average size increases with the increase of the sintering temperature. The XRD pattern for the above TiO2 powders is confirmed that the crystal form of TiO2 sintered at 500 ℃ is the mixture of amorphous and anatase TiO2, and the crystal form sintered at 550 and 650 ℃ are all of anatase TiO2. However, the crystal form sintered at 700 ℃ is the mixture of anatase and rutile TiO2. Because the sulfur in the precipitation can be eliminated at 600 ℃ and anatase TiO2 assumes good photocatalytic activity, so the sintering temperature is chosen as 600 ℃.
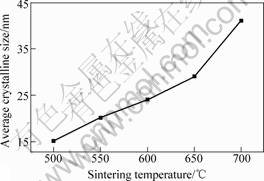
Fig.5 Effect of sintering temperature on average crystalline size
Fig.6 shows the results of photocatalytic decolorization of methyl orange by the TiO2 particles (the hydrolytic reaction temperature is 90 ℃, R =1.5,
F=80) and TiO2-P25, respectively. After illuminating with ultra-violet light for 10 min, the decolorization rate of methyl orange by the TiO2 and TiO2-P25 is 42.8% and 31.6%, respectively. After illuminating with ultra-violet light for 60 min, both of the decolorization rates are above 98%.
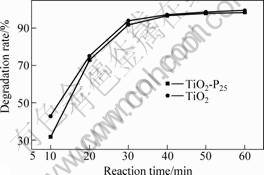
Fig.6 Decolorization of methyl orange degraded by TiO2 and TiO2-P25
4 ConclusionsTiO2 nanoparticles are prepared by using cheap HTiO3 as raw material and adopting TiOSO4 solution neutralizing process. The yield of TiO2 is determined by the hydrolytic reaction temperature and time, the molar ratio of urea to Ti precursors (R) and volume ratio of distilled water to the TiOSO4 solution (F). The average crystalline size of TiO2 nanoparticles can be controlled by adjusting R, F and the sintering temperature. The as-prepared TiO2 particles have good photocatalytic activity for decolorization of methyl orange.
References[1] FERNA’NDEZ P, BLANCO J, SICHEL C, MALATO S. Water disinfection by solar photocatalysis using compound parabolic collectors[J]. Catalysis Today, 2005, 101: 345-352.
[2] NONAMI T, HASE H, FUNAKOSHI K. Apatite-coated titanium dioxide photocatalyst for air purification[J]. Catalysis Today, 2004, 96: 113-118.
[3] ZULKARNAIN Z, LEE K H, MOHD Z H. Removal of dyes using immobilized titanium dioxide illuminated by fluorescent lamps[J]. J Hazardous Materials B, 2005, 125: 113-120.
[4] DUNLOP P S M, BYRNE J A, MANGA N, EGGINS B R. The photocatalytic removal of bacterial pollutants from drinking water[J]. J Photochemistry and Photobiology A: Chemistry, 2002, 148: 355-363.
[5] JUNGWOO M, CHANG Y Y, KYUNG W C, MIN S K, JONGHEOP Y. Photocatalytic activation of TiO2 under visible light using acid red 44[J]. Catalysis Today, 2003, 87: 77-86.
[6] TERUHISA O, MIYAKO A, TSUTOMU U, KEISUKE A, TAKAHIRO M, MICHIO M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light[J]. Applied Catalysis A: General, 2004, 265: 115-121.
[7] MARIA S, DIMITRIS I K, XENOPHON E V. Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions[J]. Applied Catalysis B: Environmental, 2004, 47: 189-201.
[8] AKIHIKO K. Photocatalyst materials for water splitting[J]. Catalysis Surveys from Asia, 2003, 7: 31-39.
[9] SARAH S W, DONIA B, JASON A S, ROSE A. The effect of preparation method on the photoactivity of crystalline titanium dioxide particles[J]. Chemical Engineering, 2003, 95: 213-220.
Foundation item: Project(NCET-04-0915) supported by Program for New Century Excellent Talents in Umiversity; Project(2005E0007M) supported by the Natural Science Foundation of Yunan Province
Corresponding author: LIU Qing-ju; Tel: +86-871-5035376; Fax: +86-871-5035376; E-mail: qjliu@ynu.edu.cn


