
J. Cent. South Univ. (2021) 28: 1279-1290
DOI: https://doi.org/10.1007/s11771-021-4696-8

ZIF-67 derived CoSx/NC catalysts for selective reduction of nitro compounds
ZHANG Guang-ji(张广吉)1, TANG Fei-ying(唐飞鹰)1, WANG Li-qiang(王立强)2,YANG Wen-jie(杨文杰)2, LIU You-nian(刘又年)1, 3
1. Hunan Provincial Key Laboratory of Micro & Nano Materials Interface Science, College of
Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Henan Province Industrial Technology Research Institute of Resources and Materials, School of
Material Science and Engineering, Zhengzhou University, Zhengzhou 450001, China;
3. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
Transition metal sulfides (TMSs)-based materials have been extensively investigated as effective non-noble catalysts for various applications. However, the exploration of TMSs-based catalysts for hydrogenation of nitro compounds is limited. Herein, CoSx/NC catalysts were prepared by solvothermal sulfurization of ZIF-67, followed by high-temperature annealing (300–600 °C) under NH3 atmosphere. It was found that the structures and compositions of the as-prepared CoSx/NC can be readily tuned by varying the annealing temperature. Particularly, CoSx/NC-500, which possesses higher degree of S defects and larger specific surface areas, can achieve high conversion, selectivity and stability for catalytic reduction of nitro compounds into amines under mild reaction conditions.
Key words:
transition metal sulfides; catalytic hydrogenation; crystalline phase; selective reduction;
Cite this article as:
ZHANG Guang-ji, TANG Fei-ying, WANG Li-qiang, YANG Wen-jie, LIU You-nian. ZIF-67 derived CoSx/NC catalysts for selective reduction of nitro compounds [J]. Journal of Central South University, 2021, 28(5): 1279-1290.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4696-81 Introduction
Catalytic hydrogenation is an essential reaction in the organic industry [1, 2]. For example, hydrogenation offers an important approach towards for reduction of nitro compounds into the corresponding amines which have been extensively used for synthesis of dye, medicine, pigment, etc [3-5]. Nowadays, noble metal-based catalysts play a dominating role in hydrogenation of nitro compounds. Nevertheless, the high price, low selectivity and easy to be poisoned features seriously limit their industrial-scale applications [6]. As an alternative, carbon-supported non-noble catalysts have received tremendous attention recently [7], since Beller found that carbon- supported FeOx or CoOx exhibited decent activity and high selectivity towards hydrogenation of nitro compounds [8-10]. Especially, transition metal sulfides (TMSs) based materials have been investigated as low cost and highly active non-noble catalysts for various applications [11]. DUAN et al [12] prepared a hybrid non-noble FeS2/NSC catalyst, and the catalyst shows excellent activity, selectivity and stability in the hydrogenation of nitro compounds. SORRIBES et al [13] prepared a nanolayered molybdenum disulfide cobalt-promoted materials (Co-Mo-S), which possess high selectivity for catalytic hydrogenation of nitro compounds into the corresponding amines under mild conditions. Our group also developed an N-doped porous carbon-supported TMSs-based catalyst that can afford high activity and selectivity for hydrogenation of nitroarenes in water/methanol mixture [14]. Though several TMSs-based catalysts have been reported in hydrogenation of nitro compounds into the corresponding amines, the catalysts with improved performance are still high required.
Recently, MOFs (metal-organic frameworks) have been proved excellent precursors towards preparing carbon-supported catalysts [15-23]. The MOFs-based catalysts often have a uniform porous structure and uniformly dispersed metal active sites, which offer the catalysts excellent catalytic activity [24-26]. For instance, MOF-derived transition metal sulfides (TMSs) have been proved excellent candidates for electrocatalysts [27-31]. To the best of our knowledge, there are only a few reports on the exploration of MOF-derived TMSs-based materials for catalytic hydrogenation of nitro compounds. Especially, there are few reports on the influence of the crystalline of TMSs on the catalytic performance of hydrogenation [32, 33].
Herein, hollow porous cobalt sulfides supported on N-doped carbon are developed as hydrogenation catalysts for the reduction of nitro compounds. Firstly, Co3S4/NC was obtained by solvothermal sulfuration of ZIF-67 (as a precursor). Co3S4/NC was annealed under NH3 atmosphere at various temperatures, affording CoSx/NC-X (X represents annealing temperature) with tunable structures and compositions (see Scheme 1). Then,the effects of annealing temperature on the compositions and structures of the catalysts were investigated. Finally, the catalytic hydrogenation performance of CoSx/NC-X was evaluated.

Scheme 1 Schematic illustration of preparation of CoSx/NC-X for reducing nitro compounds (RT: Room temperature)
2 Results and discussion
CoSx/NC was prepared by solvothermal sulfuration of ZIF-67, followed by high- temperature anneal (see Supporting information). ZIF-67 prepared according to reported method exhibits a high degree of crystallinity (Figure S1(a)) [34, 35]. After sulfuration by thioacetamide, ZIF-67 was converted into Co3S4. It was found that some residual organic materials were left and coated on the surface of Co3S4. Notably, at low sulfuration temperature, Co3S4 shows a low crystallinity. The XRD patterns of NH3-treated samples are shown in Figure 1. After treatment at 300 °C, the main crystal phase of Co3S4/NC-300 is still Co3S4, but possesses a higher degree of crystallinity. This is because high temperatures can remove the residual organic molecules and promote the crystallization (see Figure 1(a)). Raising the processing temperature to 400 °C can further improve crystallinity (see Figure 1(b)). However, when the temperature was raised to 500 °C, CoSx/NC-500 displays XRD peaks of a mixed crystal phase of Co9S8 and Co1-xS (see Figure 1(c)). As illustrated in Figure 1(d), when the pyrolysis temperature was increased from 500 °C to 600 °C, Co3S4 was completely converted into Co9S8 with a high degree of crystallinity, indicating that CoSx/NC can gradually change from Co3S4 to Co9S8 with the increase of the temperature. Such a phase transition is mainly due to the reducibility of NH3. NH3 can interact with Co within CoSx, and drive the crystal to form thermodynamically stable Co9S8 under high temperature. Of note, such a process leads to S defect, which can be explored to tune the catalytic activity of the gained CoSx.
SEM and TEM measurements were performed to provide a direct view of the morphology of CoSx/NC-X. As shown in Figure 2(a), ZIF-67 exhibits a solid regular dodecahedron structure. After sulfuration, Co3S4/NC possesses a polyhedron-like frame but with more wrinkles on its surface (see Figure 2(b)). After annealing under the NH3 atmosphere, the morphology displays negligible changes.
Specifically, Co3S4/NC-300, Co3S4/NC-400 and CoSx/NC-500 maintain the polyhedron morphology, but partial collapses occurred, and more collapses produced when the pyrolysis temperature was increased to 600 °C (see Figures 2(c)-(f)). The structure collapse is mainly caused by chemical etching effect of ammonia, which is aggravated by raising pyrolysis temperature. Such collapse is likely to bury the active sites, and consequently sacrifice the activity. Energy dispersive results of X-ray analysis (EDS) reveal that Co, S, N and C are homogeneously distributed through the whole CoSx/NC-X catalysts (see Figures 2(g), (h) and (i)).

Figure 1 XRD patterns of Co3S4/NC-300 (a), Co3S4/NC-400 (b), CoSx/NC-500 (c), and Co9S8/NC-600 (d)
TEM images of the samples are shown in Figure 3. All CoSx/NC-X samples possess hollow structures, consistent with the results reflected by SEM. The hollow structure can work as a reactor, which can improve the reaction kinetics by accelerating mass transfer and increasing the local concentration of the reactants. Similarly, NH3- caused deformation was observed in the TEM images. Meanwhile, the high-resolution transmission electron microscopy (HR-TEM) image reveals that the lattice spacing of 0.30 nm indexing to the (311) crystal plane of Co9S8 appears, meaning that there exists Co9S8 within CoSx-500.
X-ray photoelectron spectroscopy (XPS) was used to probe the chemical composition and state of CoSx/NC catalysts. As shown in Figure 4(a), the peaks of C 1s, N 1s, O 1s, S 2p and Co 2p can be observed in the XPS profiles of CoSx/NC. It shows that C 1s is divided into three peaks at 284.8, 286.4, and 288.8 eV (see Figure 4(b)), corresponding to sp2 hybridized C=C, C—N/C—O and C=O, respectively [22, 33, 34]. It can also be observed that N 1s is divided into three peaks, i.e., pyridine N (398.4 eV), Co-Nx (399.8 eV) and graphite N (401.6 eV) [35-38]. Usually, N atoms coordinate with metal ions, thus can adjust the electronic structure of the catalyst, and stabilize the metal-based nanoparticles (see Figure S4). XPS of S 2p of CoSx/NC is shown in Figure 4(c). Peaks at 161.8 and 163.2 eV can be observed, which are attributed to S 2p3/2 and S 2p1/2 of S2–, respectively [14, 32]. The peak at 168.8 eV is attributed to an oxidized sulfur species, indicating the presence of Co sulfide [27]. In the Co 2p spectra of CoSx/NC,the peak at 778.8 eV belongs to Co 2p3/2 of Co9S8. The corresponding peak intensity follows the order of Co3S4/NC-400 < CoSx/NC-500 < Co9S8/NC-600, indicating that the Co3S4 phase in the catalyst gradually changes to Co9S8 with the increase of annealing temperature. Peaks at 781.0, 793.8 and 797.2 eV are attributed to Co 2p3/2 of Co2+, Co 2p1/2 of Co3+ and Co2+, respectively (see Figure 4(d)) [20, 39-43].

Figure 2 SEM images of ZIF-67 (a), Co3S4/NC (b), Co3S4/NC-300 (c), Co3S4/NC-400 (d), CoSx/NC-500 (e, g),Co9S8/NC-600 (f), elemental mapping of Co, S, N and C in CoSx/NC-500 (h, i)

Figure 3 TEM images of Co3S4/NC-300 (a), Co3S4/NC-400 (b), CoSx/NC-500 (c), Co9S8/NC-600 (d), HR-TEM images of CoSx/NC-500 (e, f)
N2 adsorption/desorption measurements were carried out to investigate the porous structure of CoSx/NC-X. As shown in Figure 5(a) and S5, the adsorbed volume of ZIF-67 is much larger than that of CoSx/NC-X, indicating that both the sulfuration and pyrolysis can decrease the porosity of CoSx/NC-X by breaking the micropore structure. There exists a hysteresis loop between 0.6 and 0.9 (P/P0), suggesting the existence of the mesoporous structure in the sample. The pore size distribution is shown in Figure 5(b). All CoSx/NC-X samples possess mesopore structures, consistent with the results of nitrogen adsorption/desorption isotherms. Besides, there are also some micropores with sizes of 0.5 to 2 nm in the samples. The details of the pore volume and specific surface area of CoSx/NC-X are listed in Table S1. The specific pore volumes of Co3S4/NC-300, Co3S4/NC-400, CoSx/NC-500 and Co9S8/NC-600 are 0.03, 0.06,0.03 and 0.03 cm3/g, respectively. The specific surface area of CoSx/NC decreases along with raising the pyrolysis temperature, i.e., Co3S4/NC- 300 (44.5 m2/g)>Co3S4/NC-400 (38.7 m2/g)>CoSx/ NC-500 (24.6 m2/g)>Co9S8/NC-600 (9.6 m2/g), which can be contributed to the collapse of the structure of the catalysts. The micropore surface areas of Co3S4/NC-300, Co3S4/NC-400, CoSx/NC- 500 and Co9S8/NC-600 are 6.4, 12.9, 2.3 and 4.1 m2/g, respectively, demonstrating that mesopore is the main pore structure of the catalyst. Mesopore structure is conducive to the mass transfer, hence promoting the reaction.
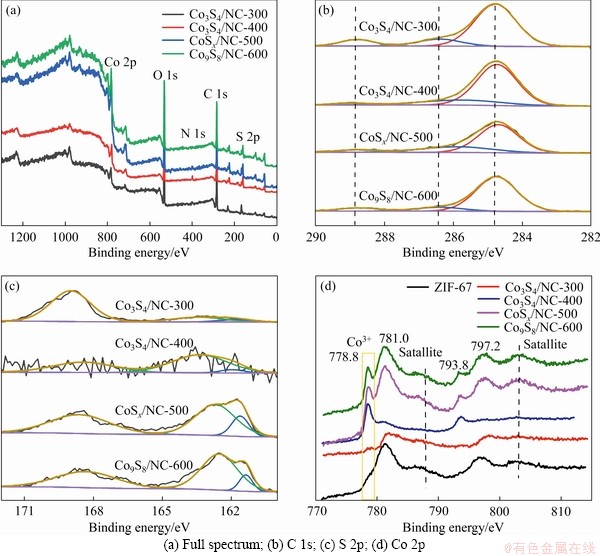
Figure 4 XPS spectra of CoSx/NC-X:

Figure 5 CoSx/NC-X of N2 adsorption/desorption curves (a) and pore size distribution (b)
The catalytic performance of the catalysts for the reduction of nitro compounds was then evaluated. First, CoSx/NC-X catalysts were used for 4-nitrophenol hydrogenation. As listed in Table 1, The precursor ZIF-67 has none catalytic activity, and Co3S4/NC exhibits poor catalytic activity (Conversion: 10%; Selectivity: 83%). The poor catalytic performance is possible because there are organic residues on the surface of Co3S4, which can poison the active sites. After annealing under the NH3 atmosphere, CoSx/NC-X samples exhibit significantly improved catalytic performance. Specifically, Co3S4/NC-400 performs better than Co3S4/NC-300 (Conversion: 93% vs. 60%; Selectivity: 99% vs. 98%). This is because high temperature is beneficial for removing the remaining organic molecules, hence improving the crystallinity of Co3S4. When the annealing temperature was raised to 500 °C, the as-prepared CoSx/NC-500 reveals reduced specific surface area but exhibits higher catalytic activity (Conversion: 99%; Selectivity: 99%), probably because there are more S defects and high crystallinity in CoSx, which could afford more active sites for adsorption and activation of the nitro compounds. The specific surface area of Co9S8/NC-600 is much lower than that of CoSx/NC-500, so is the porosity. Consequently, the catalytic activity of Co9S8/NC-600 is lower than that of CoSx/NC-500. However, the isolated Co9S8 particles only exhibit common catalytic performance. This phenomenon suggests that nitrogen-doped carbon substrates are vital to the excellent catalytic performance of CoSx/NC-500, which can modulate the electron structure of the active site for improved catalytic activity. Overall, CoSx/NC-500 performs the best among the as-prepared catalysts due to the combined effect of rich S defects and relatively large specific surface areas.
Table 1 Catalytic performance of various catalysts for hydrogenation of 4-nitrophenol

Generally, the solvent has a great impact on the catalytic performance. Ethanol (EtOH), methanol (MeOH), tetrahydrofuran (THF), acetonitrile, dioxane and water (H2O) were used as solvents for the hydrogenation. As listed in Table S2, CoSx/NC-500 performs the best in both EtOH and H2O, and the conversions of 4-aminophenol in EtOH and H2O are 99% and 99.9%, respectively. Meanwhile, the selectivity in both solutions exceeds 99%. The kinetics of catalytic reduction was further investigated. As shown in Figure S3, the conversion increases upon prolonging the reaction time (16 h, conversion 100%).
To verify the generality of CoSx/NC-500 in hydrogenation reduction of nitro compounds, different types of nitro compounds were used as reaction substrates. As listed in Table 2, catalyst CoSx/NC-500 delivers high conversion and selectivity for different nitro compounds. For example, for halogenated nitro compounds, the conversion and the selectivity are 98.9% and 99%, respectively (substrates 4 and 13), and no dehalogenation was observed. Unsaturated groups such as aldehyde groups are often sensitive to the hydrogenation and can be easily reduced on Pt/C catalysts. Comparatively, CoSx/NC-500 exhibits high selectivity and conversion,4-nitrobenzaldehyde can be efficiently converted into 4-amino benzaldehyde (Conversion: 97%; Selectivity: 99%, Entry 11) without aldehyde group being reduced. For nitro compounds containing other functional groups such as ether, ester, alkyl, carboxyl, and amino, CoSx/NC-500 can catalyze the reduction of nitro group, selectively. Similarly, nitrogen-containing heterocyclic nitro compounds can also be selectively reduced to the corresponding amines.
Table 2 Catalytic performance of CoSx/NC-500 for various substrates

The stability of the catalyst was also evaluated. As shown in Figure 6, after eight catalytic cycles, the conversion of 4-aminophenol is above 95%, and the selectivity is around 99%, meaning that CoSx/NC-500 is of high stability. The crystal structure of the recycled catalysts was monitored by XRD, where negligible changes of XRD profiles were observed.

Figure 6 Catalytic recyclability of CoSx/NC-500
3 Conclusions
In summary, diverse CoSx/NC-X catalysts were prepared by sulfidation of ZIF-67, followed by annealing at different temperatures (300-600 °C) under NH3 atmosphere. During the annealing process, Co3S4 was gradually converted into CoSx phase with a higher degree of crystallinity. The catalytic activity of CoSx/NC-X is highly related to its crystal structure and specific area. Particularly, CoSx/NC-500 with more S defects and relatively larger specific surface areas, displays excellent performance in catalytic hydrogenation of nitro compounds. CoSx/NC-500 catalyst shows high conversion and selectivity for production of amines under mild reaction conditions (100 °C, 1.6 MPa H2). Our findings provide a facile strategy for the fabrication of N-doped porous carbon-supported TMSs-based catalysts with a tunable crystalline phase, which has a great potential for industrial applications.
Supporting information
1 General information
All nitroarenes were purchased from Aladdin Chemicals (Shanghai, China). Cobalt chloride hexahydrate (CoCl2·6H2O), ethanol absolute was obtained from Adamas-beta. Chromatographic methanol and 2-methylimidazole were purchased from Tansoole under Shanghai Titan Technology. All chemicals were of analytical grade and used directly without further purification.
2 Catalyst preparation
ZIF-67 was prepared as follows: 2-methylimidazole (96 mmol) was dissolved in 200 mL methanol, then 12 mmol cobalt chloride (CoCl2·6H2O) was dispersed into 200 mL methanol. The above two solutions were mixed and stirred at room temperature for 6 h. The resulting solid was collected by centrifugation. Then it was washed 5 times with methanol and dried in an oven at 60 °C for 12 h to afford ZIF-67.
CoSx/NC-X was prepared as follows: To 500 mL flask, 0.84 g ZIF-67, 350 mL ethanol, 1.57 g thioacetamide were added to afford a blue solution. After ultrasonication for 30 min, the obtained solution was placed into a high-pressure reactor, and the solvothermal reaction was carried out in an oven at a temperature of 120 °C for 4 h. After the reaction solution was cooled to room temperature, the solid was collected by centrifugation, followed by washing with ethanol several times, and drying in an oven at 75 °C overnight, to obtain black, solid Co3S4/NC. The black and solid Co3S4/NC was placed into a tube furnace and calcined at 300, 400, 500 and 600 °C for 2 h in an NH3 atmosphere to give Co3S4/C-300, Co3S4/C-400, CoSx/C-500 and Co9S8/C-600, respectively.
3 Catalyst characterization
X-ray diffraction (XRD) was carried out on Bruker D8 (diffractometer with Cu Kα (k=1.5418  ) radiation (50 kV, 30 mA)). Scanning electron microscopy (SEM, S-4800, Hitachi), and transmission electron microscopy (TEM, Tecnai G2-20, FEI) were explored to investigate the morphologies of the catalysts. The N2 adsorption-desorption isotherms were measured to investigate the specific surface area and pore size of the catalysts (Aut absorb-IQ gas adsorption analyzer, Quantachrome). X-ray photoelectron spectra were explored to determine the compositions of materials (XPS, Thermo Scientific K-Alpha+ system).
) radiation (50 kV, 30 mA)). Scanning electron microscopy (SEM, S-4800, Hitachi), and transmission electron microscopy (TEM, Tecnai G2-20, FEI) were explored to investigate the morphologies of the catalysts. The N2 adsorption-desorption isotherms were measured to investigate the specific surface area and pore size of the catalysts (Aut absorb-IQ gas adsorption analyzer, Quantachrome). X-ray photoelectron spectra were explored to determine the compositions of materials (XPS, Thermo Scientific K-Alpha+ system).
4 Catalytic performance
The catalytic experiments were carried out in a 25 mL Teflon-lined high-pressure reactor. 0.3 mmol reaction substrate, 4 mL solvent and 7 mg catalyst were placed in the reaction kettle and then filled hydrogen of 1.6 MPa pressure. The reaction was kept at 100 °C for 16 h. After the reaction was completed, it was cooled to room temperature. GC-MS was used to detect the conversion and selectivity of the product of the reaction.

Figure S1 XRD patterns of ZIF-67 (a) and Co3S4/NC (b)

Figure S2 XRD pattern of reused CoSx/NC-500
The catalyst was recovered by centrifugation and then washed several times with methanol. After drying, it was re-used to test the catalytic recyclability.

Figure S3 Kinetic curves of Co3S4/NC-400 and CoSx/NC-500

Figure S4 XPS high-resolution N 1s spectra of Co3S4/NC-300 and CoSx/NC-500

Figure S5 N2 adsorption/desorption curves of ZIF-67
Table S1 Surface area and pore volume of CoSx/NC

Table S2 Catalytic performance of CoSx/NC-500 under various solvent

Contributors
ZHANG Guang-ji performed the experiments and wrote the first draft of the manuscript. ZHANG Guang-ji, TANG Fei-ying and WANG Li-qiang analyzed the measured data. YANG Wen-jie edited the draft of manuscript. LIU You-nian conceptualized and designed the study, coordinated and supervised data collection and analysis. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
There are no conflicts of interest to declare.
References
[1] HUANG Yun-jing, YANG Wei-jun, QIN Ming-gao, ZHAO Hao-liang. Mild and highly efficient transfer hydrogenation of aldehyde and ketone catalyzed by rubidium phosphate [J]. Journal of Central South University, 2016, 23(7): 1603-1610. DOI: 10.1007/s11771-016-3214-x.
[2] BLASER H U. A golden boost to an old reaction [J]. Science, 2006, 313(5785): 312. DOI: 10.1126/science.1131574.
[3] SAHOO B, FORMENTI D, TOPF C, BACHMANN S, SCALONE M, JUNGE K, BELLER M. Biomass-derived catalysts for selective hydrogenation of nitroarenes [J]. Chem Sus Chem, 2017, 10(15): 3035-3039. DOI: 10.1002/cssc. 201700796.
[4] SHENG Yao, WANG Xue-guang, XING Zhi-kang, CHEN Xiu-bin, ZOU Xiu-jing, LU Xiong-gang. Highly active and chemoselective reduction of halogenated nitroarenes catalyzed by ordered mesoporous carbon supported platinum nanoparticles [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(9): 8908-8916. DOI: 10.1021/acssuschemeng.9b00 948.
[5] CAI Shuang-fei, DUAN Hao-hong, RONG Hong-pan, WANG Ding-sheng, LI Lin-sen, HE Wei, LI Ya-dong. Highly active and selective catalysis of bimetallic Rh3Ni1 nanoparticles in the hydrogenation of nitroarenes [J]. ACS Catalysis, 2013, 3(4): 608-612. DOI: 10.1021/cs300689w.
[6] ZHANG Jian, WANG Liang, SHAO Yi, WANG Yan-qin, GATES B C, XIAO Feng-shou. A Pd@Zeolite catalyst for nitroarene hydrogenation with high product selectivity by sterically controlled adsorption in the zeolite micropores [J]. Angewandte Chemie International Edition, 2017, 56(33): 9747-9751. DOI: 10.1002/anie.201703938.
[7] FORMENTI D, FERRETTI F, SCHARNAGL F K, BELLER M. Reduction of nitro compounds using 3d-non-noble metal catalysts [J]. Chemical Reviews, 2019, 119(4): 2611-2680. DOI: 10.1021/acs.chemrev.8b00547.
[8] YUN Rui-rui, HONG Li-rui, MA Wan-jiao, JIA Wei-guo, LIU Shou-jie, ZHENG Bai-shu. Fe/Fe2O3@N-dopped porous carbon: A high-performance catalyst for selective hydrogenation of nitro compounds [J]. ChemCatChem, 2019, 11(2): 724-728. DOI: 10.1002/cctc.201801626.
[9] MADASU M, HSIA C F, REJ S, HUANG M H. Cu2O pseudomorphic conversion to Cu crystals for diverse nitroarene reduction [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 11071-11077. DOI: 10.1021/acssus chemeng.8b02537.
[10] WEI Zhong-zhe, WANG Jing, MAO Shan-jun, SU Die-feng, JIN Hai-yan, WANG Yi-he, XU Fan, LI Hao-ran, WANG Yong. In situ-generated Co0-Co3O4/N-doped carbon nanotubes hybrids as efficient and chemoselective catalysts for hydrogenation of nitroarenes [J]. ACS Catalysis, 2015, 5(8): 4783-4789. DOI: 10.1021/acscatal.5b00737.
[11] GUO Yan-na, PARK T, YI J W, HENZIE J, KIM J H, WANG Zhong-li, JIANG Bo, BANDO Y, SUGAHARA Y, TANG J, YAMAUCHI Y. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting [J]. Advanced Materials, 2019, 31(17): 1807134. DOI: 10.1002/adma.201807134.
[12] DUAN Yan-an, DONG Xiao-su, SONG Tao, WANG Zhao-zhan, XIAO Jian-liang, YUAN You-zhu, YANG Yong. Hydrogenation of functionalized nitroarenes catalyzed by single-phase pyrite FeS2 nanoparticles on N,S-codoped porous carbon [J]. Chem Sus Chem, 2019, 12(20): 4636-4644. DOI: 10.1002/cssc.201901867.
[13] SORRIBES I, LIU Li-chen, CORMA A. Nanolayered Co-Mo-S catalysts for the chemoselective hydrogenation of nitroarenes [J]. ACS Catalysis, 2017, 7(4): 2698-2708. DOI: 10.1021/acscatal.7b00170.
[14] ZHANG Guang-ji, TANG Fei-ying, WANG Xiao-ying, AN Ping, WANG Li-qiang, LIU You-nian. Co,N-codoped porous carbon-supported CoyZnS with superior activity for nitroarene hydrogenation [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(15): 6118-6126. DOI: 10.1021/ acssuschemeng.0c0 1300.
[15] WANG Chao-hai, KANETI Y V, BANDO Y, LIN Jian-jian, LIU Chao, LI Jian-sheng, YAMAUCHI Y. Metal–organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion [J]. Materials Horizons, 2018, 5(3): 394-407. DOI: 10.1039/C8 MH00133B.
[16] PAN Yuan, SUN Kai-an, LIU Shou-jie, CAO Xing, WU Kong-lin, CHEONG Weng-chon, CHEN Zheng, WANG Yu, LI Yang, LIU Yun-qi, WANG Ding-sheng, PENG Qing, CHEN Chen, LI Ya-dong. Core–shell ZIF-8@ZIF-67- derived CoP nanoparticle-embedded N-doped carbon nanotube hollow polyhedron for efficient overall water splitting [J]. Journal of the American Chemical Society, 2018, 140(7): 2610-2618. DOI: 10.1021/jacs.7b12420.
[17] MURUGESAN K, SENTHAMARAI T, SOHAIL M, ALSHAMMARI A S, POHL M M, BELLER M, JAGADEESH R V. Cobalt-based nanoparticles prepared from MOF–carbon templates as efficient hydrogenation catalysts [J]. Chemical Science, 2018, 9(45): 8553-8560. DOI: 10.1039/C8SC02807A.
[18] TOYAO T, FUJIWAKI M, MIYAHARA K, KIM T H, HORIUCHI Y, MATSUOKA M. Design of zeolitic imidazolate framework derived nitrogen-doped nanoporous carbons containing metal species for carbon dioxide fixation reactions [J]. Chem Sus Chem, 2015, 8(22): 3905-3912. DOI: 10.1002/cssc.201500780.
[19] JAGADEESH R V, MURUGESAN K, ALSHAMMARI A S, NEUMANN H, POHL M M, RADNIK J, BELLER M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines [J]. Science, 2017, 358(6361): 326-332. DOI: 10.1126/science.aan6245.
[20] WANG Xi, LI Ying-wei. Chemoselective hydrogenation of functionalized nitroarenes using MOF-derived Co-based catalysts [J]. Journal of Molecular Catalysis A: Chemical, 2016, 420: 56-65. DOI: 10.1016/j.molcata.2016.04.008.
[21] YANG Shu-liang, PENG Li, BULUT S, QUEEN W L. Recent advances of MOFs and MOF-derived materials in thermally driven organic transformations [J]. Chemistry-A European Journal, 2019, 25(9): 2161-2178. DOI: 10.1002/chem. 2018 03157.
[22] BAVYKINA A, KOLOBOV N, KHAN I S, BAU JEREMY A, RAMIREZ A, GASCON J. Metal-organic frameworks in heterogeneous catalysis: Recent progress, new trends, and future perspectives [J]. Chemical Reviews, 2020, 120(16): 8468-8535. DOI: 10.1021/acs.chemrev.9b00685.
[23] KONNERTH H, MATSAGAR B M, CHEN S, PRECHTL MARTIN H G, SHIEH F K, WU KEVIN C W. Metal-organic framework (MOF)-derived catalysts for fine chemical production [J]. Coordination Chemistry Reviews, 2020, 416: 213319. DOI: 10.1016/j.ccr.2020.213319.
[24] SHENG Jian-ping, WANG Li-qiang, DENG Liu, ZHANG Min, HE Hai-chuan, ZENG Ke, TANG Fei-ying, LIU You-nian. MOF-templated fabrication of hollow Co4N@N- doped carbon porous nanocages with superior catalytic activity [J]. ACS Applied Materials & Interfaces, 2018, 10(8): 7191-7200. DOI: 10.1021/acsami.8b00573.
[25] TANG Fei-ying, WANG Li-qiang, ZHANG Guang-ji, ZHANG Min, LIU You-nian. Creating coordination mismatch in MOFs: Tuning from pore structure of the derived supported catalysts to their catalytic performance [J]. Industrial & Engineering Chemistry Research, 2019, 58(14): 5543-5551. DOI: 10.1021/acs.iecr.9b01096.
[26] PARLETT C M A, WILSON K, LEE A F. Hierarchical porous materials: Catalytic applications [J]. Chemical Society Reviews, 2013, 42(9): 3876-3893. DOI: 10.1039/ C2CS353 78D.
[27] LEDOUX M J, DJELLOULI B. Hydrodenitrogenation activity and selectivity of well-dispersed transition metal sulfides of the second row on activated carbon [J]. Journal of Catalysis, 1989, 115(2): 580-590. DOI: 10.1016/0021-9517 (89)90059-6.
[28] YANG Tao, YIN Li-si, HE Mao-shuai, WEI Wen-xian, CAO Guo-jian, DING Xin-ran, WANG Yi-hui, ZHAO Zi-ming, YU Ting-ting, ZHAO Hong, ZHANG Dong-en. Yolk–shell hierarchical catalyst with tremella-like molybdenum sulfide on transition metal (Co, Ni and Fe) sulfide for electrochemical water splitting [J]. Chemical Communications, 2019, 55(95): 14343-14346. DOI: 10.1039/C9CC06244K.
[29] WENG Bai-cheng, WANG Xiao-ming, GRICE C R, XU Feng-hua, YAN Yan-fa. A new metal–organic open framework enabling facile synthesis of carbon encapsulated transition metal phosphide/sulfide nanoparticle electrocatalysts [J]. Journal of Materials Chemistry A, 2019, 7(12): 7168-7178. DOI: 10.1039/C9TA00404A.
[30] XU Hua-jie, CAO Jing, SHAN Chang-fu, WANG Bing-kai, XI Pin-xian, LIU Wei-sheng, TANG Yu. MOF-derived hollow CoS decorated with CeOx nanoparticles for boosting oxygen evolution reaction electrocatalysis [J]. Angewandte Chemie International Edition, 2018, 57(28): 8654-8658. DOI: 10.1002/anie.201804673.
[31] LI Hui, YUE Fan, XIE Hong-tao, YANG Chao, ZHANG Yi, ZHANG Liu-gen, WANG Ji-de. Hollow shell-in-shell Ni3S4@Co9S8 tubes derived from core–shell Ni-MOF-74@ Co-MOF-74 as efficient faradaic electrodes [J]. Crystengcomm, 2018, 20(7): 889-895. DOI: 10.1039/C7CE 01873H.
[32] YANG Shu-liang, PENG Li, SUN D T, OVEISI E, BULUT S, QUEEN W L. Metal–organic-framework-derived Co3S4 hollow nanoboxes for the selective reduction of nitroarenes [J]. ChemSusChem, 2018, 11(18): 3131-3138. DOI: 10.1002/cssc. 201801641.
[33] XU Yong, LV Xiao-jun, CHEN Yong, FU Wen-fu. Highly selective reduction of nitroarenes to anilines catalyzed using MOF-derived hollow Co3S4 in water under ambient conditions [J]. Catalysis Communications, 2017, 101: 31-35. DOI: 10.1016/j.catcom.2017.07.001.
[34] WU Lan-lan, WANG Qi-shun, LI Jian, LONG Yan, LIU Yu, SONG Shu-yan, ZHANG Hong-jie. Co9S8 nanoparticles- embedded N/S-codoped carbon nanofibers derived from metal–organic framework-wrapped CdS nanowires for efficient oxygen evolution reaction [J]. Small, 2018, 14(20): 1704035. DOI: 10.1002/smll.201704035.
[35] DOU Shuo, TAO Li, HUO Jia, WANG Shuang-yin, DAI Li-ming. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis [J]. Energy & Environmental Science, 2016, 9(4): 1320-1326. DOI: 10.1039/C6EE00054A.
[36] ARECHEDERRA R L, ARTYUSHKOVA K, ATANASSOV P, MINTEER S D. Growth of phthalocyanine doped and undoped nanotubes using mild synthesis conditions for development of novel oxygen reduction catalysts [J]. ACS Applied Materials & Interfaces, 2010, 2(11): 3295-3302. DOI: 10.1021/am100724v.
[37] XIAO Jun-wu, CHEN Chen, XI Jiang-bo, XU Yang-yang, XIAO Fei, WANG Shuai, YANG Shi-he. Core–shell Co@Co3O4 nanoparticle-embedded bamboo-like nitrogen- doped carbon nanotubes (BNCNTs) as a highly active electrocatalyst for the oxygen reduction reaction [J]. Nanoscale, 2015, 7(16): 7056-7064. DOI: 10.1039/C4NR 05917D.
[38] CASANOVAS J, RICART J M, RUBIO J, ILLAS F, JIMENEZ J M. Origin of the large N 1s binding energy in X-ray photoelectron spectra of calcined carbonaceous materials [J]. Journal of the American Chemical Society, 1996, 118(34): 8071-8076. DOI: 10.1021/ja960338m.
[39] WANG Xiao-xia, CULLEN D A, PAN Yung-tin, HWANG S, WANG Mao-yu, FENG Zhen-xing, WANG Jing-yun, ENGELHARD M H, ZHANG Han-guang, HE Yang-hua, SHAO Yu-yan, SU Dong, MORE K L, SPENDELOW J S, WU Gang. Nitrogen-coordinated single cobalt atom catalysts for oxygen reduction in proton exchange membrane fuel cells [J]. Advanced Materials, 2018, 30(11): 1706758. DOI: 10.1002/adma.201706758.
[40] WESTERHAUS F A, JAGADEESH R V, WIENHOFER G, POHL M M, RADNIK J, SURKUS A E, RABEAH J, JUNGE K, JUNGE H, NIELSEN M, BRUCKNER A, BELLER M. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes [J]. Nature Chemistry, 2013, 5: 537. DOI: 10.1038/nchem.1645.
[41] ZHENG Bo, WANG Jiong, WANG Feng-bin, XIA Xing-hua. Low-loading cobalt coupled with nitrogen-doped porous graphene as excellent electrocatalyst for oxygen reduction reaction [J]. Journal of Materials Chemistry A, 2014, 2(24): 9079-9084. DOI: 10.1039/C4TA01043D.
[42] MOROZAN A, JEGOU P, JOUSSELME B, PALACIN S. Electrochemical performance of annealed cobalt- benzotriazole/CNTs catalysts towards the oxygen reduction reaction [J]. Physical Chemistry Chemical Physics, 2011, 13(48): 21600-21607. DOI: 10.1039/C1CP23199E.
[43] HADJ-AISSA A, DASSENOY F, GEANTET C, AFANASIEV P. Solution synthesis of core-shell Co9S8@ MoS2 catalysts [J]. Catalysis Science & Technology, 2016, 6(13): 4901-4909. DOI: 10.1039/C6CY00311G.
(Edited by YANG Hua)
中文导读
ZIF-67衍生的CoSx/NC催化剂的制备及其对硝基化合物的选择性还原
摘要:过渡金属硫化物作为高效的非贵金属催化剂,已在化工领域得到了广泛的应用。但是,很少将其应用于硝基化合物的催化加氢反应中。在本文中,通过硫化金属有机框架材料ZIF-67,并在氨气气氛下进行退火,制备得到了CoSx/NC催化剂。研究发现,该类催化剂的结构和组成可通过改变退火温度进行调控。特别是,当退火温度为500 °C时,得到的催化剂CoSx/NC-500由于具有高的硫缺陷和比表面积,可在温和反应条件下高选择性地将硝基化合物还原成相应的氨基化合物。
关键词:过渡金属硫化物;催化加氢;晶相;选择性还原
Foundation item: Projects(21636010, 21878342) supported by the National Natural Science Foundation of China; Project(2019JJ50758) supported by the Hunan Provincial Natural Science Foundation of China; Project(2019TP1001) supported by the Hunan Provincial Science and Technology Plan Project of China; Project(CX20190097) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2020-09-25; Accepted date: 2021-01-07
Corresponding author: YANG Wen-jie, PhD, Senior Engineer; Tel: +86-15378750416; E-mail: ywj2012@zzu.edu.cn; ORCID: https://orcid.org/0000-0002-5795-2278; LIU You-nian, PhD, Professor; Tel: +86-731-88879616; E-mail: liuyounian@csu.edu.cn;ORCID: https://orcid.org/0000-0002-7078-5937
Abstract: Transition metal sulfides (TMSs)-based materials have been extensively investigated as effective non-noble catalysts for various applications. However, the exploration of TMSs-based catalysts for hydrogenation of nitro compounds is limited. Herein, CoSx/NC catalysts were prepared by solvothermal sulfurization of ZIF-67, followed by high-temperature annealing (300–600 °C) under NH3 atmosphere. It was found that the structures and compositions of the as-prepared CoSx/NC can be readily tuned by varying the annealing temperature. Particularly, CoSx/NC-500, which possesses higher degree of S defects and larger specific surface areas, can achieve high conversion, selectivity and stability for catalytic reduction of nitro compounds into amines under mild reaction conditions.


