
J. Cent. South Univ. (2020) 27: 1176-1185
DOI: https://doi.org/10.1007/s11771-020-4358-2
Preparation of magnetic Fe3O4@Cu/Ce microspheres for efficient catalytic oxidation co-adsorption of arsenic(III)
JIN Lin-feng(金林锋)1, CHAI Li-yuan(柴立元)1, 2, SONG Ting-ting(宋婷婷)3,YANG Wei-chun(杨卫春)1, 2, WANG Hai-ying(王海鹰)1, 2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control and Treatment of Heavy Metal Pollution, Changsha 410083, China;
3. Beijing Urban Construction Design and Development Group Co., Ltd., Beijing 100037, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Magnetic Fe3O4@Cu/Ce microspheres were successfully prepared by one-step solvothermal approach and further utilized to remediate toxic arsenic (As(III)) pollution. The effects of Cu/Ce elements co-doping on the crystal structure, catalytic oxidation and adsorption behaviors of magnetic microspheres were researched systematically. The results showed that with the aid of Cu/Ce elements, the grain size reduced, lattice defects increased, and the oxygen vacancies and surface hydroxyl groups were improved. Therefore, Cu/Ce elements endowed magnetic Fe3O4@Cu/Ce microspheres with excellent As(III) removal performance, whose maximum adsorption capacity reached 139.19 mg/g. The adsorption mechanism mainly involved catalytic oxidant co-adsorption. This research developed a feasible strategy for the preparation of high efficiency magnetic adsorbent to enhance the removal of As(III).
Key words:
Cu/Ce; magnetic composites; As(III); catalytic oxidation; adsorption;
Cite this article as:
JIN Lin-feng, CHAI Li-yuan, SONG Ting-ting, YANG Wei-chun, WANG Hai-ying. Preparation of magnetic Fe3O4@Cu/Ce microspheres for efficient catalytic oxidation co-adsorption of arsenic(III) [J]. Journal of Central South University, 2020, 27(4): 1176-1185.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4358-21 Introduction
Arsenic (As) pollution is seriously hazardous to human beings and ecological systems, due to its toxic and carcinogenic nature [1-4]. The World Health Organization stipulates that the maximum concentration of arsenic contamination shall not exceed 10 μg/L [5]. Arsenic mainly exists in the forms of arsenate (As(V)) and arsenite (As(III)), and the latter is more mobile and toxic than the former [6, 7]. Therefore, the remediation of As(III) pollution is extremely urgent. At present, various technologies have been reported, such as membrane separation [8], ion exchange [9] and precipitation [10]. Among them, adsorption is an alternative method because of high efficiency, low cost and easy regeneration. Therefore, the development of high-performance adsorbents for As(III) removal is of significant importance.
Magnetic composites, especially magnetite (Fe3O4) composites, have aroused increasing attention owing to its outstanding physicochemical properties, easy recycle and high efficiency in the environmental fields [11-16]. However, magnetic composites can’t effectively remove As(III) due to the uncharged property of As(III) in acid or neutral solution. In contrast, As(V) is negatively charged in a wide range of pH. Therefore, the conversion from As(III) to As(V) is a critical factor in the removal process. Accordingly, the strategy of catalytic oxidation co-adsorption is proposed. However, the performance of traditional magnetic composites converting As(III) to As(V) is unfavorable.
Generally, copper (Cu) possesses outstanding catalytic properties in the forms of isolated Cu2+ ions and highly dispersed clusters [17, 18]. In addition, Ceria (Ce) has also attracted increasing attention due to its good redox and catalytic properties [19, 20]. Also, the synergistic effects of dispersed Cu and Ce would enhance the catalytic oxidant performances of composites [21].
In this work, the strategy of enhancing catalytic oxidation co-adsorption of magnetic composites was proposed by co-doping Cu/Ce elements. The as-prepared magnetic Fe3O4@Cu/Ce microspheres were characterized in detail and applied to remove As(III). Characterizations and adsorption experiments were performed to understand the property of microspheres and As(III) adsorption behavior. Finally, the As(III) removal mechanism was proposed.
2 Material and methods
2.1 Materials
Cerium chloride heptahydrate (CeCl3·7H2O, 99.9 %), cupric chloride dihydrate (CuCl2·2H2O, AR), ferric chloride hexahydrate (FeCl3·6H2O, AR), anhydrous sodium acetate (CH3COONa, AR) and ethylene glycol (EG, AR) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (China). As(III) solution was prepared by dissolving NaAsO2 in deionized (DI) water. All other chemicals are analytical grade and do not require further purification.
2.2 Synthesis of magnetic Fe3O4@Cu/Ce microspheres
Magnetic Fe3O4@Cu/Ce microspheres with different mass ratios of Cu to Ce were synthesized through a facile solvothermal approach. Firstly, FeCl3·6H2O, CuCl2·2H2O and CeCl3·7H2O were dissolved in 36 mL EG solution, and then 3.6 g CH3COONa was added into above solution with vigorously stirring for 40 min. Secondly, the mixed solution was put into a Teflon-lined autoclave at 200 °C for 6 h. Finally, the precipitate was filtrated, washed with plenty of DI water and ethanol. The obtained composites were dried at 65 °C for 6 h. For comparison, pure Cu doped magnetic microsphere was prepared under the same condition without adding Ce. The as-prepared magnetic microspheres doped with Cu and Ce elements with different molar ratios (i.e., 1:0, 10:1, 5:1, 2.5:1, 1:1, 0.5:1) were synthesize in turn, and labeled as CCFX (X=0, 1, 2, 3, 4, 5).
2.3 Characterization
The morphology and structure of the magnetic CCFX microspheres were characterized by scanning electron microscopy (SEM-EDS, JSM-6360), X-ray diffraction (XRD, Rigaku D/Max-RB diffractometer), Raman spectroscopy (Renishow Invia, 532 nm), X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific KAlpha 1063, Al Kα), Fourier transformed infrared spectroscopy (FT-IR, Nicolet IS10), respectively. Hydrogen temperature-programmed reduction (H2-TPR) was performed in PCA-1200 instrument.
2.4 Batch experiments
Adsorption experiments were conducted at 25 °C, 180 r/min, 0.5 g/L dose. Different pH values were regulated by HCl or NaOH solution. Within the specified time, adsorbent was separated by external magnetic field. The initial and residual arsenic concentrations were detected through inductively coupled plasma-optical emission spectroscopy (ICP-OES, ICAP 7000). The regeneration experiments were conducted using 1 mol/L NaOH solution. The absorbent was shaken in NaOH solution for 12 h, and then separated by magnetic and rinsed with water. Finally, the adsorbent was applied to the next adsorption experiments.
3 Results and discussion
3.1 Materials characterization
The morphologies of CCFX microspheres are seen from Figure 1. CCFX microspheres exhibited spherical grains and the diameter of CCF0 was ~150 nm (Figure 1(a)). In contrast, CCF1-5 have the diameters range from ~120 to ~50 nm (Figure 1(b)-(f)). The decrease in the diameter of CCF1-5 was largely due to the introduction of Cu/Ce elements, which acted as an inhibitor. SEM-EDS mappings of CCF0 and CCF4 are shown in Figures 1(g) and (h), and Fe, O, Cu and Ce elements of CCF4 microsphere were more homogeneous than that of CCF0. Therefore, the existence of Cu/Ce elements enhanced the elements dispersion, which was conducive to the improvement of catalytic activity and adsorption performance [22].

Figure 1 SEM images of CCF0-5 (a-f) and SEM-EDS mappings of CCF0 (g) and CCF4 (h)
XRD, FT-IR, Raman and XPS technologies were applied to characterize the structure of CCFX microspheres, respectively. As shown in Figure 2(a), CCFX microspheres showed similar peaks with the typical peaks of Fe3O4 and Cu without the peaks of Ce or Ce oxide. The high-magnified XRD patterns of Cu(111) are shown in Figure 2(b). The Cu(111) diffraction peaks shifted obviously, which probably originated from the strong interaction between Cu and Ce elements [23]. In addition, XRD patterns also revealed that the characteristic peaks of CCF1-5 microspheres are broad and strong than that of CCF0. The phenomenon was attributed to the decrease of grain size and strain, as well as the generation of oxygen vacancies in the lattice. Meanwhile, the reverse trend was observed when further increasing the molar ratio of Ce to Cu (CCF4 and CCF5) because of the tight aggregation of microspheres [24].
As shown in Figure 2(c), the peaks of O—H bond (3000-3600 cm-1), H—O—H bond (1637 cm-1) and hydroxyl groups on metal oxides (M—OH) (1060 cm-1) showed increased intensity, which originated from the enhanced oxygen vacancies [25]. Raman spectra are seen from Figure 2(d). The peaks of CCF0 at 218 and 282 cm-1 were attributed to Cu2O and CuO, respectively [26]. The peak at 662 cm-1 was attributed to the magnetite band [27]. After co-doping Cu/Ce elements, the peaks of CCF1-5 at 218 and 282 cm-1 missed, indicating that the introduction of Ce element led to microscopic strain effects and high disorder. Meanwhile, the peaks at 677 cm-1 were strengthened. Furthermore, these peaks shifted slightly to a high frequency, indicating the formation of lattice defects and oxygen vacancies [28].
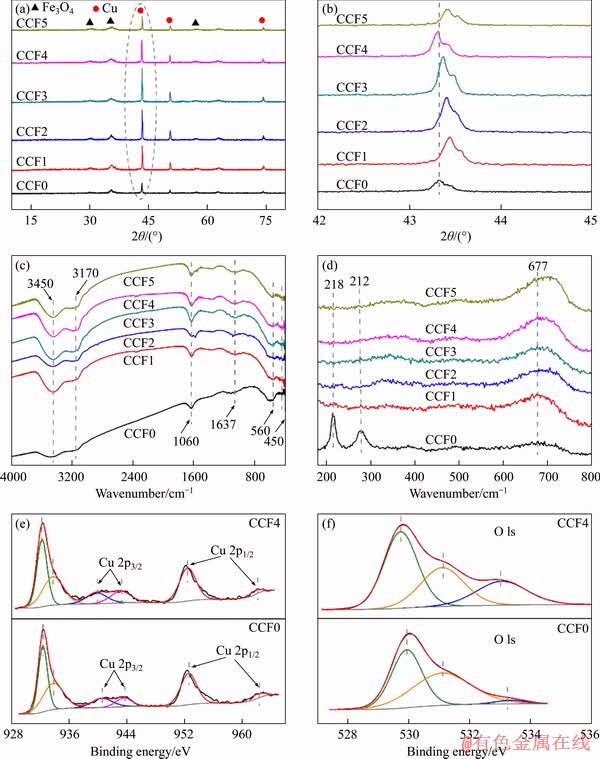
Figure 2 XRD patterns (a), high-magnified XRD patterns (b), FT-IR spectra (c), Raman spectra (d), XPS spectra of CFX: (e) Cu 2p; (f) O 1s
The chemical states of magnetic CCFX microspheres were further analyzed, and Cu 2p XPS spectra were shown in Figure 2(e). The Cu 2p3/2 peaks of CCF0 and CCF4 were observed at 932.5 and 933.9 eV respectively, and the two satellite peaks at 940.6 and 943.7 eV implied the existence of CuO, Cu2O and Cu. The peak at 952.6 eV was assigned to Cu 2p1/2, and the peak at 961.8 eV belonged to the satellite peaks of Cu 2p1/2 [29]. Hence, the introduction of Ce element induced an imbalance charge and unsaturated chemical bonds on the CCF4 surface, which would enhance oxygen vacancies and bring large amounts of hydroxyl groups [29, 30]. The O 1s spectra (Figure 2(f)) were also divided into three peaks (530.0, 531.1 and 533.1 eV), which were originated to lattice oxygen, surface adsorbed oxygen as well as chemisorbed water, respectively [31]. The shift of lattice oxygen peak to low binding energy would facilitate to catalytic oxidation of As(III) [24].
3.2 Adsorption experiments
3.2.1 Adsorption isotherms
Adsorption isotherms of CCFX microspheres with different initial As(III) concentrations were investigated. As shown in Figure 3(a), CCF0 had the lowest As(III) adsorption capacity. However, CCF1-4 microspheres exhibited the enhanced adsorption performance for As(III), implying that the co-doping Cu/Ce elements facilitated the removal of As(III). It was noted that the adsorption performance of CCF5 microspheres was decreased, probably due to the serious aggregation of microspheres, as shown in Figure 1(f). The adsorption data were matched by Langmuir and Freundlich isotherm models, which were depicted in Eqs. (1) and (2), respectively [32].
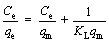 (1)
(1)
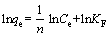 (2)
(2)
The calculated isotherm parameters were shown in Table 1. The data possessed favorable correlation to Freundlich model, which described the multilayer adsorption process [33]. In addition, the maximum As(III) removal performance of CCF4 microsphere was 139.19 mg/g, which was compared with that of other typically magnetic adsorbents, as seen from Table 2, indicating the bright prospects of CCF4 microspheres in the field of As(III) remediation.
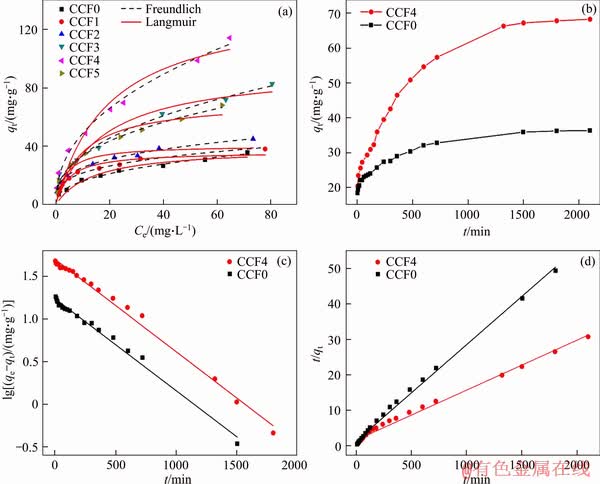
Figure 3 Adsorption isotherms of CCFX microspheres (a), adsorption kinetics of CCF0 and CCF4 microspheres (b), pseudo-first-order (c) and pseudo-second order models (d) of CCF0 and CCF4 microspheres
3.2.2 Adsorption kinetics
The effect of time on the removal performance of CCF0 and CCF4 microspheres was explored, as shown in Figure 2(b). The uptake amounts of As(III) quickly increased at the initial stage, and then slowly increased until the adsorption equilibrium. In addition, CCF4 microspheres showed a favorable As(III) removal performance compared with CCF0. Pseudo-first-order and pseudo-second-order kinetic models were utilized to simulate the removal process (Figures 2(c), (d)). The mathematical expressions of pseudo-first-order and pseudo-second-order adsorption models are depicted in Eqs. (3) and (4), respectively [39, 40].
ln(qe-qt)=lnqe-k1t (3)
 (4)
(4)
The corresponding kinetic parameters of CCF0 and CCF4 microspheres were presented in Table 3. According to the coefficient values, pseudo-second- order model was more suitable to describe the kinetic process, suggesting that As(III) adsorption process was a chemical adsorption process [41].
Table 1 Langmuir and Freundlich coefficients of magnetic CCFX microspheres
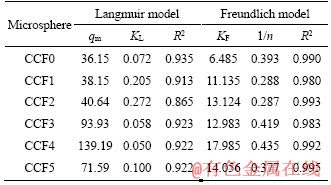
Table 2 Comparison of removal performance between magnetic CCF4 microspheres and other typically magnetic adsorbents
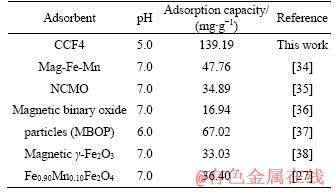
Table 3 Coefficients of pseudo-first-order and pseudo- second-order kinetic models of CCF0 and CCF4 microspheres

3.2.3 Regeneration
Recyclability is a crucial factor in the practical application of adsorbents. Regeneration experiments were performed to examine the recyclability of CCF4 microspheres. As seen from Figure 4, there is a slight decrease in the adsorption efficiency of CCF4 microsphere when increasing recycles. The decreased adsorption performance may be attributed to the reduction of binding sites, which were occupied by adsorbed As(III). Nevertheless, the adsorption performance of CCF4 microspheres remained about 75 % after five cycles, revealing a promising future.
3.3 As(III) adsorption mechanism
H2-TPR and XPS technologies were performed to investigate the As(III) adsorption mechanism, as shown in Figure 5(a). The H2-TPR of CCF0 microsphere presented three reduction peaks at ~220, ~600 and ~710 °C, which were ascribed to the reduction of copper ions (Cu2+ → Cu+ → Cu0), iron ions (Fe3O4 → FeO → Fe0) and bulk oxygen, respectively [42]. Interestingly, the synergistic interactions of Cu/Ce elements obviously decreased hydrogen consumption and reduction temperatures of CCF4 microsphere. These changes not only decreased the consumption of active sites by lower the temperature, but also promoted the migration of oxygen in the crystal structures. Furthermore, the oxygen vacancies would improve the reducibility and catalytic ability of CCF4 microspheres.
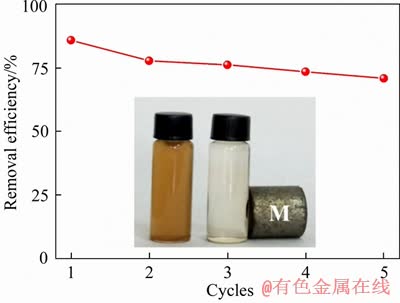
Figure 4 Regeneration of CCF4 microsphere (insets: photograph of As(III) solution before and after adsorption)
XPS spectra of CCF0 and CCF4 microspheres treated with As(III) were analyzed and the chemical state of As(III) on the surface of the microspheres were investigated, as presented in Figure 5(b). The As 3d peak was divided into two peaks at ~44.1 and ~45.3 eV [43, 44], indicating the existence of As(III) and As(V) species after treatment. In addition, As(V) contributed up to 80% of adsorbed As species on the surface of CCF4 microsphere, which was higher than CCF0 microsphere (63.7%). This effect indicated that Ce/Cu elements facilitated the catalytic oxidation of As(III) to As(V).
XPS spectra of CCF4 microsphere before and after As(III) adsorption were recorded to further investigate the adsorption mechanism, as seen from Figures 5(c) and (d). The Fe 2p peaks of CCF4 were similar to CCF4-As(III), suggesting no phase transformation of Fe3O4. The hydroxyl groups decreased from 37.2 % to 28.0 % after As(III) adsorption, revealing the significant interactions in the adsorption process. Moreover, the binding energy of lattice oxygen shifted to a low energy region, and the proportion of M-O increased from 51.0 % to 54.0 %. The improvement may be ascribed to the formation of M-O, As-O and As-O-M species on the CCF4 surface after adsorption.
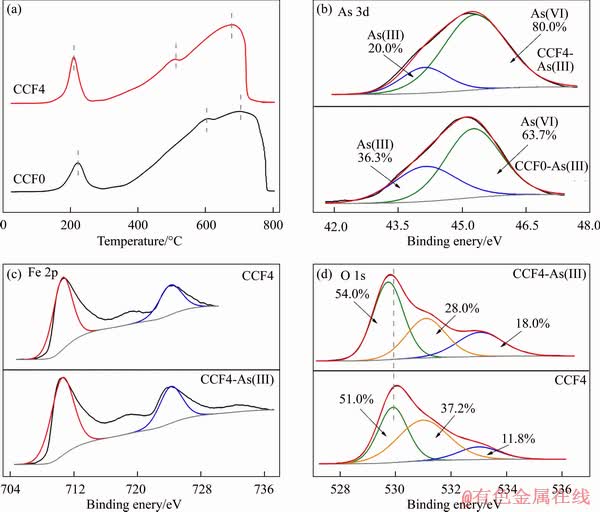
Figure 5 H2-TPR spectra (a), As 3d spectra after treating As(III) (b), Fe 2p spectra (c) and O 1s spectra (d) before and after As(III) adsorption
4 Conclusions
This study illustrated the catalytic oxidation co-adsorption of magnetic Fe3O4@Cu/Ce microspheres for efficient As(III) removal from aqueous solutions. The co-doping Cu/Ce elements obviously improved the concentration of surface oxygen vacancies on the surface of magnetic Fe3O4@Cu/Ce microspheres. Furthermore, magnetic Fe3O4@Cu/Ce microspheres possessed excellent As(III) adsorption capacity (139.19 mg/g), far higher than other typical magnetic adsorbents. Profoundly, the effective catalytic activity to convert As(III) to As(V) was successfully realized by dissolved oxygen and lattice oxygen with the aid of Cu/Ce elements. In summary, co-doping Cu/Ce elements provided a feasible strategy for efficient removal and detoxification of As(III) from aqueous solution.
References
[1] SHAKOOR M B, NIAZI N K, BIBI I, SHAHID M, SAQIB Z A, NAWAZ M F, SHAHEEN S M, WANG H, TSANG D C W, BUNDSCHUH J, OK Y S, RINKLEBE J. Exploring the arsenic removal potential of various biosorbents from water [J]. Environment International, 2019, 123: 567-579. DOI: 10.1016/j.envint.2018.12.049.
[2] XU Fang-nan, CHEN Hu-xing, DAI Yu-xia, WU Shuang-lei, TANG Xian-jin. Arsenic adsorption and removal by a new starch stabilized ferromanganese binary oxide in water [J]. Journal of Environment Management, 2019, 245: 160-167. DOI: 10.1016/j.jenvman.2019. 05.071.
[3] ASERE T G, STEVENS C V, DU LAING G. Use of (modified) natural adsorbents for arsenic remediation: A review [J]. Science of the Total Environment, 2019, 676: 706-720. DOI: 10.1016/j.scitotenv.2019.04.237.
[4] SUN Tian-yi, ZHAO Zhi-wei, LIANG Zhi-jie, LIU Jie, SHI Wen-xin, CUI Fu-yi. Efficient degradation of p-arsanilic acid with arsenic adsorption by magnetic CuO-Fe3O4 nanoparticles under visible light irradiation [J]. Chemical Engineering Journal, 2018, 334: 1527-1536. DOI: 10.1016/ j.cej.2017.11. 052.
[5] XI Yun-hao, ZOU Jing-tian, LUO Yong-guang, LI Jing, LI Xi-teng, LIAO Tian-qi, ZHANG Li-bo, WANG Chen, LIN Guo. Performance and mechanism of arsenic removal in waste acid by combination of CuSO4 and zero-valent iron [J]. Chemical Engineering Journal, 2019, 375: 121928. DOI: doi.org/10.1016/j.jcis.2018.02.046.
[6] HE Xin-yu, DENG Fang, SHEN Ting-ting, YANG Li-ming, CHEN De-zhi, LUO Jian-feng, LUO Xu-biao, MIN Xiao-ye, WANG Fang. Exceptional adsorption of arsenic by zirconium metal-organic frameworks: Engineering exploration and mechanism insight [J]. Journal of Colloid and Interface Science, 2019, 539: 223-234. DOI: 10.1016/ j.jcis.2018.12. 065.
[7] YU Yang, YU Ling, SHIH Kai-min, CHEN J P. Yttrium-doped iron oxide magnetic adsorbent for enhancement in arsenic removal and ease in separation after applications [J]. Journal of Colloid and Interface Science, 2018, 521: 252-260. DOI: doi.org/10.1016/j.jcis.2018. 02.046.
[8] NASIR A M, GOH P S, ISMAIL A F. Highly adsorptive polysulfone/hydrous iron-nickel-manganese (PSF/HINM) nanocomposite hollow fiber membrane for synergistic arsenic removal [J]. Separation and Purification Technology, 2019, 213: 162-175. DOI: 10.1016/j.seppur.2018. 12.040.
[9] CHEN M, SHAFER-PELTIER K, RANDTKE S J, PELTIER E. Modeling arsenic (V) removal from water by micellar enhanced ultrafiltration in the presence of competing anions [J]. Chemosphere, 2018, 213: 285-294. DOI: 10.1016/j. chemosphere.2018.09.046.
[10] JAHROMI F G, GHAHREMAN A. In-situ oxidative arsenic precipitation as scorodite during carbon catalyzed enargite leaching process [J]. Journal of Hazardous Materials, 2018, 360: 631-638. DOI: 10.1016/j.jhazmat.2018. 08.019.
[11] CAI Gui-yuan, ZHU Xing, LI Kong-zhai, QI Xian-jin, WEI Yong-gang, WANG Hua, HAO Feng-yan. Self-enhanced and efficient removal of arsenic from waste acid using magnetite as an in situ iron donator [J]. Water Research, 2019, 157: 269-280. DOI: 10.1016/j.watres.2019.03.067.
[12] WU Kun, JING Chun-yang, ZHANG Jin, LIU Ting, YANG Sheng-jiong, WANG Wen-dong. Magnetic Fe3O4@CuO nanocomposite assembled on graphene oxide sheets for the enhanced removal of arsenic(III/V) from water [J]. Applied Surface Science, 2019, 466: 746-756. DOI: 10.1016/j.apsusc. 2018.10.091.
[13] QI Peng-fei, LUO Rong, PICHLER T, ZENG Jian-qiang, WANG Yan, FAN Yu-hua, SUI Kun-yan. Development of a magnetic core-shell Fe3O4@TA@UiO-66 microsphere for removal of arsenic(III) and antimony(III) from aqueous solution [J]. Journal of Hazardous Materials, 2019, 378: 120721. DOI: 10.1016/j.jhazmat.2019.05.114.
[14] WANG Hai-ying, HE Ying-jie, CHAI Li-yuan, LEI Huang, YANG Wei-chun, HOU Lan-jing, YUAN Tao, JIN Lin-feng, TANG Chong-jian, LUO Jian. Highly-dispersed Fe2O3@C electrode materials for Pb2+ removal by capacitive deionization [J]. Carbon, 2019, 153: 12-20. DOI: 10.1016/ j.carbon.2019. 06.066.
[15] WU Jin, ZHU Hong-shan, LIU Ge, TAN Li-qiang, HU Xiao-ye, CHEN Chang-lun, ALHARBI N S, HAYAT T, TAN Xiao-li. Fabrication of core–shell CMNP@PmPD nanocomposite for efficient As(V) adsorption and reduction [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(5): 4399-4407. DOI: 10.1021/ acssuschemeng.7b00468.
[16] WU Jin, CHEN Ke, TAN Xiao-li, FANG Ming, HU Xiao-ye, TANG Zhen-wu, WANG Xiang-ke. Core-shell CMNP@PDAP nanocomposites for simultaneous removal of chromium and arsenic [J]. Chemical Engineering Journal, 2018, 349: 481-490. DOI: 10.1016/j.cej.2018.05.114.
[17] PENG Bing, SONG Ting-ting, WANG Ting, CHAI Li-yuan, YANG Wei-chun, LI Xiao-rui, LI Chao-fang, WANG Hai-ying. Facile synthesis of Fe3O4@Cu(OH)2 composites and their arsenic adsorption application [J]. Chemical Engineering Journal, 2016, 299: 15-22. DOI: 10.1016/j.cej. 2016.03.135.
[18] XIE Wu-ming, ZHOU Feng-ping, BI Xiao-lin, CHEN Dong-dong, HUANG Zi-jun, LI Yu-hui, SUN Shui-yu, LIU Jing-yong. Decomposition of nickel(II)- ethylenediaminetetraacetic acid by fenton-like reaction over oxygen vacancies-based Cu-doped Fe3O4@γ-Al2O3 catalyst: A synergy of oxidation and adsorption [J]. Chemosphere, 2019, 221: 563-572. DOI: 10.1016/j.chemosphere.2019.01. 083.
[19] AASHIMA A, UPPAL S, ARORA A, GAUTAM S, SINGH S, CHOUDHARY R J, MEHTA S K. Magnetically retrievable Ce-doped Fe3O4 nanoparticles as scaffolds for the removal of azo dyes [J]. RSC Advances, 2019, 9(40): 23129-23141. DOI: 10.1039/C9RA03252E.
[20] GOGOI A, NAVGIRE M, SARMA K C, GOGOI P. Fe3O4-CeO2 metal oxide nanocomposite as a Fenton-like heterogeneous catalyst for degradation of catechol [J]. Chemical Engineering Journal, 2017, 311: 153-162. DOI: 10.1016/ j.cej.2016.11.086.
[21] MORETTI E, STORARO L, TALON A, RIELLO P, MOLINA A I, RODRIGUEZ-CASTELLON E. 3-D flower like Ce–Zr–Cu mixed oxide systems in the CO preferential oxidation (CO-PROX): Effect of catalyst composition [J]. Applied Catalysis B: Environmental, 2015, 168-169: 385-395. DOI: 10.1016/j.apcatb.2014.12.032.
[22] ZHANG Yan-ping, FLYTZANI-STEPHANOPOULOS M. Hydrothermal stability of cerium modified Cu-ZSM-5 catalyst for nitric oxide decomposition [J]. Journal of Catalysis, 1996, 164: 131-145. DOI: 10.1006/jcat. 1996.0369.
[23] CAI Wei, ZHONG Qin, YU Yang, DAI Sheng. Correlation of morphology with catalytic performance of CrOx/Ce0.2Zr0.8O2 catalysts for NO oxidation via in-situ STEM [J]. Chemical Engineering Journal, 2016, 288: 238-245. DOI: 10.1016/j.cej. 2015.12.009.
[24] JAMPAIAH D, IPPOLITO S J, SABRI Y M, REDDY B M, BHARGAVA S K. Highly efficient nanosized Mn and Fe codoped ceria-based solid solutions for elemental mercury removal at low flue gas temperatures [J]. Catalysis Science Technology, 2015(5): 2913-2924. DOI: 10.1039/ C5CY00231A.
[25] PENG Cong, CHAI Li-yuan, TANG Chong-jian, MIN Xiao-bo, SONG Yu-xia, DUAN Cheng-shan, YU Cheng. Study on the mechanism of copper-ammonia complex decomposition in struvite formation process and enhanced ammonia and copper removal [J]. Journal of Environmental Sciences, 2017, 51: 222-233. DOI: 10.1016/j.jes.2016. 06.020.
[26] CHOU M H, LIU S B, HUANG C Y, WU S Y, CHENG C L. Confocal Raman spectroscopic mapping studies on a single CuO nanowire [J]. Applied Surface Science, 2008, 254(23): 7539-7543. DOI: 10.1016/j.apsusc.2007.12.065.
[27] THI T M, TRANG N T H, van ANH N T. Effects of Mn, Cu doping concentration to the properties of magnetic nanoparticles and arsenic adsorption capacity in wastewater [J]. Applied Surface Science, 2015, 340: 166-172. DOI: 10.1016/j.apsusc. 2015.02.132.
[28] YEN H, SEO Y, KALIAGUINE S, KLEITZ F. Tailored mesostructured copper/ceria catalysts with enhanced performance for preferential oxidation of CO at low temperature [J]. Angewandte Communications, 2012, 51(48): 12032-12035. DOI: 10.1002/anie.201206505.
[29] MALLESHAM B, SUDARSANAM P, REDDY B, VENKAT S, REDDY B M. Development of cerium promoted copper–magnesium catalysts for biomass valorization: Selective hydrogenolysis of bioglycerol [J]. Applied Catalysis B: Environmental, 2016, 181: 47-57. DOI: 10.1016/j.apcatb. 2015.07.037.
[30] SCHAUB R, THOSTRUP P, LOPEZ N, LAEGSGAARD E, STENSGAARD I, NORSKOV J K, BESENBACHER F. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110) [J]. Physical Review Letters, 2001, 87(26): 266104. DOI: 10.1103/PhysRevLett.87.266104.
[31] CAI Mei-qiang, ZHU Yi-zu, WEI Zong-su, HU Jian-qiang, PAN Sheng-dong, XIAO Rui-yang, DONG Chun-ying, JIN Mi-cong. Rapid decolorization of dye orange G by microwave enhanced fenton-like reaction with delafossite-type CuFeO2 [J]. Science of the Total Environment, 2017, 580: 966-973. DOI: 10.1016/j.scitotenv. 2016.12.047.
[32] JIN Lin-feng, CHAI Li-yuan, REN Li-li, JIANG Yu-xin, YANG Wei-chun, WANG Sheng, LIAO Qi, WANG Hai-ying, ZHANG Li-yuan. Enhanced adsorption-coupled reduction of hexavalent chromium by 2D poly(m-phenylenediamine)- functionalized reduction graphene oxide [J]. Environ Sci Pollut R, 2019, 26(30): 31099-31110. DOI: 10.1007/ s11356-019-06175-x.
[33] WANG Ting, ZHANG Li-yuan, LI Chao-fang, YANG Wei-chun, SONG Ting-ting, TANG Chong-jian, MENG Yun, DAI Shuo, WANG Hai-ying, CHAI Li-yuan, LUO Jian. Synthesis of core-shell magnetic Fe3O4@poly(m- Phenylenediamine) particles for chromium reduction and adsorption [J]. Environmental Science and Technology, 2015, 49(9): 5654-5662. DOI: 10.1021/ es5061275.
[34] SHAN Chao, TONG Mei-ping. Efficient removal of trace arsenite through oxidation and adsorption by magnetic nanoparticles modified with Fe-Mn binary oxide [J]. Water Research, 2013, 47(10): 3411-3421. DOI: 10.1016/j.watres. 2013.03.035.
[35] GUPTA K, BHATTACHARYA S, NANDI D, DHAR A, MAITY A, MUKHOPADHYAY A, CHATTOPADHYAY D J, RAY N R, SEN P, GHOSH U C. Arsenic(III) sorption on nanostructured cerium incorporated manganese oxide (NCMO): A physical insight into the mechanistic pathway [J]. Journal of Colloid and Interface Science, 2012, 377(1): 269-276. DOI: 10.1016/ j.jcis.2012.01.066.
[36] DHOBLE R M, LUNGE S, BHOLE A G, RAYALU S. Magnetic binary oxide particles (MBOP): A promising adsorbent for removal of As (III) in water [J]. Water Research, 2011, 45(16): 4769-4781. DOI: 10.1016/j.watres. 2011.06.016.
[37] LIN Sen, LU Dian-nan, LIU Zheng. Removal of arsenic contaminants with magnetic γ-Fe2O3 nanoparticles [J]. Chemical Engineering Journal, 2012, 211-212: 46-52. DOI: 10.1016/j.cej.2012.09.018.
[38] YU Lian, PENG Xian-jia, NI Fan, LI Jin, WANG Dong-sheng, LUAN Zhao-kun. Arsenite removal from aqueous solutions by gamma-Fe2O3-TiO2 magnetic nanoparticles through simultaneous photocatalytic oxidation and adsorption [J]. Journal of Hazardous Materials, 2013, 246-247: 10-17. DOI: 10.1016/j.jhazmat.2012.12.007.
[39] JIN Lin-feng, HUANG Lei, REN Li-li, HE Ying-jie, TANG Jing-wen, WANG Sheng, YANG Wei-chun, WANG Hai-ying, CHAI Li-yuan. Preparation of stable and high-efficient poly(m-phenylenediamine)/reduced graphene oxide composites for hexavalent chromium removal [J]. J Mater Sci, 2019, 54(1): 383-395. DOI: 10.1007/s10853-018- 2844-9.
[40] PENG Cong, CHAI Li-yuan, SONG Yu-xia, MIN Xiao-bo, TANG Chong-jian. Thermodynamics, kinetics and mechanism analysis of Cu(II) adsorption by in-situ synthesized struvite crystal [J]. Journal of Central South University, 2018, 25(5): 1033-1042. DOI: 10.1007/s11771- 018-3803-y.
[41] HABIBA U, AFIFI A M, SALLEH A, ANG B C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+ [J]. Journal of Hazardous Materials, 2017, 322(Pt A): 182-194. DOI: 10.1016/j.jhazmat.2016.06.028.
[42] WANG Teng, LI Cai-ting, ZHAO Ling-kui, ZHANG Jun-yi, LI Shan-hong, ZENG Guang-ming. The catalytic performance and characterization of ZrO2 support modification on CuO-CeO2/TiO2 catalyst for the simultaneous removal of Hg0 and NO [J]. Applied Surface Science, 2017, 400: 227-237. DOI: 10.1016/j.apsusc.2016. 12.192.
[43] WANG Ting, ZHANG Li-yuan, WANG Hai-ying, YANG Wei-chun, FU Ying-chun, ZHOU Wen-li, YU Wan-ting, XIANG Kai-song, SU Zhen, DAI Sshuo, CHAI Li-yuan. Controllable synthesis of hierarchical porous Fe3O4 particles mediated by poly(diallyldimethylammonium chloride) and their application in arsenic removal [J]. ACS Applied Materials Interfaces, 2013, 5(23): 12449-12459. DOI: 10.1021/ am403533v.
[44] WEN Zhi-pan, ZHANG Ya-lei, WANG Yu, LI Li-na, CHEN Rong. Redox transformation of arsenic by magnetic thin-film MnO2 nanosheet-coated flowerlike Fe3O4 nanocomposites [J]. Chemical Engineering Journal, 2017, 312: 39-49. DOI: 10.1016/j.cej.2016.11.112.
(Edited by HE Yun-bin)
中文导读
磁性Fe3O4@Cu/Ce微球催化氧化协同吸附重金属三价砷
摘要:本文通过一步溶剂法成功制备了磁性Fe3O4@Cu/Ce微球,并将其应用于毒性重金属三价砷的处理。系统考察了Cu/Ce元素共掺杂对复合材料结构晶型、催化氧化性能以及对三价砷的去除性能的影响。揭示了Cu/Ce元素的共掺杂效应可以显著降低磁性微球的晶粒尺寸,增加晶格缺陷,提高氧空缺位和丰富活性官能团。研究表明磁性Fe3O4@Cu/Ce微球对重金属三价砷具有较强的去除性能,最大吸附量可达139.19 mg/g。三价砷的去除机理主要涉及到催化氧化协同吸附,将三价砷氧化为五价砷的同时将其吸附在微球上。本文提出了一种可行的高性能磁性复合材料的制备方法,为三价砷的处理提供了技术思路。
关键词:铜/铈;磁性材料;三价砷;催化氧化;吸附
Foundation item: Project(2018YFC1802204) supported by the National Key R&D Program of China; Project(51634010) supported by the Key Project of National Natural Science Foundation of China; Project(2018SK2026) supported by the Key R&D Program of Hunan Province, China
Received date: 2019-11-14; Accepted date: 2020-03-18
Corresponding author: WANG Hai-ying, PhD, Professor; Tel: +86-731-88830875; E-mail: haiyw25@yahoo.com; ORCID: 0000-0002- 5446-3217
Abstract: Magnetic Fe3O4@Cu/Ce microspheres were successfully prepared by one-step solvothermal approach and further utilized to remediate toxic arsenic (As(III)) pollution. The effects of Cu/Ce elements co-doping on the crystal structure, catalytic oxidation and adsorption behaviors of magnetic microspheres were researched systematically. The results showed that with the aid of Cu/Ce elements, the grain size reduced, lattice defects increased, and the oxygen vacancies and surface hydroxyl groups were improved. Therefore, Cu/Ce elements endowed magnetic Fe3O4@Cu/Ce microspheres with excellent As(III) removal performance, whose maximum adsorption capacity reached 139.19 mg/g. The adsorption mechanism mainly involved catalytic oxidant co-adsorption. This research developed a feasible strategy for the preparation of high efficiency magnetic adsorbent to enhance the removal of As(III).


