
Effect of surface free energy of ceramic glaze on oil droplet shape and its behavior in water
LIANG Jin-sheng(梁金生), MENG Jun-ping(孟军平), LIANG Guang-chuan(梁广川),
WANG Li-juan(王丽娟), ZHANG Jin((张 晋), LI Ji-yuan(李计元)
Institute of Power Source and Ecomaterials Science, Hebei University of Technology, Tianjin 300130, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
A super-hydrophilic functional ceramic was prepared by adjusting the chemical components of ceramic glaze. Effect of surface free energy of ceramic glaze on oil droplet shape and its behavior in water were studied. The results show that water can spread on ceramic surface with high surface free energy, and oil droplet can aggregate rapidly and separate from the ceramic surface in water. For the ceramic with lower surface free energy, the polar shares are dependant on its easy-cleaning property. The higher the polar shares, the better the easy-cleaning property, and the easier the droplet separates from the ceramic surface in water.
Key words:
functional ceramic; physicochemical property; easy-cleaning property; surface free energy; polar shares; disperse shares; contact angles;
1 Introduction
Self-cleaning technology has attracted great attention with increasing problems such as cleaning laboring, and direct harms of detergent to environment and human health. At present, there are five methods to realize antifouling ability and self-cleaning on ceramic surface as follows. 1) Super-hydrophobic coatings. The wetting angle of water was increased by the coatings with low surface free energy so that the contaminant couldn’t be adhered to the sanitaryware[1,2]. 2) Super-smoothness surface. A super-smooth layer was glazed by ToTo Company of Japan to inhibit the contaminants tightly adhering to the sanitaryware. 3) Nano-semiconductor films[3,4]. The coated nano-meter TiO2 thin films not only could photocatalytically degrade the organic but also formed a water film that could clean out the degraded products automatically. 4) Antibacterial and self-cleaning technique[5-7]. The metal antimicrobial was added into the ceramic glaze. 5) Ion intelligent cleaning technique. A special ceramic glaze developed by ToTo Company was sintered on the surface, thus the ceramic surface was not only super-smooth, which decreased the room containing dirt, but formed an isolated wall on the sanitaryware. As soon as the dirt touched the wall, it had been bounced and stopped by an ion force layer.
However, there is little knowledge about the cleaning degree. There are no scientific description and effective examining means either. So, the easy-cleaning was presented. And the experimental method and evaluating criterion of easy-cleaning were set up, respectively[8,9]. On the basis of the past research, the effect of surface free energy of ceramic glaze on oil droplet shape and its behavior in water was studied. It provided a theoretical and experimental gist for developing easy-cleaning functional ceramic.
2 Experimental
2.1 Sample preparation
The sample 40 mm×40 mm×10 mm was made of ceramic body. The ceramic glaze with easy-cleaning property was coated on the body surface sintered beforehand, then dried by ventilation and sintered at
1 210 ℃. Finally, sample A was achieved. The glaze was 150-200 μm in thickness. The main chemical components of glaze are as follows: SiO2: 40-70; Al2O3: 10-14; K2O: 2-5; Na2O: 5-15; CaO: <5; MgO: 2-6; ZnO: <5; Others: <5. Sample B was common sanitaryware.
2.2 Characterization of surface free energy
The ceramic surface free energy and its components were characterized by Germany Dataphysics OCA-30.
2.3 Characterization of oil droplet shape and its behavior
As shown in Fig.1, 15 μL edible salad oil was dropped on the surface of samples A and B, respectively. After the oil droplet was stable, the samples were put into the transparent vessel filled with deionizing water respectively. The oil droplet shape and its behavior in water were recorded. And the contact angle between oil droplet and the ceramic was measured.
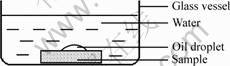
Fig.1 Schematic diagram of characterization of oil droplet
3 Results and discussion3.1 Surface free energy of ceramic glaze
The testing results of samples A and B are shown in Table 1, thereinto SE is the surface free energy calculated by EMIL et al[10] and ZHANG et al[11] according to the contact angle tested.
Table 1 Surface free energy of easy-cleaning ceramic and common sanitaryware

It can be seen from Table 1 that the surface free energy of easy-cleaning ceramic is higher than that of common sanitaryware, and its proportion of polar shares is 92%, which is higher significantly.
3.2 Oil droplet shape and its behavior
3.2.1 Oil droplet behavior on surface of easy-cleaning ceramic
Fig.2 shows the oil droplet shape and its behavior on ceramic surface in 4 s, after the easy-cleaning ceramic with oil droplet was immerged into water.
It can be seen that, as soon as the sample is put into water, the oil droplet on ceramic surface has aggregated. The water spread rapidly on ceramic surface, entering into the solid-liquid interface of ceramic and oil droplet and continually compelling oil droplet to aggregate. As the water entering, the contact area between oil droplet and ceramic interface decreases continually, leading to decreased interaction between oil droplet and ceramic interface, and the oil droplet trends to turn into ball. The floating trend of oil droplet becomes more and more stronger, accompanying with an increasing floating speed. Because more and more water is crushed into the interface of water and oil droplet, the volume of oil becomes bigger and bigger, correspondingly the buoyancy increases rapidly. However, there is continuous interaction between the ceramic surface and oil droplet, though the interaction deceases as the decreasing contact area, the bottom part of oil forms a bottleneck near the ceramic surface due to the floating speed over that of bottom part crushed into the top, as shown in Figs.2(c) and 2(d). When the floating speed of top bottleneck exceeds that of aggregating of bottom, the oil droplet beging to separate from the bottleneck. At this time, the oil droplet is divided into two parts. The top part concerns almost the entire quality of oil droplet and is separated from the ceramic surface to float on the water surface.
After the big oil part separated from the ceramic surface, there leaves a less part on its surface due to the remnant adhesive action between the glaze surface and the oil droplet. Then the less part shrinks rapidly into a ball, at this time the buoyancy of less part is less than that of the adhesive action, thus the less part only forms the little ball and stays steadily on the ceramic surface. The balance could be broken only given a little outer disturbance such as shaking. Tables 2 and 3 show the contact angles(CA) changes of oil droplet and remaining oil on ceramic surface at different times, respectively.
Table 2 Contact angle changes of oil on ceramic surface

Table 3 Contact angle changes of remaining oil on ceramic surface
![]()
It can be seen from the contact angle changes that, in 2.5 s, the contact angle increases rapidly meanwhile the contact area decreases dramatically so that the oil droplet aggregates into ball and floates rapidly from the ceramic surface. After 2.5 s, the most part of oil droplet had separated from the surface, thus the contact angle couldn’t be measured. At 2.5 s, the diameter of bottleneck is 1/10 that of top part droplet. It is thought that the bottleneck is the boundary that the oil droplet is separated from the ceramic surface. At this time, the
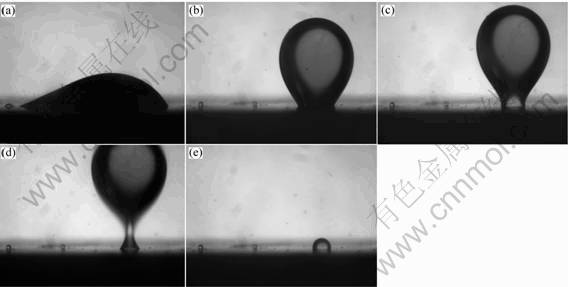
Fig.2 Oil dropket shape and its behavior in water on easy-cleaning ceramic surface after different times: (a) 0.4 s; (b) 1.5 s; (c) 2.5 s; (d) 3.5 s; (e) 4 s
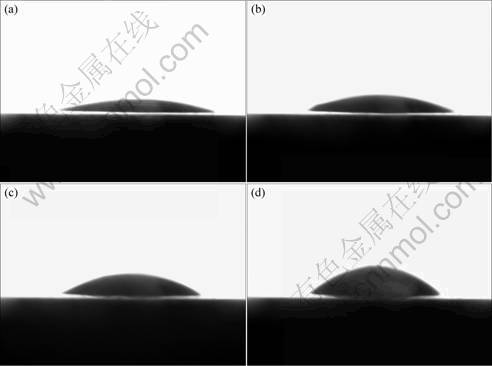
Fig.3 Oil droplet shape and its behavior in water on sanitaryware surface after different time: (a) 0.4 s; (b) 1.5 s; (c) 2.5 s; (d) 3.5 s
bottleneck part couldn’t supply enough stretch force and broke off, causing the thorough separation of top part droplet from the surface. The less part shrink rapidly into ball, at this time the buoyancy of the less part is less than the adhesive action, thus the less part only forms little ball and stays steadily on the ceramic surface.
3.2.2 Oil droplet behavior on surface of common sanitaryware
Due to the lower surface free energy, the interaction between water and surface of common sanitaryware was weaker, leading to the slower change of contact angle. Fig.2 shows the oil droplet shape and its behavior on surface of common sanitaryware. Table 4 shows the contact angle changes.
Table 4 Contact angle changes of oil on sanitaryware surface

From Fig.3 and Table 4, it is concluded that the contact angle increases slowly and the oil droplet slightly aggregates upwards with respect to the float. However, due to the lower surface free energy of sanitaryware and weaker interaction with water, the water couldn’t enter into the interface between oil and ceramic. After the interaction among water, oil and ceramic achieves the balance, the water couldn’t compel the contact area to decrease continually, thus when the top droplet shrink to some extent, for the bottom of oil couldn’t float continually, the top volume couldn’t increase continually, the trend of float couldn’t go on, correspondingly the oil couldn’t break off and separate from the surface. some extent, for the bottom of oil couldn’t float continually, the top volume couldn’t increase continually, the trend of float couldn’t go on, correspondingly the oil couldn’t break off and separate from the surface.
3.2.3 Effect mechanism of surface free energy for oil droplet shape and its behavior in water
The solid surface includes two kinds: the high and low energy surfaces. The high-energy surface means the solid surface with over 500 mJ/m2 and the low one means that with below 100 mJ/m2. Most ceramic glaze surfaces belong to the latter.
According to the wettability condition, the surface free energy decreases greatly owing to the wettability of liquid on the solid surface, thereby the liquid can spread automatically on a high-energy surface. Whereas, for a low one, due to the surface free energy of liquid is similar to that of solid, the wettability differs from the high-energy surface. It was thought by Zisman, the critical surface tension γc is an important constant reflecting the wettability. Only when surface tension less than γc, the liquid can spread on the solid surface. The less the γc of solid, the lower the corresponding surface tension satisfying the wettability and the more difficult the solid was wetted.
The γc value concerns about its components. For ceramic, it is dependant on the characteristics of outermost atom or groups of ceramic glaze. According to elastic equation, the contact angle (θ) is related to γsv, γsl and γlv. From the study and discussion for the wettability by Zisman, the wettability of water for solid is related to the γc value instead of the solid-liquid surface free energy. This is mainly because that in the hydrophilic part or groups between water and solid surface, there is stronger interaction. Thus, the remaining force among the molecules of solid-liquid surface, correspondingly, the less γsl value is which could be omitted during the wettability.
From the surface free energy testing, it is indicated the energy value of ceramic with good easy-cleaning property, is 70 mJ/m2 or so which is similar to the water surface tension, so it is easier for the water to spread on the ceramic surface. From the surface free energy components, the polar shares are mainly which suggests that the polar groups are predominant. Thus, the surface
free energy dramatically increased with increasing polar proportion. The polar water has great interaction with high polar ceramic surface, correspondingly that of oil and ceramic surface is decreased. Thereinto, it is easier for the water to spread and substitute the oil on the ceramic surface.
4 ConclusionsThrough modifying the physical chemistry characteristic of ceramic surface, a super-hydrophilic functional ceramic was prepared by adjusting the chemical components of ceramic glaze, on the basis of the fundamental principles of physical chemistry on solid surface. Water can spread on easy-cleaning ceramic surface, and the oil droplet can aggregate rapidly and separate from the ceramic surface in water. The higher the surface free energy, the better the easy-cleaning property. The easy-cleaning property depends on the polar shares of ceramic with low energy, i.e. the higher the polar shares the better the easy-cleaning property.
References[1] SUSAN A, CHARLES E, FORNALIK J, BRAUNSTEIN G, SRIVIDYA C, BABU S V. Composition and surface energies of plasma-deposited multiplayer fluorocarbon thin films[J]. Surf Coat Technol, 1997, 96(2-3): 210-222.
[2] ATSUSHI H, OSAMU T. Preparation of ultra water-repellent films by microwave plasma-enhanced CVD [J]. Thin Solid Films, 1997, 303(1-2): 222-225.
[3] YU J G, ZHOU M H, YU H G, ZHANG Q J, Y U. Enhanced photoinduced super-hydrophilicity of the sol-gel-derived TiO2 thin films by Fe-doping[J]. Mater Chemi Physi, 2006, 95(2-3): 193-196.
[4] SUISALU A, ARIK J, ANDA H, SILDOS I. Spectroscopic study of nanocrystalline TiO2 thin films grown by atomic layer deposition[J]. Thin Solid Films,1998,336: 295-298.
[5] LIANG J S, LIANG G H, QI H F, JI Z J, JIN Z Z. Influence of composite phosphate inorganic antibacterial materials containing rare earth on activated water property of ceramics[J]. J Rare Earths, 2004, 22(3): 436-439.
[6] KEIJIRO F. Silver-ceramic bactericide[J]. Funct Mater, 1997, 17(4): 41.
[7] LIANG J S, ZHANG J, LIANG G C, WANG L J, LI G S, MENG J P. Effect of rare earth phosphate composite materials on the cleanout oil-dirty property of ceramics[J]. J Rare Earths, 2004, 22(S2): 176-179.
[8] LIANG J S, LIANG G C, WEI C Z, et al. A Method for Testing the Easy-cleaning Property of Easy-cleaning Anti-bacterial Ceramics[P]. CN699956A, 2005.
[9] LIANG J S, LIANG G C,WEI C Z, et al. Super-hydrophilic Easy-cleaning Functional Ceramic Material and Its Prepration[P]. CN/686919A.2005.
[10] EMIL C, RAFAEL P C. Problems of contact angle and solid surface free energy determination[J]. Adv Colloid Interface Sci, 2002, 98: 245-264.
[11] ZHANG J, LIANG J S, OU X Q, WANG L J, LI J Y, PENG Y M. Influence of rare earth composite phosphate on surface free energy of ceramics glaze[J]. J Rare Earths, 2005, 23(S): 156-159.
Corresponding author: LIANG Jin-sheng; Tel: +86-22-26562575; Fax: +86-22-26564850; E-mail: liang_jinsheng@sina.com


