
Synthesis and characterization of novel cathode material Li3V2(PO4)3 by carbon-thermal reduction method
ZHONG Sheng-kui(钟胜奎)1,2, YIN Zhou-lan(尹周澜)1, WANG Zhi-xing(王志兴)3;
GUO Hua-jun(郭华军)3, LI Xin-hai(李新海)3
1.School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2.Department of Material and Chemistry, Guilin University of Technology, Guilin 541004, China;
3.School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Li3V2(PO4)3 cathode material was prepared by a carbon-thermal reduction (CTR) process. V2O5, LiOH?H2O, NH4H2PO4 and C were used as starting materials to synthesize Li3V2(PO4)3 by sintering the mixture at 800 ℃ for 24 h. The property of the Li3V2(PO4)3 sample was investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM) and electrochemical measurement. The results show that the Li3V2(PO4)3 sample has the same monoclinic structure as the Li3V2(PO4)3 sample synthesized by hydrogen reduction method. The particle size is about 1.5 μm together with homogenous distribution. The initial discharge capacity of Li3V2(PO4)3 powder is 120 mA?h?g-1 at the rate of 0.1C,and the capacity retains 112 mA?h?g-1 after 30 cycles.
Key words:
lithium ion batteries; cathode material; Li3V2(PO4)3; carbon-thermal reduction; cyclic voltammetry;
1 Introduction
Currently, LiCoO2 is a cathode material most widely used in lithium-ion batteries. However it is relatively expensive, especially for large-scale applications such as backup power systems and hybrid electric vehicles. Many researchers are trying to find a cheap, effective replacement for LiCoO2[1-7]. Since the demonstration of reversible electrochemical lithium insertion-extraction for LiFePO4 in 1997[8], lithium transition metal phosphates have attracted much great interest as a new promising cathode material for lithium-ion batteries[9-13]. Recently, it was shown that Li could be extracted from Li3V2(PO4)3, its capacity corresponded to a theoretical capacity of 197 mA?h?g-1[14]. Considering the very strong stability of these materials and the voltage range of operation, these compounds can be used as useful positive electrode materials commercially. Usually, Li3V2(PO4)3 was synthesized by hydrogen reduction method[15]. Hydrogen reduction method needs high reaction conditions. It is very difficult to obtain Li3V2(PO4)3 sample with small particle and homogenous distribution, which is crucial to its electrochemical performance, especially the cyclic stability. In this study, Li3V2(PO4)3 sample was synthesized by carbon-thermal reduction, the leaving residual carbon favors stabilization of the vanadium as V3+, which is useful in the subsequent electrode processing, and its electrochemical performance was evaluated.
2 Experimental
Stoichiometric V2O5,LiOH?H2O, NH4H2PO4 and 25%(mass fraction) excess C were first decomposed at 300 ℃ for 4 h to disperse the gas, reground, and then calcined at 800 ℃ for 24 h to obtain Li3V2(PO4)3.
The powder X-ray diffraction (XRD, Rint-2000, Rigaku) measurement using Cu Kα radiation was employed to identify the crystalline phase of the synthesized materials, recorded at room temperature. The particle size and morphology of the Li3V2(PO4)3 powders was observed with scanning electron microscope (JEOL, JSM-5600LV) at an accelerating voltage of 20 kV.
The electrochemical characterizations were performed using CR2025 coin-type cell. For positive electrode fabrication, the prepared powders were mixed with 10% of carbon black and 10% of polyvinylidene fluoride in N-methyl pyrrolidinone until slurry was obtained. And then, the blended slurries were pasted onto an aluminum current collector, and the electrode was dried at 120 ℃ for 10 h in vacuum. The test cell consisted of the positive electrode and lithium foil negative electrode separated by a porous polypropylene film, and 1 mol/L LiPF6 in EC, EMC and DMC(1∶1∶1 in volume) as the electrolyte. The assembly of the cells was carried out in a dry Ar-filled glove box. The cells were charged and discharged over a voltage range of 3.0 V to 4.2 V versus Li/Li+ electrode at room temperature. Cyclic voltammograms were tested at a scanning rate of 0.1 mV/s in the voltage ranges of 3.0-4.4 V.
3 Results and discussion
Fig.1 shows the XRD pattern collected for the prepared Li3V2(PO4)3 powders. It’s evident that all fundamental peaks can be indexed to the monoclinic structure(space group P21/n).
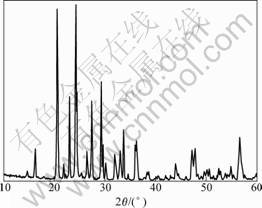
Fig.1 XRD pattern of Li3V2(PO4)3
The SEM morphology of the Li3V2(PO4)3 sample SEM is shown in Fig.2. Fine particles can be observed, and the particle size is about 1.5 μm together with homogenous distribution. The SEM micrograph indicates the characteristics of the composite product morphology, revealing the presence of carbon in the Li3V2(PO4)3 powders.
The first charge-discharge curves of Li3V2(PO4)3 cycled between 3.0 V and 4.2 V at the rate of 0.1C are shown in Fig.3. Three regions are seen in the charge and discharge curves. These regions span the following composition ranges: x=0-0.5, 0.5-1.0 and 1.0-2.0 in Li(3-x)V2(PO4)3, respectively. In each region, plateaus are observed, substantiating the two phase characteristics of the electrochemical reaction in this range, with little or no lithium solubility in each phase. Under the current regime used, the first two lithiums are extracted at an average voltage of 3.65 and 4.08 V versus Li/Li+, respectively. In turn, the first lithium is removed in two steps,i.e.3.60 and 3.70 V versus Li/Li+, followed by a charge capacity of 28 and 34 mA?h?g-1, respectively. The second lithium, however, is extracted over one single step, 4.08 V versus Li/Li+, followed by a charge capacity of 63 mA?h?g-1. The discharge step consists of the re-insertion of both lithiums with a small reduction in the reversible charge return. The last two lithiums are inserted at an average voltage of 4.00 and 3.62 V versus Li/Li+, followed by a charge capacity of 60 and 60 mA?h?g-1, respectively. Namely, the Li3V2(PO4)3 sample exhibits a charge capacity about 125 mA?h?g-1, and a discharge capacity about 120 mA?h?g-1 in the initial charge/discharge cycle, indicating a high coulombic efficiency of about 96%.

Fig.2 SEM image of Li3V2(PO4)3
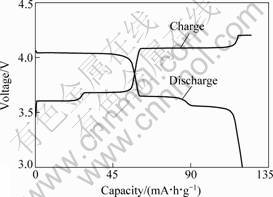
Fig.3 First charge-discharge curves of Li3V2(PO4)3 at the rate of 0.1C
The electrochemical cycling performance of the Li3V2(PO4)3 compound was evaluated in the Li/Li3V2(PO4)3 cell configuration in the voltage range of 3.0-4.2 V at room temperature. Fig.4 shows the cyclic charge/discharge profiles of the Li3V2(PO4)3 cathode materials at rate 0.1C. As seen in Fig.4, the initial discharge capacity of Li3V2(PO4)3 is about 120 mA?h?g-1, and the discharge capacity drops to about 112 mA?h?g-1 after 30 cycles. The capacity loss is about 6.67% after 30 cycles.
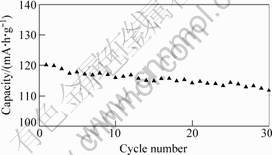
Fig.4 Electrochemical cycling performance of Li3V2(PO4)3
The cyclic voltammetry (CV) for the Li3V2(PO4)3 electrode in the first cycle at a scanning rate of 0.1 mV/s are shown in Fig.5. The cyclic voltammetry profile indicates the oxidation/reduction potential at which lithium ion was extracted from the lattice or inserted into the lattice. As shown in Fig.5, the electrode of Li3V2(PO4)3 exhibits three oxidation peaks around 4.11, 3.71 and 3.63 V and three reduction peaks around 4.00, 3.67 and 3.59 V, which are in good agreement with the first charge-discharge results.
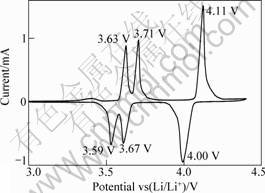
Fig.5 CVs of Li3V2(PO4)3 electrode in first cycle
4 Conclusions
Li3V2(PO4)3 powder are prepared successfully by a carbon-thermal reduction (CTR) process under Ar atmosphere. X-ray diffraction (XRD) patterns show that Li3V2(PO4)3 has monoclinic structure. Scanning electron microscopic (SEM) images show that the particle size is about 1.5 μm together with homogenous distribution. Initial charge and discharge capacities are approximately 125 mA?h?g-1 and 120 mA?h?g-1, respectively. The discharge capacity retains 112 mA?h?g-1 after 30 cycles.In summary, the demonstrated performance of the Li3V2(PO4)3 synthesized by a carbon-thermal reduction may promote the application of this cathode material to commercial lithium-ion batteries.
References
[1] BARKER J, SAIDI M Y, SWOYER J, et al. Electrochemical insertion properties of the novel lithium vanadium fluorophosphates, LiVPO4F[J]. J Electrochem Soc, 2003, 150(10): A1394-A1398.
[2] TAKAHASHI K, SAITOH M, ASAKURA N, et al. Electrochemical properties of lithium manganese oxides with different surface areas for lithium ion batteries[J]. J Power Sources, 2004, 136: 115-121.
[3] ARACHI Y, KOBAYASHI H, EMURA S, et al. Li de-intercalation mechanism in LiNi0.5Mn0.5O2 cathode material for li-ion batteries[J]. Solid State Ionics, 2005, 176(9-10): 895-903.
[4] LIU Q Y, LIU H W, ZHOU X W, et al. A Soft chemistry synthesis and electrochemical properties of LiV3O8 as cathode material for lithium secondary batteries[J]. Solid State Ionics, 2005, 176(17-18): 1549-1554.
[5] PARK S H, SUN Y K. Synthesis and electrochemical properties of 5V spinel LiNi0.5Mn0.5O4 cathode material prepared by ultrasonic spray pyrolysis method[J]. Electrochemica Acta, 2004, 50(2-3): 427-430.
[6] PARK S H, OH S W, MYUNG S T, et al. Effects of synthesis condition on LiNi1/2Mn3/2O4 cathode material for prepared by ultrasonic spray pyrolysis method[J]. Solid State Ionics, 2005, 176(5-6): 481-486.
[7] DENG Ling-feng, LI Xin-hai, XIAO Li-xin, et al. Synthesis and electrochemical properties of polyradical cathode material for lithium second batteries[J]. J Cent South Univ Techno, 2003, 10(3): 190-193.
[8] PANHI A K, NANJUNDASWAMY K S, GOODENPUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. J Electrochem Soc, 1997, 144(4): 1188-1194.
[9] HU Y Q, DOEFF M M, KOSTECKI R, et.al. Electrochemical Performance of Sol-Gel Synthesized LiFePO4 in lithium batteries[J]. J Electrochem Soc, 2004, 151(8): A1279-A1285.
[10] PATOUX S, WURM C, MORCRETTE M, et al. A comparative structural and electrochemical study of monoclinic Li3Fe2(PO4)3 and Li3V2(PO4)3[J]. J Power Sources, 2003, 119-121: 278-284.
[11] RICHARDSON T J. Phosphate-stabilized lithium intercalation compounds[J]. J Power Sources, 2003, 119-121: 262-265.
[12] ZHOU F, KANG K, MAXISCH T, et al. The electronic structure and band gap of LiFePO4 and LiMnPO4[J]. Solid State Communications, 2004, 132(3-4): 181-186.
[13] ZANE D, CAREWSKA M, SCACIA S, et al. Factor affecting rate performance of undoped LiFePO4[J]. Electrochimica Acta, 2004, 49(5): 4259-4271.
[14] BARKER J, SAIDI M Y.Lithium-containing phosphates,method of preparation, and use thereof[P]. USP: 005871866A, 1999.
[15] MORGAN D, CEDER G, SAIDI M Y, et al. Experimental and computational study of the structure and electrochemical properties of monoclinic LixM2(PO4)3 compounds[J]. J Power Sources, 2003, 119-121: 755-759.
Foundation item: Project(50302016) supported by the National Natural Science Foundation of China
Corresponding author: ZHONG Sheng-kui; Tel: +86-132-07497471; E-mail: zskui74@163.com


