J. Cent. South Univ. Technol. (2007)02-0181-05
DOI: 10.1007/s11771-007-0036-x ![]()
Ni-Cr alloy electrodepositing technology on Fe substrate and
coating performance
XU Li-jian(许利剑)1, 2, GONG Zhu-qing(龚竹青)1, TANG Jian-xin(汤建新)2,
HE Quan-guo(贺全国)2, HE Nong-yue(何农跃)2, DU Jing-jing(杜晶晶)2
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Green Packaging and Biological Nanotechnology Laboratory, Hunan University of Technology,
Zhuzhou 412008, China)
Abstract:
The Ni-Cr alloy electrodepositing technology on iron substrate in the chlorid-sulfate solution and the impacts of main processing parameters on coating composition were studied. The optimal Ni-Cr alloy electrodepositing conditions are that the cathode current density is 16 A/dm2,the plating solution temperature is 30 ℃ and the pH value is 2.5. The bright, compact coating gained under the optimal conditions has good cohesion and 24.1% Cr content. The results show that the coating is composed of crystalline, the average grain size is 82 nm and the higher the Cr content of coating, the larger the rigidity, and the higher the corrosion resistance. The rigidity of coating reaches 78.6(HR30T) and the passivation area broadens to 1.4 V when the Cr content of coating is 24.1%.
Key words:
electrodeposition; plating; Ni-Cr alloy; corrosion resistance ;
1 Introduction
Ni-Cr alloy has many advantages such as high resistance to abrasion and corrosion, high resistance to oxidation at elevated temperature and good decoration virtue[1]. The service life of steel component that is used most broadly in human beings’ life can be increased greatly and good decoration virtue can be gained if Ni-Cr alloy is electrodeposited on the surface. Ni-Cr alloy electrodepositing technology on Cu substrate and its coating performance were studied successively by ZHANG[2-3], CHEN [4] and ZHANG[5] in recent years, whereas there are few reports on Ni-Cr alloy electrodepositing on Fe or other metal substrate. The experiments show that it’s easier to electrodeposit Ni-Cr alloy on Cu substrate than other metals due to easy pretreatment, convenient polish, slow oxidation rate and fine cohesion. On the contrary, it is harder to electrodeposit Ni-Cr alloy on Fe substrate because iron is more rigid and active than copper. It is easier to be corroded in the process of removing rust and easier to be oxidized again because of its coarse surface when it is exposed to atmosphere for a long time. In this paper, the bright and compact Ni-Cr alloy was gained on iron substrate in chlorid-sulfate solution, and the optimal technology conditions were gained through studying the impacts of primary processing parameters on the coating composition. The pattern, crystal structure, rigidity and corrosion resistance of coating were analysed and tested in this study.
2 Experimental
.1 Experimental materials and main instruments
A Ni piece and a Fe piece were taken as anode and cathode, respectively. The size of cathode was 30 mm×20 mm×1 mm. There kinds of reagents—NiSO4·6H2O, NiCl2·6H2O and CrCl3·6H2O were analytically pure.
ZD-30A/50V direct current power supply, electric- heated thermostatic water bath, PHS-25 dogmatic pH-meter, CHI600A electrochemistry workstation, JHS- 1/90 electronic constant-speed beater, KYKY2800 sweeping-electron microscope, HSRD-45 surface Rockwell hardometer, XD98-X radial diffraction apparatus and energy spectrometer were employed.
2.2 Experimental methods
Fe/Cu pieces need pretreatment before plating to clear all of the greasy dirt and oxide coating on the surface. In this paper, the pieces were dipped in the solution containing 185 mL/L thick hydrochloric acid, 5.0-7.5 g/L OP emulsifier and 5 g/L 6-methenyl-4- ammonium at 50-60 ℃ for 30 min.
The plating solution components were ascertained by groping experiments, and their concentrations were 50 g/L NiSO4·6H2O, 45 g/L NiCl2·6H2O, 50-135 g/L CrCl3·6H2O, 50 g/L boracic acid, 70 g/L citric acid, 105 g/L natrium citrate, 0.15-0.20 g/L lauryl sodium sulfate, 4 g/L WHT and 7.5-15 mL/L 791 brightener. The main factors such as cathode current density, temperature, pH value and CrCl3·6H2O concentration were studied in this study. The plating time was 20 min in each test.
The crystal structure of coating was measured by XD98-X radial diffractometer(made by Beijing Science and Technology University Machine Corporation) under the conditions that the radial target was copper and the sweeping rate was 4 (?)/min. The anode polarization curves of 10% sulfuric acid solution containing Ni-Cr alloy with different components were described by the CHI600A electrochemistry workstation to study the corrosion resistance of coating. The rigidity test was operated on HSRD-45 surface Rockwell hardometer under the conditions that the load was 30 N and the time was 10 s, and the rigidity value was the average value of five tests.
3 Results and discussion3.1 Impacts of main processing parameters on coating composition
3.1.1 pH value
The impacts of pH value on the coating composition are shown in Fig.1 when the temperature is 30 ℃, the current density is 14 A/dm2 and CrCl3·6H2O concentration is 100 g/L. It is presented in Fig.1 that Cr content of coating increases with increasing pH value firstly, which gets the max when pH value is 2.5; and decreases with increasing pH value when pH value is more than 2.5. And the higher the pH value, the higher the citric acid dissociating speed; also the higher the concentration of Cr3+ compound, the higher Cr3+ discharging speed and the higher the Cr content. However, Cr3+ ions participate in the polymerization reaction[6] more easily when pH value is too high, and the hydrogen on the anode surface increases in the alloy electrodepositing process, which results in increasing of the pH value of double layer between the electrode and solution interface, accelerating of Cr3+ polymerization reaction, and hindering of Cr3+ discharging on the electrode surface due to the hydroxide film. Consequently, Cr content of coating decreases and the coating quality gets bad. Therefore, pH value should be maintained at 2.5 in Ni-Cr alloy plating on Fe substrate.
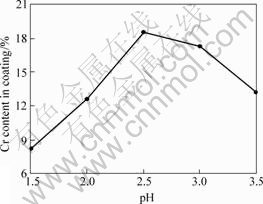
Fig.1 Impacts of pH value of solution on coating composition
3.1.2 Temperature
The impacts of temperature on coating composition are shown in Fig.2 when pH value is 2.5 and other conditions are fixed. It is presented in Fig.2 that the impacts of temperature on Cr content of coating are obvious. Cr contents of coating are 19.8% and 4.1% when temperatures are 30 ℃ and 50 ℃, respectively. Generally speaking, the metal ion is easily deposited on the substrate with increasing the temperature because the diffusion and transfer speed of metal ion is accelerated, the activation energy of metal deposition decreases and cathode polarization decreases when the plating temperature increases. However, it is harder for Cr3+ to discharge than Ni2+ and the trends of increasing Cr3+ diffusion speed and decreasing the cathode polarization are smaller than Ni2+ with the temperature increasing, which decreases Cr content of coating. When the temperature is too low, the ion diffusion and deposition speed gets slow and the coating gets bad because there exists consistency polarization. Therefore, the plating temperature should be controlled at 30 ℃ in Ni-Cr alloy plating on Fe substrate.
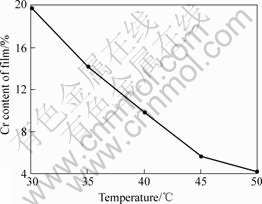
Fig.2 Impacts of temperature on coating composition
3.1.3 Current density
The impacts of current density on coating composition are shown in Fig.3 when the above conditions are fixed. It is presented in Fig.3 that Cr content increases with increasing the anode current density. The larger the current density, the easier the cathode polarization, the higher the Cr3+ depositing speed, and the higher the Cr content of coating. However, when the anode current density is more than 16 A/dm2, the crystal of coating is coarse, the coating brim burns and surface quality of coating gets bad because the electrochemistry reaction turns into the blended area of electrochemistry polarization and consistency polarization, even into the consistency polarization area if the current density is too high. Therefore, the current density should be controlled at 16 A/dm2 in Ni-Cr alloy plating on Fe substrate.

Fig.3 Impacts of cathode current density on coating composition
3.1.4 CrCl3·6H2O concentration
The impacts of CrCl3·6H2O concentration on coating composition are shown in Fig.4 when the other conditions are fixed. It is presented that Cr content of coating increases with increasing the CrCl3·6H2O concentration when the concentration is less than 75 g/L; then decreases with increasing greatly CrCl3·6H2O concentration when the concentration is more than 75 g/L. The concentrations of Cr3+ ion and Cr3+ compound increase with increasing CrCl3·6H2O concentration, which benefits Cr3+ discharge and improves Cr content of coating. However, the polymerization reaction is easier [7-8], the coating surface quality gets bad and Cr content of coating decreases when Cr3+ concentration is too high. Therefore, CrCl3·6H2O concentration should be controlled at about 75 g/L in Ni-Cr alloy plating.
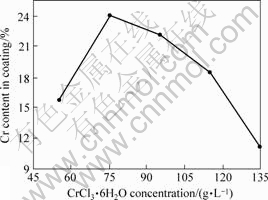
Fig.4 Impacts of CrCl3·6H2O concentration on coating component
The optimal processing parameters of the electrodepositing Ni-Cr alloy on Fe substrate are listed in Table 1 according to the above results.
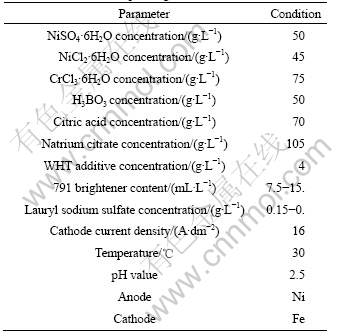
Table 1 Optimal processing parameters of Ni-Cr alloy electrodepositing on Fe substrate
The bright, even and compact coating obtained under the above optimal conditions has fine cohesion and is 15 μm in thickness. The component of coating was analyzed by EDS, and the result shows that Cr and Ni contents of coating are 24.1% and 75.9%, respectively.
3.2 Performance analysis of Ni-Cr alloy coating
3.2.1 Pattern and crystal structure of Ni-Cr alloy coating
XRD result of Ni-Cr alloy coating obtained under the optimal conditions is shown in Fig.5.
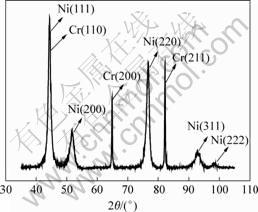
Fig.5 XRD pattern of Ni-Cr alloy coating
It is presented in Fig.5 that the component of coating is Ni and Cr, and its structure is composed ofcrystalline. The Ni diffraction maximum for Ni is found when 2θ is 44.4?, where the relevant texture is crystal plane (111). The diffraction maximum for Cr is found when 2θ is 44.599?, where the relevant texture is crystal plane (110). The average size of crystal grain of coating is estimated as 82 nm according to Scherrer formula[9].
3.2.2 Corrosion resistance of Ni-Cr alloy coating
The anode polarization curves of 10% sulfuric acid solution containing Ni-Cr alloy with different components were described to study the corrosion resistance of coating. The curves were measured by the CHI600A electrochemistry workstation under the conditions that the solution temperature was 25℃, the assistant electrode was platinum electrode and the reference electrode was calomel electrode in the "H" model electro-bath. The results are shown in Fig.6 and Table 2.
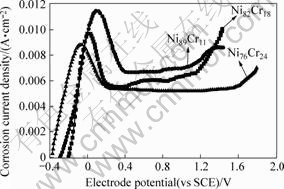
Fig.6 Anode polarization curve of 10% sulfuric acid solution containing Ni-Cr alloy of different components
Table 2 Anode polarization parameters of 10% sulfuric acid solution containing Ni-Cr alloy of different components

It is presented in Fig.6 and Table 2 that φcir moves to negative aspect, Jin decreases, Jmain decreases, φover increases and the passivation area broadens with increasing Cr content of coating. The passivation area reaches 1.4 V when Cr content of coating is 24.1%. It is testified that it is easier to form passivation film and the capacity of resisting to destroy passivation film increases with increasing Cr content of coating. Therefore, the corrosion resistance of coating increases with increasing Cr content of coating[10-11].
3.2.3 Rigidity of Ni-Cr alloy coating
The rigidity is a kind of integrative performance and an important reference index of abrasion resistance although it can not reflect the accurate value [12]. It is used in practical manufacture broadly due to the convenient measurement. The relation between the rigidity and Cr content of coating is shown in Fig.7. It is presented that the rigidity of coating increases with increasing Cr content of coating, which reaches 78.6(HR30T) when Cr content of coating is 24.1%.
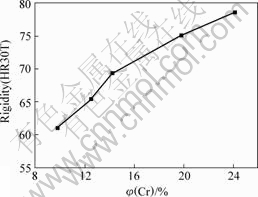
Fig.7 Relation between Cr content and rigidity of coating
3.2.4 Cohesion of Ni-Cr alloy coating and Fe substrate
The cohesion of coating is a kind of important physical performance of coating. The coating loses the ability to protect the substrate, induces primary cell corrosion and accelerates the corrosion rate of base metal when the cohesion is bad. The bend and steel pin scoring method was adopted to measure the cohesion in this study. The coating does not detach from the substrate and there is no wrinkle on the surface when the piece is bent even to 180? until it is broken or scored to naked substrate. It is testified that the cohesion between coating and substrate is fine[13-14].
4 Conclusions
1) The plating solution chemical components of electrodepositing Ni-Cr alloy on Fe substrate and the impacts of main processing parameters on coating composition were studied. The optimal Ni-Cr alloy electrodepositing conditions are that the cathode current density is 16 A/dm2, the plating solution temperature is 30 ℃ and pH value is 2.5. The bright and compact coating gained under the optimal conditions has good cohesion and 24.1% Cr content.
2) The anode polarization curves of 10% sulfuric acid solution containing Ni-Cr alloy with different compositions were described. The results show that the more the Cr content of coating, the better the corrosion resistance. The HSRD-45 surface Rockwell hardometer test shows that the larger the Cr content of coating, the larger the rigidity.
3) The microstructure of coating was studied by XRD, and the results show that the component of coating is Ni and Cr. Its structure is composed of crystalline and the average size of crystal grain is 82 nm.
References
[1] CHI Hong-zhong, LIU Shen-zhong. Nickel-chromium alloy electroplating[J]. Plating and Finishing, 2001, 23(3): 23-26. (in Chinese)
[2] ZHANG Pi-jian, ZOU Li-zhuang, WANG Xiao-ling. Influencing factors on Cr content in Ni-Cr deposit[J]. Materials Protection, 1997, 30(9): 22-23. (in Chinese)
[3] ZHANG Pi-jian, ZOU Li-zhuang, WANG Xiao-ling. Ni-Cr alloy electrodeposition[J]. Materials Protection, 1997, 30(3): 16-18. (in Chinese)
[4] CHEN Lei, GONG Zhu-qing, HUANG Zhi-jie, et al. Study of nickel-chromium electrodeposit[J]. Plating and Finishing, 1998, 17(4): 4-7. (in Chinese)
[5] ZHANG Sheng-li, ZHU Yu-fa, FENG Shao-bin, et al. Cr-Ni alloy electroplating technology in trivalent chromium bath and coating performance[J]. Materials Protection, 2005, 38(5): 35-38. (in Chinese)
[6] HE Xiang-zhu, GONG Zhu-qing, JIANG Han-ying. Electroplating of amorphous chromium with Cr(III) aqueous solution[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(1): 95-100.(in Chinese)
[7] HE Xiang-zhu, GONG Zhu-qing, JIANG Han-ying. Electro- deposition of amorphous chromium from Cr(III) aqueous solution[J]. The Chinese Journal of Nonferrous Metals, 1999, 9(3): 646-650. (in Chinese)
[8] WASTSON A, ANDSON A M H, EL-SHARIF M R, et al. The role of chromium Ⅱ catalyzed oblation reactions in the sustained deposition of chromium and its alloys from environmentally acceptable chromium Ⅲ electrolytes[J]. Trans IMF, 1991, 69(Part 1): 26-32.
[9] DENG Shu-hao. Studies on Technology and Basic Mechanism of Pulse Plating Nanocrystalline Cr-Ni-Fe Alloy[D]. Changsha: School of Metallurgical Science and Engineering, Central South University, 2003. (in Chinese)
[10] MA Zheng-qing, LI Wen-xian, TAN Dun-qiang, et al. Corrosion resistance of the Fe-Ni-Cr alloy coating[J]. Corrosion and Protection, 2001, 22(10): 417-423. (in Chinese)
[11] CHEN Jun, HU Yao-jun, LIU Guo-jia, et al. Impacts of β stable element on the electrochemistry characteristic of 3.5%NaCl solution with Ti alloy[J]. The Chinese Journal of Nonferrous Metals, 1998, 8(2): 328-332. (in Chinese)
[12] GAO Cheng-hui. Hardness control in electrodepositing Ni-Fe-P alloy[J]. Electroplating and Protection Pollution, 1997, 17(3): 3-5. (in Chinese)
[13] ZHANG Yun-cheng. Plating Handbook[M]. Beijing: National Defense Industry Press, 1977. (in Chinese)
[14] WANG Yun-yan, SHU Yu-de, PENG Wen-jie. Study of additives for zinc-iron alloy plating in alkaline zincate bath[J]. Electroplating and Finishing, 2002, 21(5): 11-16. (in Chinese)
Foundation item: Project (59674025) supported by the National Natural Science Foundation of China
Received date: 2006-05-21; Accepted date: 2006-07-27
Corresponding author: XU Li-jian, Doctoral candidate; Tel: +86-731-8830752; E-mail: xlj235@163.com
(Edited by YANG Bing)
Abstract: The Ni-Cr alloy electrodepositing technology on iron substrate in the chlorid-sulfate solution and the impacts of main processing parameters on coating composition were studied. The optimal Ni-Cr alloy electrodepositing conditions are that the cathode current density is 16 A/dm2,the plating solution temperature is 30 ℃ and the pH value is 2.5. The bright, compact coating gained under the optimal conditions has good cohesion and 24.1% Cr content. The results show that the coating is composed of crystalline, the average grain size is 82 nm and the higher the Cr content of coating, the larger the rigidity, and the higher the corrosion resistance. The rigidity of coating reaches 78.6(HR30T) and the passivation area broadens to 1.4 V when the Cr content of coating is 24.1%.