- Abstract:
- 1 Introduction▲
- 2 Experimental ▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Effect of HCl concentration on electrical conductivity of microemulsion
- Fig.2 Cyclic voltammograms of K3Fe(CN)6 in W/O micro- emulsions at scan rate of 50 mV/s with 0 mol/L (a), 3 mol/L (b), 6 mol/L (c) and 7 mol/L (d) HCl in water phase
- Fig.3 Cyclic voltammograms of K3Fe(CN)6 in W/O micro- emulsion system (a) and aqueous solution system (b)
- Fig.4 Electrochemical impedance spectroscopy curves of K3Fe(CN)6 in W/O microemulsion system (a) and aqueous solution system (b)
- Fig.5 Scheme of electrochemical reaction of Fe(CN)63-/ Fe(CN)64- at electrode/microemulsion interface
J. Cent. South Univ. Technol. (2009) 16: 0061-0065
DOI: 10.1007/s11771-009-0010-x![]()
Electrochemical behavior of K3Fe(CN)6 in water-in-oil microemulsion
Zhou Hai-hui(周海晖), Zeng Wei(曾 伟), Ying Xiao-fang(英晓芳),
Zeng Jin-xiang(曾金祥), LI Dan(李 丹), Chen Jin-hua(陈金华), Kuang Ya-fei(旷亚非)
(College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China)
Abstract:
A water-in-oil (W/O) microemulsion composed of Triton X-100, n-hexanol, n-hexane and water solution with hydrochloric acid was prepared. K3Fe(CN)6 was added in as a water-soluble electroactive probe, and its electrochemical behavior was investigated by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). It is found that the H+ concentration of the water phase has a great effect on the conductivity of the W/O microemulsion, and hence influences the electrochemical behavior of K3Fe(CN)6. When the pH value of water phase is about 7, the electrical conductivity of the W/O microemulsion is only 1.2×10-6 S/cm, and K3Fe(CN)6 almost cannot react at the glassy carbon electrode. But when the H+ concentration is more than 3 mol/L, the W/O microemulsion has a good electrical conductivity and K3Fe(CN)6 shows good electrochemical performance in it. The results of CV and EIS studies indicate that the electrochemical behavior of Fe(CN)63-/Fe(CN)64- in the W/O microemulsion is different from that in the aqueous solution. This may be due to the unique liquid structure of the W/O microemulsion and the unique mass transfer in the W/O microemulsion.
Key words:
K3Fe(CN)6; electrochemical behavior; W/O microemulsion; electrical conductivity;
1 Introduction
Microemulsions are defined as isotropic, thermo- dynamically stable and transparent dispersions of water, oil, and surfactant, usually with a cosurfactant. There are three types of microemulsion: oil-in-water (O/W), water-in-oil (W/O), and bicontinuous (BC). In the W/O microemulsions, the nanosized water droplets disperse in continuous oil phase uniformly, and are stabilized by the interfacial film composed of surfactant (or surfactant and cosurfactant). The highly dispersive water droplets offer a special microenvironment for the formation of nanoparticles, and the surfactant-stabilized microcavities provide a cage-like effect that limits the particles nucleation, growth, and agglomeration. As a result, the particles obtained in such a medium are generally very fine and monodisperse. A series of nanoparticles, such as metal particles, metal oxide particles, magnetic particles and polymer particles, were synthesized by W/O microemulsions [1-5].
In order to investigate the microstructure and the application of microemulsions, many techniques have been employed, for example, quasi-elastic light scattering [6], nuclear magnetic resonance [7], small angle X-ray scattering [8] and neutron scattering [9]. Besides, electrochemical measurements are also useful and efficient for characterizing organized surfactant solutions. Cyclic voltammetry (CV), rotation disk voltammetry and chronocoulometry have been successfully applied in the study of physicochemical properties and microstructures of aqueous surfactant solutions and microemulsions [10-12]. Commonly, an oil-soluble or a water-soluble or an amphiphilic compound electroactive probe is employed in the electrochemical research and its diffusion coefficient is measured. According to the diffusion coefficient, much information can be obtained, such as the dynamics, the microstructure and structural transition of microdroplets [11-14].
However, due to the poor electrical conductivity of the W/O microemulsion, the electroactive probe in it usually has a low current density [13-14]. Normally, W/O microemulsion can hardly be used as a good conductive electrolyte in the electrochemical field. Recently, we have found that the electrical conductivity of W/O microemulsion can be greatly improved by adding hydrochloric acid with high concentration to the water droplets. It can be expected that, as W/O microemulsion has the unique microstructure and the unique way of mass transfer, the electrochemical behavior of K3Fe(CN)6 in it must be much different from that in aqueous solution. And this research work may promote the application of microemulsion in the electro- chemical field.
2 Experimental
2.1 Chemicals
Triton X-100 (C.P. grade), n-hexanol (A.R. grade), n-hexane (A.R. grade) and potassium ferricyanide (A.R. grade) were obtained from Sinopharm Chemical Reagent Co., Ltd. All other chemicals used in this work were A.R. grade. Water for the preparation of all solutions was double distilled.
2.2 Apparatus and procedures
For preparing the microemulsion, Triton X-100 was used as the surfactant, with n-hexanol as the cosurfactant, n-hexane as the organic phase and hydrochloric acid solution as the aqueous phase. K3Fe(CN)6 was employed as the electroactive probe. After mixing 10 mL TritonX-100, 20 mL n-hexanol and 20 mL n-hexane, appropriate amounts of HCl solution and K3Fe(CN)6 solution were added in under stirring respectively and the water phase was kept at 4 mL, and then a transparent microemulsion was obtained. The microemulsion was centrifuged by a high-speed centrifuge (Eppondorf Centrifuge 5804 R, German) with a speed of 10 000 r/min at 25 ℃ for 10 min. Fortunately, the micro- emulsion was still transparent and without any phase separation, indicating that the microemulsion used in this work was quite stable. The structure of the microemulsion was characterized by dynamic light scattering equipment (ALV/CGS-5022F, ALV/Laser Vertriebsgesellschaft m.b.H Company) with a He-Ne laser (λ0=632.8 nm) with a 23 mW power. A conductometer (DDS-307, Shanghai Precision and Scientific Instrument Co., Ltd) was used to measure the electrical conductivity of the microemulsion.
The electrochemical measurement was carried out with a CHI model 660C electrochemical workstation (Shanghai Chenhua Instrument Factory, China). A glassy carbon (GC) electrode was used as the working electrode, a platinum wire and a saturated calomel electrode (SCE) were used as the counter electrode and the reference electrode, respectively. Prior to use, GC electrode (d=4 mm) was first polished with alumina emery paper. Then, it was cleaned with an ultrasonator in 50% (volume fraction) alcohol aqueous solution and 50% (volume fraction) nitric acid solution for 5 min to remove the remnant organic and aluminum oxide particles on the electrode surface, and subsequently it was washed with double distilled water. After that, the GC electrode was electrochemically activated in 0.5 mol/L H2SO4 solution using CV scan with potential varying from -1 to 1 V until no peak appeared in the cyclic voltammogram curves (CVs). Finally, it was washed with double distilled water and allowed to air dry.
The electrochemical behavior of K3Fe(CN)6 in the W/O microemulsion was studied by CV and electro- chemical impedance spectroscopy (EIS). EIS was performed under open circuit potential of the working electrode in the A.C. range of 1×10-2-1×105 Hz with an excitation signal of 10 mV. For comparison, an aqueous solution system with the same apparent concentrations of HCl (c(HCl)apparent=0.44 mol/L) and K3Fe(CN)6 (c(K3Fe(CN)6)apparent=7.4 mmol/L) was also employed. The apparent concentration of A is defined as c(A)apparent=nA/V, where nA is the mole amount of A, and V is the whole volume of the W/O microemulsion or aqueous solution.
3 Results and discussion
3.1 Electrical conductivity of microemulsion
There are two different opinions on the conductive behaviors of the microemulsion. One considers that droplets fluctuate and collide under the effect of electric field, which makes the surfactant molecules transit, so the microemulsion is conductive [15]. The other opinion claims that the conductivity of microemulsion is due to the transition of electrolyte ions in water pools at the oil phase/water phase interface film [16]. The electrical conductivities of microemulsions of 10 mL TritonX-100, 20 mL n-hexanol, 20 mL n-hexane and 4 mL water phase with different HCl concentrations were measured, and the results are shown in Fig.1. It is found that as the concentration of HCl increases, the electrical conductivity of the microemulsion greatly increases. There are two conductive ions (H+ and Cl-) in the microemulsion and H+ ions are the main conductive ions due to their small volume and fast transfer rate. As H+ ions almost come from HCl in the water phase, the electrical conductivity of the microemulsion is enhanced by the high concentration of HCl apparently.

Fig.1 Effect of HCl concentration on electrical conductivity of microemulsion
In order to characterize the structure of microemulsion, a microemulsion of 10 mL TritonX-100, 20 mL n-hexanol, 20 mL n-hexane and 4 mL water phase with 6 mol/L HCl was prepared, and measured by dynamic light scattering. It is found that the average hydrodynamic radius (Rh) is 17.1 nm with a distribution from 3.2 to 87.1 nm. Therefore, the microemulsion with the electrical conductivity of 424.8×10-6 S/cm was a W/O one with nanometer water droplets even if the concentration of HCl in the water phase reaches 6 mol/L.
3.2 Electrochemical behavior of K3Fe(CN)6 in W/O microemulsion
K3Fe(CN)6 was added to the W/O microemulsions with different HCl concentrations in the water phase as a water-soluble electroactive probe, and its CVs are shown in Fig.2. It can be seen from Figs.1 and 2 that in the absence of HCl in the water phase (the pH value of the water phase is about 7), the electrical conductivity of the W/O microemulsion is only 1.2×10-6 S/cm, so, no peak can be observed from the CVs and the current is very low (Fig.2, Curve a). When the water phase contains 3 mol/L HCl (Fig.2, Curve b), the electrical conductivity of the W/O microemulsion is 242.2×10-6 S/cm, and Fe(CN)63-/Fe(CN)64- peaks appear. The oxidation and reduction peak potentials are 0.62 and 0.46 V, respectively, namely, the peak separation ΔEp (ΔEp=Epa- Epc) is 160 mV, and the corresponding peak currents are 13 μA and 16 μA. When HCl concentration in the water phase reaches 6 mol/L (Fig.2, Curve c), the electrical conductivity of the W/O microemulsion increases to 424.8×10-6 S/cm. As a result, the oxidation and reduction peak potentials shift to 0.69 and 0.56 V, respectively, namely, ΔEp is 130 mV, and the corresponding peak currents increase to 17 and 19 μA. But when HCl concentration is more than 6 mol/L (Fig.2, Curve d), the peak currents decrease. This is because H+ and Fe(CN)63- can form H3Fe(CN)6 at high concentration of H+, and hence the concentration of reactive particles decreases. The results above indicate that with a lot of H+ in the water phase, the electrical conductivity of the W/O microemulsion is good and Fe(CN)63-/Fe(CN)64- can react efficiently.
In order to further investigate the electrochemical reaction characteristic of K3Fe(CN)6 in W/O microemulsion, the CV behavior of K3Fe(CN)6 in W/O microemulsion was compared with that in aqueous solution. Fig.3 shows the CVs of K3Fe(CN)6 in the W/O microemulsion and aqueous solution with the same apparent concentrations of HCl and K3Fe(CN)6. As shown in Fig.3, the CVs are quite different for the two systems. In the aqueous solution system, the electro- chemical reaction of K3Fe(CN)6 is a typical reversible process (Fig.3(b)). Taking the curve obtained at the scan rate of 20 mV/s for example, the open circuit potential is 0.44 V, the oxidation and reduction peak potentials are 0.46 and 0.40 V, respectively, namely, ΔEp is 60 mV, and the corresponding peak currents are 110 and 100 μA. It can also be seen that the anodic curve first goes up quickly with increasing anodic potential until peak potential appears, and then goes down. The cathodic curve first goes down with decreasing cathodic potential, and then goes up beyond reduction peak potential. Additionally, for all the CVs, the oxidation and reduction peak potentials almost do not shift with the increase of the scan rate, and the peak currents increase linearly with the increase of the square root of scan rate, which suggests that the electrochemical reaction of K3Fe(CN)6 in the aqueous solution system is a reversible process and is controlled by the diffusion. While in the W/O microemulsion system (Fig.3(a)), the open circuit potential is 0.62 V, which is higher than that in the aqueous solution system. From Fig.3(a), it can be observed that the oxidation and reduction peak potentials are 0.67 and 0.55 V (ΔEp=120 mV), and the corresponding peak currents are 9 and 10 μA (about 1/10 as those in the aqueous solution system) at a scan rate of 20 mV/s. It can also be seen that the anodic curve first goes up quickly with increasing anodic potential, but differing from Fig.3(b), the curve only goes down a little after the peak potential. The cathodic curve first goes down with decreasing cathodic potential and then goes up a little beyond the reduction peak potential. Additionally, for all the CVs, the oxidation and the reduction peak potentials shift with the increase of the scan rate.
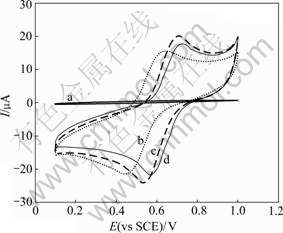
Fig.2 Cyclic voltammograms of K3Fe(CN)6 in W/O micro- emulsions at scan rate of 50 mV/s with 0 mol/L (a), 3 mol/L (b), 6 mol/L (c) and 7 mol/L (d) HCl in water phase
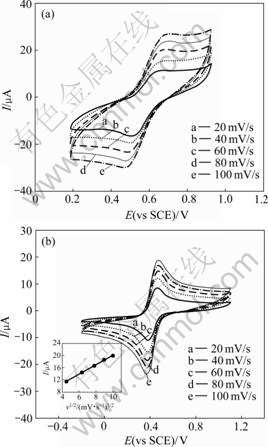
Fig.3 Cyclic voltammograms of K3Fe(CN)6 in W/O micro- emulsion system (a) and aqueous solution system (b)
The EIS curves of K3Fe(CN)6 in the W/O microemulsion and aqueous solution were also measured. As can be seen from Fig.4 (Curve b), the EIS curve is a beeline crossing the original point in the aqueous solution system, which indicates that the redox reaction of Fe(CN)63-/Fe(CN)64- is controlled by the diffusion. While in the microemulsion system, the EIS curve is made up of a capacitance loop at high frequency and a Warburg resistance at low frequency (Fig.4, Curve a). The charge transfer resistance Rct, the solution resistance Rs, and the electrochemical capacitance C in the W/O microemulsion are about 918.0 W, 95.8 W and 8.0×10-4 F/cm2, respectively.

Fig.4 Electrochemical impedance spectroscopy curves of K3Fe(CN)6 in W/O microemulsion system (a) and aqueous solution system (b)
Why is the electrochemical behavior of K3Fe(CN)6 in the W/O microemulsion so different from that in the aqueous solution? The reason may be that the W/O microemulsion has a unique liquid structure. Fig.5 shows the scheme of the electrochemical reaction of Fe(CN)63-/ Fe(CN)64- at the electrode/microemulsion interface. In the W/O microemulsion, a lot of nanosized water droplets surrounded by a surfactant and cosurfactant monolayer disperse uniformly in a continuous oil phase. As K3Fe(CN)6 can only dissolve in the water phase and cannot dissolve in the oil phase, Fe(CN)63- ions in the nanosized water droplets of bulk microemulsion can move to the vicinity of the GC electrode by thermal movement and “droplet collision” [17-19]. In addition, the redox reaction of Fe(CN)63-/Fe(CN)64- occurs only when the nanosized water droplets crash onto the electrode/microemulsion interface. However, the probability of crash is quite small, so, although the true concentration of K3Fe(CN)6 in the water phase of the microemulsion is higher than that in the aqueous solution, the oxidation and reduction currents of Fe(CN)63-/ Fe(CN)64- in the W/O microemulsion system are much lower than those in the aqueous solution system. On the other hand, the organic compound molecules, such as surfactant molecules, can adsorb onto the GC electrode surface and form a block film, which will increase the resistance of charge transfer of reactive ions between the electrode and the microemulsion. As a result, it is more difficult for the electrochemical reaction of Fe(CN)63-/ Fe(CN)64- to occur in the W/O microemulsion than that in aqueous solution. Now, it is clear that, due to the unique liquid structure of W/O microemulsion and the unique mass transfer in the W/O microemulsion, the electrochemical reaction of K3Fe(CN)6 in W/O microemulsion is markedly different from that in aqueous solution.
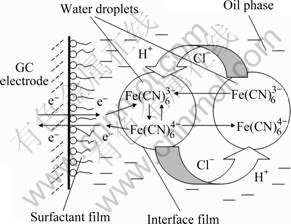
Fig.5 Scheme of electrochemical reaction of Fe(CN)63-/ Fe(CN)64- at electrode/microemulsion interface
4 Conclusions
(1) The effect of HCl concentration on the electrical conductivity of the W/O microemulsion is investigated. With a lot of H+ in the water phase, the electrical conductivity of the W/O microemulsion is good and Fe(CN)63-/Fe(CN)64- can react efficiently.
(2) The CV and EIS curves of K3Fe(CN)6 obtained from the microemulsion system are quite different from those obtained from the aqueous solution system. The redox peak currents of Fe(CN)63-/Fe(CN)64- in the W/O microemulsion system are 1/10 of those in the aqueous solution system with the same apparent concentrations of K3Fe(CN)6 and HCl. The electrochemical reaction of Fe(CN)63-/Fe(CN)64- is a quasi-reversible process and is controlled by both the charge transfer and the diffusion in the W/O microemulsion system.
(3) A scheme of the electrochemical reaction of Fe(CN)63-/Fe(CN)64- at the electrode/microemulsion interface is proposed.
References
[1] ZHANG X, CHAN K Y. Water-in-oil microemulsion synthesis of platinum-ruthenium nanoparticles, their characterization and electrocatalytic properties [J]. Chemistry of Materials, 2003, 15(2): 451-459.
[2] CAPEK I. Preparation of metal nanoparticles in water-in-oil (W/O) microemulsions [J]. Advances in Colloid and Interface Science, 2004, 110(1/2): 49-74.
[3] CASON J P, ROBERTS C B. Metallic copper nanoparticle synthesis in AOT reverses micelles in compressed propane and supercritical ethane solutions [J]. Journal of Physical Chemistry B, 2000, 104(6): 1217-1221.
[4] Zhan Zi-li, Song Wen-hui, Jiang Deng-gao. Preparation of nanometer-sized In2O3 particles by a reverse microemulsion method [J]. Journal of Colloid and Interface Science, 2004, 271(2): 366-374.
[5] RONDINONE A J, SAMIA A C S, ZHANG Z J. A chemometric approach for predicting the size of magnetic spinel ferrite nanoparticles from the synthesis conditions [J]. Journal of Physical Chemistry B, 2000, 104(33): 7919-7922.
[6] SINGHAI M, CHHABRA V, KANG P. Synthesis of ZnO nanoparticles for varistor application using Zn-substituted aerosol of microemulsion [J]. Materials Research Bulletin, 1997, 32(2): 239-247.
[7] ANTALEK B, WILLIAMS A J, GARCIA E, TEXTER J. NMR analysis of interfacial structure transitions accompanying electron-transfer threshold transition in reverse microemulsions [J]. Langmuir, 1994, 10(12): 4459-4467.
[8] EMANUEL K, CHRISTIAV S, AVETT G, STEFAN K. Synthesis and catalytic properties of microemulsion derived cerium oxide nanoparticles [J]. Journal of Solid State Chemistry, 2008, 18(7): 1614-1620.
[9] EASTOE J. Small-angle neutron scattering from dilute didodecyldimethylammonium bromide water-in-oil microemulsions: Evidence for polymer-like aggregates [J]. Langmuir, 1992, 8(6):1503-1506.
[10] HERRERO R, BARRIADA J L, LOPEZ J M, MONCELLI M R, SASTRE M E. Effect of ionic strength on the electrochemical behavior of glutathione on a phospholipid self-assembled monolayer on mercury [J]. Langmuir, 2000, 16(11): 5148-5153.
[11] Mo Chun-sheng. 1.5-Order differential electroanalysis on Triton X-100 microemulsion [J]. Langmuir, 2002, 18(10): 4047-4053.
[12] Mo Chun-sheng, Li Xiao-ge. Microstructure and structural transition in coconut oil microemulsion using semidifferential electroanalysis [J]. Journal of Colloid and Interface Science, 2007, 312(2): 355-362.
[13] CHARLTON I D, DOHERTY A P. Simultaneous observation of attractive interaction, depletion forces, and “sticky” encounters between AOT reverse micelles in isooctane using microelectrode voltammetry [J]. Journal of Physical Chemistry B, 2000, 104(33): 8061-8067.
[14] MOLINA P G, SILBER J J, CORREA N M, SERENO L. Electrochemistry in AOT reverse micelles. A powerful technique to characterize organized media [J]. Journal of Physical Chemistry C, 2007, 111(11): 4269-4276.
[15] SHEN Xing-hai WANG Wen-qing GU Guo-xing. Studies on the activation energies and the mechanism of electric conductivity of W/O type microemulsions [J]. Chemical Research in Chinese Universities, 1993, 14(5): 717-719. (in Chinese)
[16] MUKHOPADHYAY L, BHATTACHARYA P K, MOULIK S P. Additive effects on the percolation of water/AOT/decane microemulsion with reference to the mechanism of conduction [J]. Colloids and Surfaces, 1990, 50(1): 295-308.
[17] LANG J, LALEM N, ZANA R. Quaternary water in oil microemulsions (1): Effect of alcohol chain length and concentration on droplet size and exchange of material between droplets [J]. Journal of Physical Chemistry, 1991, 95(23): 9533-9541.
[18] WANG J. Analytical electrochemistry [M]. 3rd ed. Toronto: John Wiley & Sons, Inc, 2007.
[19] FU Chao-peng, ZHOU Hai-hui, KUANG Ya-fei. Comparison of eletroposition of silver in ionic liquid microemulsions [J]. Electro- chemistry Communications, 2008, 10(5): 806-809
Foundation item: Projects(20673036, J0830415) supported by the National Natural Science Foundation of China; Projects(05JT1026, 2007JT2013) supported by the Science Technology Project of Hunan Province, China
Received date: 2008-07-08; Accepted date: 2008-10-15
Corresponding author: ZHOU Hai-hui, Professor; Tel: +86-731-8821874; E-mail: haihuizh@163.com
- Electrochemical behavior of K3Fe(CN)6 in water-in-oil microemulsion


