DOI: 10.11817/j.ysxb.1004.0609.2021-36569
水体中锑的形态及转化规律研究进展
刘晓芸1,刘晶晶1, 3,柯 勇1, 2, 4,王庆伟1, 2, 4,颜 旭1, 2, 4
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 国家重金属污染防治工程技术研究中心,长沙 410083;
3. 长沙环境保护职业技术学院,长沙 410004;
4. 水污染控制技术湖南省重点实验室,长沙 410004)
摘 要:
锑是一种具有潜在毒性和致癌性的有色金属元素,随着锑及其化合物的广泛应用,大量含锑污染物进入水体中,给生态环境和人类健康带来风险。锑的毒性及其在水中的含量与形态结构密切相关,锑在复杂水体环境中的迁移转化规律是锑污染控制的理论基础。综述了近年来国内外对水中锑的形态及其氧化和还原、水解和配位、吸附和解吸、溶解和沉淀等迁移转化规律的研究进展,基于此对水体中锑的污染防治及未来研究重点作了展望。
关键词:
文章编号:1004-0609(2021)-05-1330-17 中图分类号:X52 文献标志码:A
引文格式:刘晓芸, 刘晶晶, 柯 勇, 等. 水体中锑的形态及转化规律研究进展[J]. 中国有色金属学报, 2021, 31(5): 1330-1346. DOI: 10.11817/j.ysxb.1004.0609.2021-36569
LIU Xiao-yun, LIU Jing-jing, KE Yong, et al. Research progress on speciation of antimony in natural water[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(5): 1330-1346. DOI: 10.11817/j.ysxb.1004.0609.2021-36569
锑在自然界中广泛存在,但丰度较低(0.2~0.3 μg/g),在地壳93种自然元素中含量排第64位,是一种痕量元素[1]。锑与砷的性质接近,常被视为半金属或准金属,也是一种重要的有色金属。金属锑性脆,长期未在工业上有所应用,直至1839年出现巴比特铅锑耐磨合金,锑在工业上的应用才开始推广[2]。含锑化合物的应用可追溯到至少6000年以前,古埃及用锑酸盐作为玻璃着色剂和妇女画眉的修饰品。随着19世纪工业技术的发展,锑及其化合物在工业生产和生活上的应用得到了空前发展,可用于药物、印刷、铅蓄电池、颜料和陶瓷釉彩等方面,也是锑系阻燃剂和机动车刹车片的主要成分,近期还被用于气体传感器、红外探测器、二极管、催化剂、弹药强化剂中[3],需求与日俱增。我国是世界上发现、利用锑最早的国家之一,进入20世纪以来,我国的锑产量常居世界首位,2018年我国锑产量19.2万t,约占世界总产量的80%。
由于人类活动的影响和锑的广泛应用,环境中的锑污染问题也越来越严重,例如我国广西、湖南、云南和贵州等地,锑的大量开采和冶炼使锑被释放进入水体中[4]。研究指出我国矿区水体中锑的浓度可达到7000 μg/L[5]。锑具有较强的毒性及潜在的致癌性,被我国、欧盟和美国列为优先控制污染物,在巴塞尔公约关于危险废物越境迁移限定中将锑列为危险废物[1]。锑对人类、环境的危害均与水中锑的形态密切相关,通常单质锑的毒性远大于锑的化合物,无机态锑的毒性大于有机态锑,水溶态锑的毒性大于难溶态,Sb(Ⅲ)毒性是Sb(Ⅴ)毒性的10倍,但迁移性不及Sb(Ⅴ)。水体中锑的形态特征和迁移转化规律是锑污染控制的理论基础,本文对近年来相关研究进行了综述,并对水中锑的污染控制研究作了展望。
1 自然界中锑的矿物形态
锑是一种亲硫金属,硫化物是锑在自然界中最主要的矿物形态(如辉锑矿),分布最广且产量最大。锑的原生矿物大多不稳定,外界环境改变时会转变为多种次生矿物。锑的氧化矿主要存在于辉锑矿床的露头部分或近地表处,是硫化锑矿风化产物。目前在自然界中已发现100余种含锑矿物,主要形式包括[1]:① 锑银、锑金等合金(如锑银矿Ag3Sb、方锑金矿AuSb2);② 硫化物和硫氧化物(如辉锑矿Sb2S3、红锑矿Sb2S2O);③ 含硫盐,主要是由络合阴离子与银、铜、铅、铁、镍、汞等金属形成的含硫盐矿物(如车轮矿PbCuSbS3);④ 氧化矿和含氧矿物(如锑华Sb2O3、黄锑矿Sb2O4)。锑挥发性较强,在火山活动及构造差异运动过程中,锑会在空间上重新分配并聚集成矿。锑矿物中锑含量通常为1.25%~12%,氧化物和硫化物矿中锑含量最高。除含锑矿物外,锑还会进入到硅酸盐矿物、方铅矿、闪锌矿和黄铁矿等矿物晶格中,或吸附夹杂在其他天然矿物中。此外,少量锑还会存在于煤炭和石油中,锑含量分别约为0.05~10 μg/g和<1 μg/g[6]。
2 水体中锑的含量及污染特征
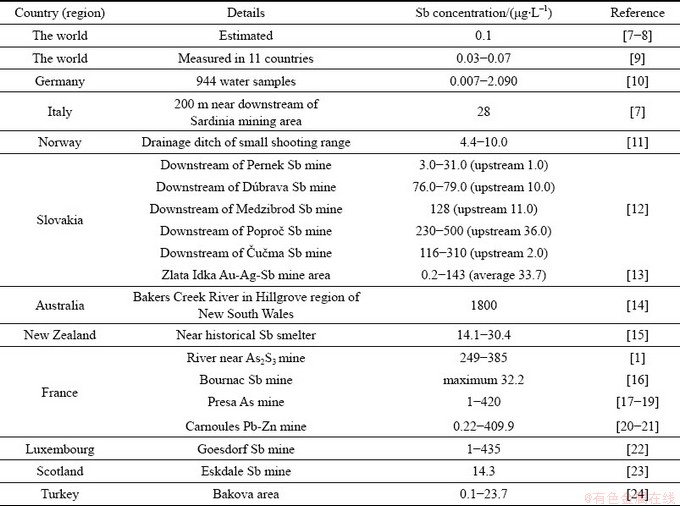
水体中锑的天然来源包括岩石风化、土壤径流、大气沉降。全世界范围内未受污染地表水中锑含量的平均值<1 μg/L。当受到金属矿区采选冶炼等人类活动影响时,水体中锑含量会显著增加。世界各地及典型金属矿区地表水中锑含量分析结果汇总于表1中。
湖南锡矿山是我国最大的锑矿区,具有“世界锑都”之称,该地含锑矿物在天然氧化、淋滤、风化过程中会释放一定量的锑,导致该地区资江水体背景值(约0.53 μg/L)略高于长江流域的其他水系。此外,该地锑矿采选冶炼历史悠久,含锑污染物排放问题突出,导致地表水中锑含量普遍较高。GUO等[34]发现湖南锡矿山地区涟溪河中锑含量高达942~2134 μg/L,间接导致附近饮用水源地水体锑含量(344~415 μg/L)超标。与湖南类似,贵州、湖北、广西、云南以及国外的金属矿产丰富地区地表水中也出现了锑污染问题。例如,HILLER等[12]分析了斯洛伐克五个废弃锑矿区河水中锑含量,最大值可达到500 μg/L。ASHLEY等[14]发现澳大利亚含锑尾矿堆存地附近河水中锑含量可达到1800 μg/L,锑污染也十分严重。
表1 国内外典型地区地表水体含量及污染情况
Table 1 Content of Sb in surface water in typical areas at home and abroad
(To be continued)
(Continued)
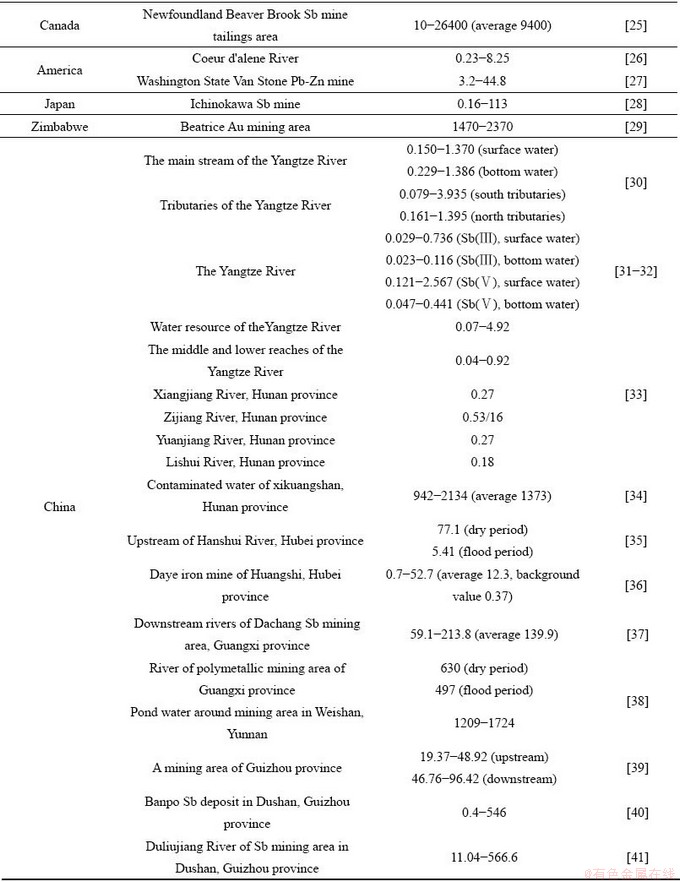
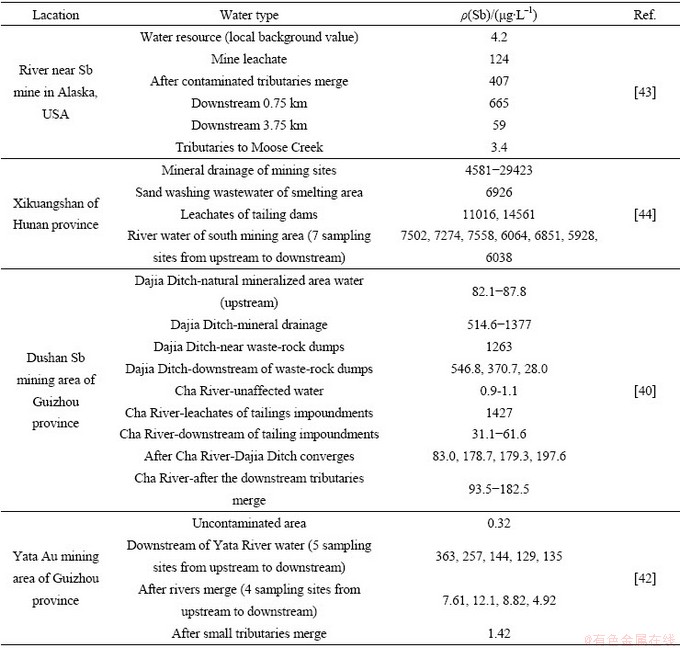
地表水中锑含量与污染源距离、地理位置及水体物理、化学环境相关,表2列举了典型矿区水体锑含量衰减变化情况。通常,排污口附近锑含量最高,随距离增加,锑可以通过吸附、共沉淀等方式自然衰减,当与其他水体汇合时可通过稀释作用发生二次衰减,后者通常是天然水体中锑衰减的主要原因。例如,张国平[42]研究发现丫他河流天然弱碱性水体中以含氧阴离子存在的 不易吸附而呈溶解态,自然衰减量有限;而当有河流汇入时,锑含量会通过稀释作用而大幅度降低至安全范围以下,类似现象也发生在美国阿拉斯加州某锑矿山、湖南锡矿山的河流中。然而,当交汇河流的物理/化学环境差别较大时,也有可能使已吸附或沉积的锑重新释放,进而导致锑含量升高(如贵州独山锑矿区)。由此可见,掌握复杂水环境中的锑形态特征和转化行为有助于理解水体中锑含量衰减规律。
不易吸附而呈溶解态,自然衰减量有限;而当有河流汇入时,锑含量会通过稀释作用而大幅度降低至安全范围以下,类似现象也发生在美国阿拉斯加州某锑矿山、湖南锡矿山的河流中。然而,当交汇河流的物理/化学环境差别较大时,也有可能使已吸附或沉积的锑重新释放,进而导致锑含量升高(如贵州独山锑矿区)。由此可见,掌握复杂水环境中的锑形态特征和转化行为有助于理解水体中锑含量衰减规律。
表2 典型矿区水体锑含量衰减变化情况
Table 2 Attenuation of Sb content in water of typical mining area
3 水体中锑的形态及其转化规律
3.1 锑的氧化和还原
锑在水体中以+3价和+5价为主。根据热力学数据φ-pH图可知,在中性富氧表层水体中,锑常以+5价的 形态存在;随着水深增加,总锑中Sb(Ⅴ)比例逐渐降低;而在缺氧环境中,锑大部分以+3价的Sb(OH)3形态存在,有硫存在时会形成Sb2S3或
形态存在;随着水深增加,总锑中Sb(Ⅴ)比例逐渐降低;而在缺氧环境中,锑大部分以+3价的Sb(OH)3形态存在,有硫存在时会形成Sb2S3或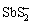 [6, 45]。但是很多研究发现在缺氧水体中也会存在Sb(Ⅴ),而在富氧水体中也会检测到Sb(Ⅲ),说明水体中锑的价态及其氧化还原反应受到诸多因素影响。
[6, 45]。但是很多研究发现在缺氧水体中也会存在Sb(Ⅴ),而在富氧水体中也会检测到Sb(Ⅲ),说明水体中锑的价态及其氧化还原反应受到诸多因素影响。
3.1.1 Sb(Ⅲ)的氧化
富氧水体中锑以Sb(Ⅴ)形态为主,通常认为是Sb(Ⅲ)氧化形成的,但研究表明均相溶液中Sb(Ⅲ)被O2氧化过程十分缓慢。LEUZ等[46]指出在pH为3.6~9.8的条件下,Sb(Ⅲ)与O2的氧化在200 d内均未发生。基于当前研究,实际水体中Sb(Ⅲ)的快速氧化包括以下几个途径:① 提高溶液pH值,促进Sb(Ⅲ)水解。随着pH升高,溶液中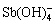 的含量增加,
的含量增加,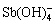 氧化转化为
氧化转化为 比Sb(OH)3更容易进行,因此当pH>10时,Sb(Ⅲ)被O2氧化的速率可明显加快;② 其他氧化剂作用。天然水体中会存在少量的H2O2(10–8~10–6 mol/L)或碘酸盐(约0.45 μmol/L),这些物质对Sb(Ⅲ)的氧化速率明显快于O2[3, 46-47]。③ 水体中共存金属作用。水体中的Cu(Ⅱ)、Mn(Ⅱ)、Pb(Ⅱ)、Fe(II)和Fe(Ⅲ)等金属离子存在会提高水溶液离子强度,还会通过催化氧化作用产生羟基自由基,促进Sb(Ⅲ)氧化[3, 48]。④ 矿物颗粒的吸附-氧化作用。LU等[49]采用铁掺杂酸性水钠锰矿吸附水中的锑,在吸附反应初期Sb(Ⅲ)可快速被生物锰矿吸附,48 h时超过96%的Sb(Ⅲ)被氧化为Sb(Ⅴ)[50]。这可能源于锑与矿物中金属间的电子转移,或者矿物固相表面的催化氧化作用。⑤ 其他物质或作用,如腐殖酸、光催化作用等。BUSCHMANN等[51-52]研究发现,Sb(Ⅲ)与腐殖酸配位结合后氧化速率会增加,而适宜的光照可使Sb(Ⅲ)氧化速率进一步提高。WU[53]近期研究了醌类单体对Sb(III)的氧化作用,结果发现1,4-对苯二酚、没食子酸、咖啡酸、原儿茶酸均可以促进Sb(Ⅲ)的氧化。
比Sb(OH)3更容易进行,因此当pH>10时,Sb(Ⅲ)被O2氧化的速率可明显加快;② 其他氧化剂作用。天然水体中会存在少量的H2O2(10–8~10–6 mol/L)或碘酸盐(约0.45 μmol/L),这些物质对Sb(Ⅲ)的氧化速率明显快于O2[3, 46-47]。③ 水体中共存金属作用。水体中的Cu(Ⅱ)、Mn(Ⅱ)、Pb(Ⅱ)、Fe(II)和Fe(Ⅲ)等金属离子存在会提高水溶液离子强度,还会通过催化氧化作用产生羟基自由基,促进Sb(Ⅲ)氧化[3, 48]。④ 矿物颗粒的吸附-氧化作用。LU等[49]采用铁掺杂酸性水钠锰矿吸附水中的锑,在吸附反应初期Sb(Ⅲ)可快速被生物锰矿吸附,48 h时超过96%的Sb(Ⅲ)被氧化为Sb(Ⅴ)[50]。这可能源于锑与矿物中金属间的电子转移,或者矿物固相表面的催化氧化作用。⑤ 其他物质或作用,如腐殖酸、光催化作用等。BUSCHMANN等[51-52]研究发现,Sb(Ⅲ)与腐殖酸配位结合后氧化速率会增加,而适宜的光照可使Sb(Ⅲ)氧化速率进一步提高。WU[53]近期研究了醌类单体对Sb(III)的氧化作用,结果发现1,4-对苯二酚、没食子酸、咖啡酸、原儿茶酸均可以促进Sb(Ⅲ)的氧化。
3.1.2 Sb(Ⅴ)的还原
水体中Sb(Ⅴ)的非生物还原研究较少。生化学家发现硫醇类物质(如半胱氨酸、谷胱氨酸)可还原Sb(Ⅴ)[54],但这类反应在天然水体中发生的可能性和程度还不得而知。Fe2+是水中常见还原剂,其对Sb(Ⅴ)的还原是热力学可行的,但在ILGEN等[55]为期2个月的研究中未观测到两者间的反应,反应速率极慢。MITSUNOBU等[56]发现另一种含Fe2+的物质—绿锈(Fe4(II)Fe2(III)-(OH)12·SO4·3H2O)对Sb(Ⅴ)具有吸附和还原作用,其反应过程推测为(pH=7.0±0.1):
Sb(V)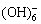 +Fe4(II)Fe2(III)(OH)12·SO4·3H2O
+Fe4(II)Fe2(III)(OH)12·SO4·3H2O  Sb(III)(OH)3+
Sb(III)(OH)3+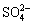 +6γ-FeOOH+6H2O+3H+ (1)
+6γ-FeOOH+6H2O+3H+ (1)
由于绿锈常存在于水体底泥中,上述过程可能是引起缺氧底层水体中Sb(V)还原的诱因之一。ZHAO[57]近期研究了紫外/亚硫酸盐辅助的电还原体系中Sb(Ⅴ)的还原,反应6 h后,95%的Sb(Ⅴ) (50 mg/L)被还原成金属锑,并沉积在电极表面,对于实际含锑废水,还原效率也可以达到60%以上。
3.1.3 锑的生物氧化与生物还原
生物作用是影响水体中锑价态及形态转化的重要原因。全球已在锑矿区和污染土壤中分离获得了60余种Sb(Ⅲ)氧化菌,主要从属于Pseudomonas、Comamonas、Agrobacterium、Acinetobacter、Stenotrophomonas等17个菌属,与抗锑和锑氧化相关的基因、酶及分子生物学机制也被较好阐明[58]。近期,LONI等[59]在我国锡矿山地区分离获得了一株细菌Paraccocus versutus XT0.6,可促进Sb2S3矿石中Sb(Ⅲ)的溶解,并将其全部氧化为Sb(Ⅴ),部分Sb(Ⅴ)还可与水体中Na+形成NaSb(OH)6沉淀物,使锑被再次固定。
在水体底层等缺氧环境中,会发生Sb(Ⅴ)的生物还原。海洋大型藻类Sargassum sp.是最早被报道可在海洋中还原Sb(Ⅴ)的生物[60]。近期,ZHANG[61]证实了海洋细菌Shewanella sp. CNZ-1对Sb(Ⅴ)的吸附和还原作用,且CNZ-1可将水体中的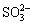 或
或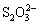 还原为S2–,与还原产生的Sb(Ⅲ)结合形成Sb2S3沉淀,促进水体中总锑去除。WANG等[62]从土壤中获得了包含Alkaliphilus、Clostridium、Tissierella和Lysinibacillus等菌属的微生物群落,在缺氧水体中该群落可将
还原为S2–,与还原产生的Sb(Ⅲ)结合形成Sb2S3沉淀,促进水体中总锑去除。WANG等[62]从土壤中获得了包含Alkaliphilus、Clostridium、Tissierella和Lysinibacillus等菌属的微生物群落,在缺氧水体中该群落可将 还原为Sb(OH)3,
还原为Sb(OH)3,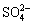 还原为S2–,形成黄色或橙黄色的Sb2S3沉淀。
还原为S2–,形成黄色或橙黄色的Sb2S3沉淀。
3.2 锑的水解和配位
3.2.1 锑的水解与无机配体结合形态
锑与其他p区金属元素类似,高价态(+5价)的化合物为共价型;而与同主族的磷和砷不同的是锑具有明显的金属性,具体表现为锑的低价态(+3价)可以Sb3+形式存在,形成离子化合物(如Sb2(SO4)3),但Sb3+仅在酸度非常高的条件下才存在,否则会强烈水解。前人已经对Sb(Ⅲ)-H2O和Sb(Ⅴ)-H2O体系中锑的水解反应及其形态变化规律进行了研究,离子分率图和主要形态如图1所示,但随着研究的深入,其他复杂水解形态被发现和证实。
Sb(Ⅲ)-H2O体系中,Sb(Ⅲ)会以Sb(OH)30(或SbO(OH)、HSbO2)形式存在,在一定条件下会分解形成Sb2O3胶体态不溶物。Sb(OH)3的水解反应可以表达为:
Sb(OH)3+yH2O=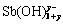 +yH+ (2)
+yH+ (2)
酸性条件(pH<~2)水解产物主要为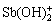 (y=-1)或SbO+,碱性条件(pH>~12)水解产物为
(y=-1)或SbO+,碱性条件(pH>~12)水解产物为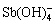 (y=1)或
(y=1)或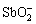 。SCHOLDER和MERBETH[65]在2 mol/L和16 mol/L的NaOH溶液中反应生成了锑酸钠类物质,包括NaSbO2、Na2Sb4O7和Na2Sb6O10·2H2O。在弱酸性或中性条件下(pH=2~10),锑以
。SCHOLDER和MERBETH[65]在2 mol/L和16 mol/L的NaOH溶液中反应生成了锑酸钠类物质,包括NaSbO2、Na2Sb4O7和Na2Sb6O10·2H2O。在弱酸性或中性条件下(pH=2~10),锑以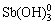 或SbO(OH)形式存在,二价和三价阳离子形式的Sb(Ⅲ)较少被单独检测 到[65]。
或SbO(OH)形式存在,二价和三价阳离子形式的Sb(Ⅲ)较少被单独检测 到[65]。
Sb(Ⅴ)-H2O体系中,Sb(Ⅴ)会以一元强酸—— 锑酸形式存在,化学式H[Sb(OH)6](或Sb(OH)5,HSbO3)。Sb(V)含氧酸的成酸原子配位数为6,呈八面体配位结构,而同主族As(Ⅴ)和P(Ⅴ)含氧酸的成酸原子配位数为4,为四面体配位结构,这种差异源于Sb(Ⅴ)半径较大,5d轨道成键倾向较强,可以激发态的sp3d2杂化轨道形成八面体配位结构[66],锑酸微溶于水,在强酸性条件下会水解形成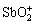 ,在弱酸性、中性及碱性条件下水解形成[Sb(OH)6]–或
,在弱酸性、中性及碱性条件下水解形成[Sb(OH)6]–或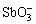 。金属锑酸盐的化学式可写为Me(I)[Sb(OH)6]、Me(I)SbO、Me(II)Sb2O6、Me(III)SbO4、Me(II)2Sb2O7。Sb(Ⅴ)的水解反应可表达为:
。金属锑酸盐的化学式可写为Me(I)[Sb(OH)6]、Me(I)SbO、Me(II)Sb2O6、Me(III)SbO4、Me(II)2Sb2O7。Sb(Ⅴ)的水解反应可表达为:
xSb(OH)5+yH2O=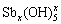 +y y–+yH+ (3)
+y y–+yH+ (3)
除 外,Sb(Ⅴ)水解还可形成一系列十二核物质,如
外,Sb(Ⅴ)水解还可形成一系列十二核物质,如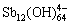 、
、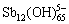 、
、 和
和 等。
等。
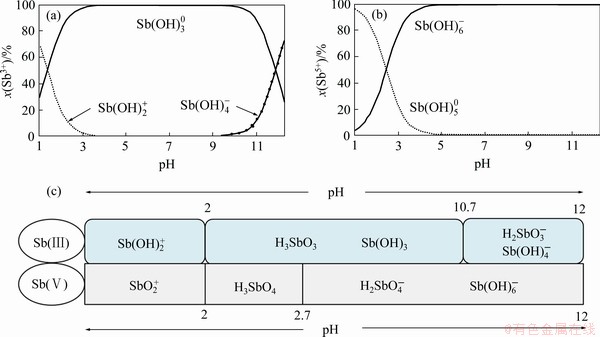
图1 Sb(Ⅲ)和Sb(Ⅴ)在水中水解的离子分率图及其主要存在形态[63-64]
Fig. 1 Ion fraction diagram of Sb(Ⅲ)(a) and Sb(Ⅴ)(b) in water, and main chemical speciation(c) of Sb in water[63-64]
卤族元素是水中锑的常见配体,其中以Sb(Ⅲ)-Cl-H2O体系研究最为深入。Sb(Ⅲ)与氯的配合物可表达为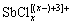 ,主要包括SbCl2+、
,主要包括SbCl2+、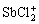 、
、 、
、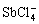 等,Sb(Ⅴ)与氯的配合物则为
等,Sb(Ⅴ)与氯的配合物则为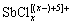 。随着pH值的升高,Sb(Ⅲ)和Sb(Ⅴ)与氯的配合物会发生强烈水解,水解产物分别为氯化氧锑SbOCl和
。随着pH值的升高,Sb(Ⅲ)和Sb(Ⅴ)与氯的配合物会发生强烈水解,水解产物分别为氯化氧锑SbOCl和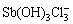 。OZTURK等[67]合成了5种碘化锑配合物,配合物中Sb可与3个硫原子和3个碘原子形成八面体几何构型,还可形成方形金字塔、三角双锥等几何构型,配合物具有稳定的双层结构。锑与卤族元素的配合物通常在强酸性或高浓度的环境中才能稳定存在,在自然水体环境中并不常见。
。OZTURK等[67]合成了5种碘化锑配合物,配合物中Sb可与3个硫原子和3个碘原子形成八面体几何构型,还可形成方形金字塔、三角双锥等几何构型,配合物具有稳定的双层结构。锑与卤族元素的配合物通常在强酸性或高浓度的环境中才能稳定存在,在自然水体环境中并不常见。
锑为亲硫元素,水中锑会与硫发生复杂的配位反应。根据FILELLA等[45]的总结,在含硫缺氧水体中,锑会形成不溶态的Sb2S3和溶解态的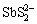 。目前已发现了多种形态的锑-硫配合物形态,包括
。目前已发现了多种形态的锑-硫配合物形态,包括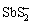 、
、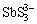 、
、 、
、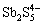 、
、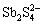 、
、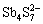 、
、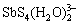 、
、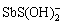 、H2Sb2S4、
、H2Sb2S4、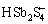 、SbS2O3–、
、SbS2O3–、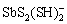 、Sb2S2(SH)2、
、Sb2S2(SH)2、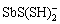 、
、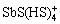 等。这些可溶态锑-硫配合物大多在锑浓度较高或pH较高条件下存在,在天然水体中存在以及对锑含量的影响还需进一步验证。
等。这些可溶态锑-硫配合物大多在锑浓度较高或pH较高条件下存在,在天然水体中存在以及对锑含量的影响还需进一步验证。
3.2.2 锑与有机配体的结合形态
水中天然有机物(Natural organic matter, NOM)主要包括腐殖酸、富里酸、微生物的代谢分泌物(如氨基酸、碳水化合物、脂肪以及低分子酸等)等,含有多种含氧、含硫、含氮基团。根据软硬酸碱理论,Sb(Ⅲ)是一种交界酸,可与软碱(如—SH)和硬酸(如—COOH)结合;Sb(Ⅴ)是一种硬酸,与硬酸类有机配体结合性较强。水中Sb(Ⅲ)和Sb(Ⅴ)会分别与NOM形成复杂多变的配合物[68],直接影响锑在水中的溶解度。
对于Sb(Ⅴ),早前的研究认为天然水体NOM通常带负电,难以对以阴离子形式存在的 起作用[68]。但后续研究证实Sb(Ⅴ)在中性水体中会与多种小分子配体配位结合。TELLA等 [63, 69]发现当水体中(pH=3~9)存在多重羧基、羟基羧酸、脂肪族羟基和酚羟基时,中心Sb(Ⅴ)原子会与6个羟基氧原子结合形成八面体配位结构,进而可通过两个Sb—O—C键与羟基相邻的苯环、脂肪酸羟基和羧基等官能团形成两个五边或六边螯合环。Sb(Ⅴ)与草酸、乳酸和柠檬酸等发生配位反应的化学计量比通常为1:1或1:3,具体的配位反应及配合物形态如表3所示。
起作用[68]。但后续研究证实Sb(Ⅴ)在中性水体中会与多种小分子配体配位结合。TELLA等 [63, 69]发现当水体中(pH=3~9)存在多重羧基、羟基羧酸、脂肪族羟基和酚羟基时,中心Sb(Ⅴ)原子会与6个羟基氧原子结合形成八面体配位结构,进而可通过两个Sb—O—C键与羟基相邻的苯环、脂肪酸羟基和羧基等官能团形成两个五边或六边螯合环。Sb(Ⅴ)与草酸、乳酸和柠檬酸等发生配位反应的化学计量比通常为1:1或1:3,具体的配位反应及配合物形态如表3所示。
Sb(Ⅲ)的有机配位体结构十分多变,三配位时为三角锥形,四配位时为四面体或畸变四面体;五配位时为三角双锥形或正方锥形,六配位时为八面体形[70]。TELLA等[63]研究发现Sb(Ⅲ)在pH=3~9条件下会与多重羧基、羟基羧酸和芳香基等形成稳定配合物,配位反应和配位形态列于表3中。配合过程中,三角锥体型的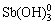 会改变配位形式,Sb(Ⅲ)会与两个临近的功能基团(O=C—OH、C—OH)中的四个氧原子结合,形成畸变三角双锥形配位结构,并形成五元螯合环,其中Sb—O—C键夹角接近90°。Sb(Ⅲ)理论上与腐殖酸中含硫基团的配位能力比含氧基团更强,但证据还不充分。水体中氨基酸等物质中含氮官能团与Sb(Ⅲ)的配位反应通常可忽略。BUSCHMANN等[51]研究发现当Sb(Ⅲ)与溶解有机碳的含量比例较低时,Sb(Ⅲ)会优先与巯基结合;当该比例较高时,Sb(Ⅲ)则会优先与含氧基团(羧基、酚羟基)形成单齿或多齿配合物;氢键和疏水作用也会促进Sb(Ⅲ)与腐殖酸间的配合 反应。
会改变配位形式,Sb(Ⅲ)会与两个临近的功能基团(O=C—OH、C—OH)中的四个氧原子结合,形成畸变三角双锥形配位结构,并形成五元螯合环,其中Sb—O—C键夹角接近90°。Sb(Ⅲ)理论上与腐殖酸中含硫基团的配位能力比含氧基团更强,但证据还不充分。水体中氨基酸等物质中含氮官能团与Sb(Ⅲ)的配位反应通常可忽略。BUSCHMANN等[51]研究发现当Sb(Ⅲ)与溶解有机碳的含量比例较低时,Sb(Ⅲ)会优先与巯基结合;当该比例较高时,Sb(Ⅲ)则会优先与含氧基团(羧基、酚羟基)形成单齿或多齿配合物;氢键和疏水作用也会促进Sb(Ⅲ)与腐殖酸间的配合 反应。
总的来说,在强酸性环境中,电中性的Sb(OH)50和Sb(OH)30均会与有机配体发生结合,而Sb(Ⅴ)形成配合物构型较灵活,配位程度更大,此条件下以配合物形式存在的Sb(Ⅴ)可达到40%。但在天然水体中(pH=4~9),Sb(V)则以带负电的 形态为主,而Sb(Ⅲ)仍为Sb(OH)30形态。受到电荷排斥作用影响,Sb(Ⅴ)此时配位程度则远低于Sb(Ⅲ)[63]。研究表明,中性水体中以配合物形态存在的Sb(Ⅴ)仅约5%,而以配合物存在的Sb(Ⅲ)可达到30%~80%。由此可见,虽然Sb(Ⅴ)毒性比Sb(Ⅲ)小,但Sb(Ⅴ)在呈中性天然水体中大多以离子形式存在,且难以配位和吸附,这是Sb(Ⅴ)迁移性比Sb(Ⅲ)强的主要原因。因此,需要关注如何调控水体中锑的形态转化,从而达到降低锑毒性和迁移性的双重目的。
形态为主,而Sb(Ⅲ)仍为Sb(OH)30形态。受到电荷排斥作用影响,Sb(Ⅴ)此时配位程度则远低于Sb(Ⅲ)[63]。研究表明,中性水体中以配合物形态存在的Sb(Ⅴ)仅约5%,而以配合物存在的Sb(Ⅲ)可达到30%~80%。由此可见,虽然Sb(Ⅴ)毒性比Sb(Ⅲ)小,但Sb(Ⅴ)在呈中性天然水体中大多以离子形式存在,且难以配位和吸附,这是Sb(Ⅴ)迁移性比Sb(Ⅲ)强的主要原因。因此,需要关注如何调控水体中锑的形态转化,从而达到降低锑毒性和迁移性的双重目的。
3.3 锑的吸附和解吸
3.3.1 锑在天然矿物表面的吸附形态和机理
天然水体中存在大量的无机矿物颗粒,包括针铁矿、赤铁矿、伊利石、蒙脱石、水合氧化锰、水合氧化铝、水合氧化铁、膨润土等,对锑具有吸附作用。矿物对锑的吸附能力与锑的形态、pH值及矿物类型、结构、比表面积等特征相关。天然含铁
表3 Sb(Ⅴ)和Sb(Ⅲ)与小分子有机物的配合反应及其配合物的稳定常数[63, 71]
Table 3 Coordination reactions of Sb(Ⅴ) and Sb(Ⅲ) with small organic compounds and stability constants of their complexes[63, 71]
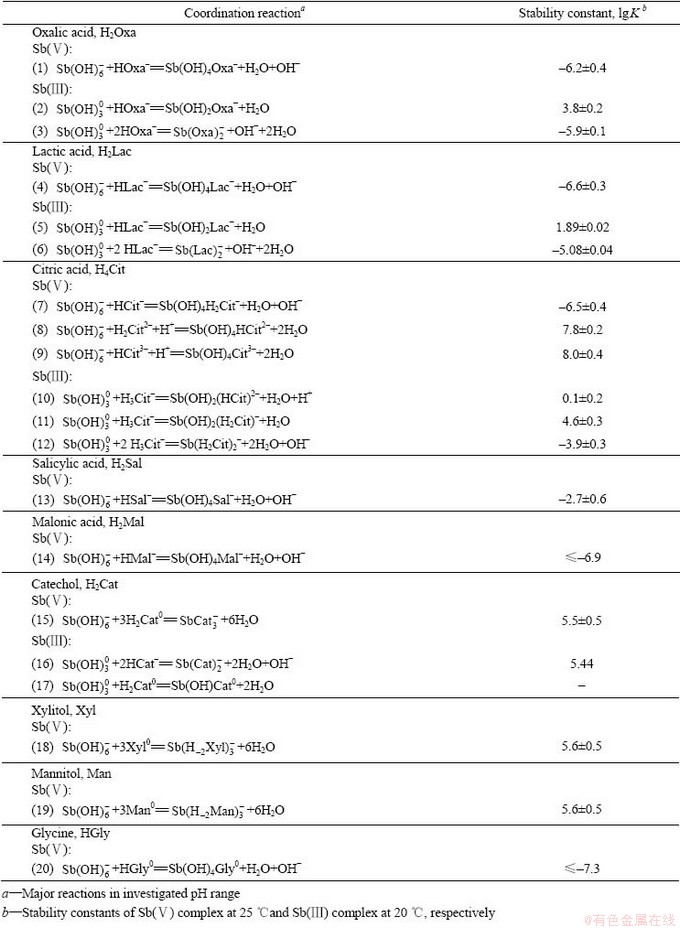
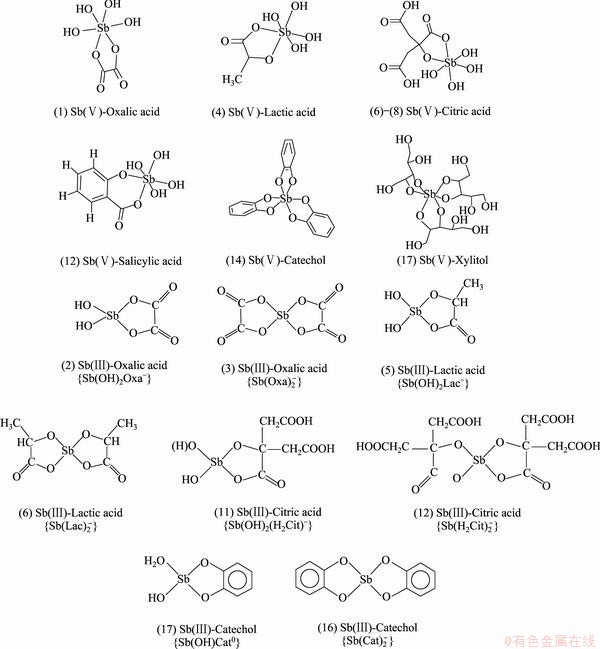
图2 Sb(Ⅴ)和Sb(Ⅲ)与有机配体形成配合物的典型结构[63, 71]
Fig. 2 Typical structures of complexes of Sb(Ⅴ) and Sb(Ⅲ) with organic ligands[63, 71]
矿物具有较大比表面积,对锑的吸附能力较强。王利[72]总结了酸性条件下矿物表面铁羟基对Sb(Ⅴ)的4步吸附过程(见图3),包括:
① 铁羟基的质子化作用,使颗粒表面带正电荷:
≡Fe—OH+H+→≡Fe—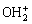 (4)
(4)
② 阴离子 通过静电吸引吸附到质子化颗粒周围;
通过静电吸引吸附到质子化颗粒周围;
③ 铁羟基与静电吸附的 发生离子交换反应,形成外层配合物:
发生离子交换反应,形成外层配合物:
≡Fe—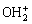 +
+ →≡Fe—
→≡Fe—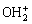 ·
· (5)
(5)
④ 外层配合物继续与表面铁羟基发生配位作用形成内层配合物,实现Sb(Ⅴ)高效吸附:
单齿:≡Fe—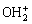 ·
· →≡Fe—O—
→≡Fe—O—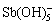 +H++H2O (6)
+H++H2O (6)
双齿:(≡Fe—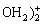 ·
· →(≡Fe)2—O—
→(≡Fe)2—O—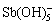 +H++2H2O (7)
+H++2H2O (7)
由于Sb(Ⅲ)和Sb(Ⅴ)在不同pH条件下的水解和配位形态不同,对两者的吸附条件和吸附形态也会有所差别。例如,Sb(Ⅲ)在pH =3~12时均可被赤铁矿吸附,而 仅能在pH<7时才能被有效吸附。
仅能在pH<7时才能被有效吸附。
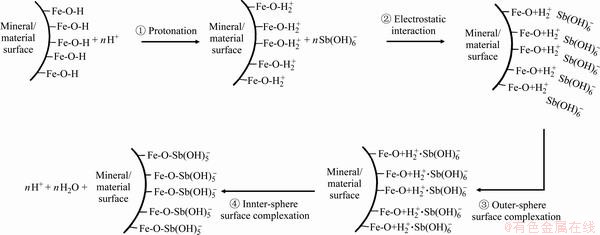
图3 酸性条件下表面铁羟基对Sb(Ⅴ)的单齿内层吸附机理[72]
Fig. 3 Inner-sphere monodentate adsorption mechanism of Sb(Ⅴ) by surface iron hydroxyl under acid condition[72]
YAN等[73]指出Sb(Ⅲ)在铁氧化物表面形成双齿共角络合结构,而Sb(Ⅴ)则是双齿共边络合结构占主导。
锰氧化物和铝氧化物在环境中也广泛存在。WANG等[74]研究指出MnOOH、Al(OH)3和FeOOH三种矿物对锑的吸附能力依次降低。LIU等[75]发现Mn(Ⅳ)矿物还原溶解形成Mn2+并暴露出更多的羟基,Mn2+会重新吸附在矿物表面增强表面正电性,促进对 的吸附。ILGEN和TRAINOR[76]研究表明,Sb(Ⅲ)和Sb(Ⅴ)在水合氧化铝、高岭石、绿脱石上的吸附均为内层吸附,其中以双齿共角配位为主,还会形成单齿配位形态和多核配位结构,但其吸附过程较慢,需要数天才能完成。
的吸附。ILGEN和TRAINOR[76]研究表明,Sb(Ⅲ)和Sb(Ⅴ)在水合氧化铝、高岭石、绿脱石上的吸附均为内层吸附,其中以双齿共角配位为主,还会形成单齿配位形态和多核配位结构,但其吸附过程较慢,需要数天才能完成。
3.3.2 功能吸附材料对锑的吸附强化机理
近些年,各国学者都在致力于开发高吸附容量的功能吸附材料。一方面可在天然矿物的基础上,通过掺杂、复合和改性等方式提高吸附能力。另一方面是开发适用于水体中锑吸附的新型功能吸附材料,包括碳材料(如活性炭、生物炭等)、无机材料(如TiO2、纳米零价铁等)、有机材料(如纤维素类、甲壳质类、聚乙烯缩丁酯等)等。
基于目前的研究,提高功能材料锑吸附性能的途径主要包括:① 增大材料的比表面积和吸附结合位点。LUO等[77]采用纳米纤维状的α-MnO2吸附水中的锑,水中的Sb(Ⅲ)和Sb(Ⅴ)可在其表面形成稳定的单齿和双齿配合物,纳米特性强化了锑的吸附效果。REN等[78]和WANG等[79]将Fe2O3装载于多孔碳纳米球中,促进了对Sb(Ⅲ)的吸附能力,还可将吸附后的材料制备成含锑催化剂,避免了锑的解吸和二次污染;② 引入金属活性位点,促进锑的结合。LI等[80]采用Zr-MOFs吸附水中的锑,MOFs具有较大比表面积,Zr-O作为吸附位点可促进Sb(Ⅲ)和Sb(Ⅴ)的吸附,还可与其中的—OH和—NH3离子交换进一步强化吸附过程。③ 引入功能基团,强化对锑的亲和力。HE等[81]发现表面氨基可通过形成—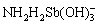 和—
和—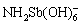 促进Sb(Ⅲ)和Sb(Ⅴ)的吸附。TU等[82]近期合成了一种负载大量胺肟的聚丙烯腈有机吸附剂,可通过静电吸引和配体交换作用实现对Sb(Ⅲ)和Sb(Ⅴ)的高效吸附。
促进Sb(Ⅲ)和Sb(Ⅴ)的吸附。TU等[82]近期合成了一种负载大量胺肟的聚丙烯腈有机吸附剂,可通过静电吸引和配体交换作用实现对Sb(Ⅲ)和Sb(Ⅴ)的高效吸附。
3.4 锑的溶解和沉淀
3.4.1 含锑矿物的氧化溶解
天然水体中含锑矿物的氧化溶解是水中锑背景值高的主要原因,通常Sb2S3和Sb2O3的溶解度都非常低,溶解速率缓慢,其溶解过程受到水体温度、pH值、氧化还原电位、光辐射、溶解氧含量以及铁矿物、Ca2+、Mg2+和小分子有机酸等因素影响[59, 83]。

图4 辉锑矿氧化溶解过程示意图[14, 84]
Fig 4 Schematic diagram of oxidation and dissolution process of stibnite [14, 84]
辉锑矿是锑最常见的含锑矿物,溶解通常需在弱还原或氧化环境中发生,即氧化溶解,具体可通过两种途径来实现(见图4),一种途径为Sb2S3直接氧化溶解形成 :
:
Sb2S3+24H2O=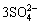 +2
+2 +28e+36H+ (8)
+28e+36H+ (8)
另一种途径为先形成Sb2O3中间产物,再水解氧化形成 ,具体涉及三个反应历程[84]:① 辉锑矿表面Sb2S3与外界接触,氧化形成Sb2O3,由于Sb2O3溶解度较低,新形成的Sb2O3会先以固体薄膜形式覆盖在辉锑矿表面:
,具体涉及三个反应历程[84]:① 辉锑矿表面Sb2S3与外界接触,氧化形成Sb2O3,由于Sb2O3溶解度较低,新形成的Sb2O3会先以固体薄膜形式覆盖在辉锑矿表面:
Sb2S3+6O2+3H2O=Sb2O3+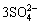 +6H+ (9)
+6H+ (9)
② 表面覆盖的Sb2O3水解形成Sb(OH)3,完成锑从固相到液相的迁移:
Sb2O3+H2O=Sb(OH)3 (10)
③ 含氧水体中,Sb(OH)3进一步被氧化,形成 :
:
Sb(OH)3+O2+4H2O=2 +2H+ (11)
+2H+ (11)
上述三个步骤的总反应方程式为:
Sb2S3+7O2+10H2O=2 +
+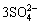 +8H+ (12)
+8H+ (12)
在锑氧化溶解过程中,由于反应不完全还会形成类似Sb(III)Sb(V)2O6(OH)的中间产物,覆盖在Sb2O3表面,但详细反应机理还不清楚[14]。另外,辉锑矿在氧化溶解过程中同时产生H2SO4。WEN等[84-85]发现碳酸盐岩地层中存在的方解石和白云石等可以中和产生的H+而不形成H2SO4,具体反应如下:
Sb2S3+8CaCO3+7O2+10H2O=
2 +8Ca2++
+8Ca2++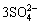 +
+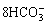 (13)
(13)
Sb2S3+4CaMg(CO3)2+7O2+10H2O=
2 +4Ca2++4Mg2++
+4Ca2++4Mg2++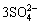 +
+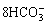 (14)
(14)
此外,HU等[86]发现Sb2S3本身具有半导体催化特性,在光照条件下会促进形成超氧自由基等强氧化剂,反过来促进自身氧化溶解。YAN等[87]近期发现光照条件下FeS2的存在会使Sb2S3的氧化溶解速率提高11.4倍,其中羟基自由基和超氧自由基发挥了重要作用。
3.4.2 天然水体中锑的沉淀物
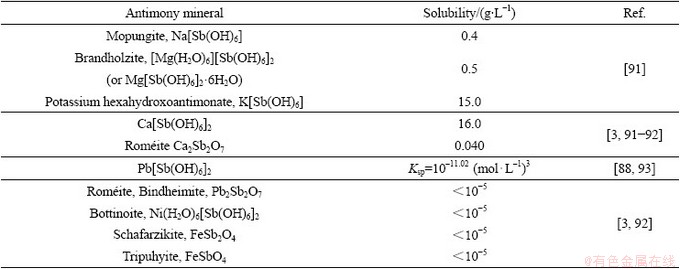
自然界中存在的碱金属元素,可与水中溶解的锑反应形成沉淀物,如Na[Sb(OH)6]、[Mg(H2O)6][Sb(OH)6]2(或Mg[Sb(OH)6]2·6H2O)、K[Sb(OH)6]、Ca[Sb(OH)6]2和Ca2Sb2O7,其溶解度分别为0.4、0.5、15.0、16.0和0.040 g/L。JOHNSON等[88]研究发现,在25 ℃条件下,Ca[Sb(OH)6]2溶度积的数量级为10–12.55,沉淀形成后主要以无定形状态为主,最终则会转变为roméite(Ca2Sb2O7)矿物晶体形态。RENARD等[89]发现酸性环境下方解石会溶解形成蚀坑并释放Ca2+,Sb(V)会与Ca2+反应形成Ca2Sb2O7,进一步通过成核、聚集生长而固定于方解石表面。
锑还可与水体中的铅、镍、铁等重金属结合形成难溶物,溶解度通常小于10 μg/L。铅可与锑形成无定形态的Pb[Sb(OH)6]2(溶度积10–11.02),随后转变为roméite类矿物沉淀Pb2Sb2O7[89]。锑与铁可以形成红锑铁矿FeSb2O4和锑铁矿FeSbO4,溶解度最低,锑-铁二次矿物往往是自然界中锑的最终去向[64]。RADKOVA等[90]研究发现,锑在水体中自然衰减时仅有1.3%的锑会吸附或进入到含铁矿物中;虽然在镁浓度较高时,会形成Mg[Sb(OH)6]2·6H2O沉淀,但易于返溶;而锑与铁形成的类似锑铁矿(FeSbO4)的沉淀物可在水体中稳定存在。总的来说,锑在水体的沉淀形态已有了初步了解(如表4所示),但沉淀结晶机理及其矿相转化机制还未阐明,需进一步研究[3]。
表4 常见含锑沉淀物的种类及其溶解度
Table 4 Types and solubility of common Sb-containing precipitates
4 结论与展望
锑对人类健康和生态环境造成的潜在危害值得重视,尽管已有锑污染控制的相关方法,但与其他金属元素(如砷)相比,关于锑的研究较少,相关理论缺乏。了解锑及其化合物在环境中的存在形态,分析其在环境中的迁移规律是有必要的。如何调控锑的形态转化行为(包括氧化和还原、水解和配位、吸附和解吸、溶解和沉淀等)来控制锑的毒性、迁移性和去除,是降低水体中锑污染风险的根本途径。当前锑形态及其转化规律的研究多以室内模拟为主,但天然水体的离子强度、杂质、pH等条件更加复杂,锑在天然水体中的形态转化规律还需进一步验证,尤其是天然水体中富含的矿物颗粒和天然有机质对锑的形态会起到决定性作用,与锑的相互作用机制还需要深入探明。在了解水体中锑污染规律与形态调控基础上,针对国内水中锑污染现状,尤其是矿区水中锑含量严重超标情况,需在涉锑行业和企业大力推广清洁生产技术,亟待开发锑污染阻控与水中微污染锑的深度净化关键技术,以保障我国地表水及饮用水的水质安全。
REFERENCES
[1] 客绍英, 石洪凌, 刘冬莲. 锑的污染及其毒性效应和生物有效性[J]. 化学世界, 2005(6): 382-384.
KE Shao-ying, SHI Hong-ling, LIU Dong-lian. Antimony pollution and its toxic effect and bioavailability[J]. Chemical World, 2005(6): 382-384.
[2] 《冶金常识》编写组. 十种常用有色金属 锡锑汞(修订本)[M]. 北京: 冶金工业出版社, 1972.
Compiling Group of General Knowledge of Metallurgy. Ten common non-ferrous metals: Sn, Sb, Hg (Revised Edition)[M]. Beijing: Metallurgical Industry Press, 1972.
[3] MULTANI R S, FELDMANN T, DEMOPOULOS G P. Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options[J]. Hydrometallurgy, 2016, 164: 141-153.
[4] 胡亚琛. 流域锑污染如何综合防治?[J]. 环境经济, 2013(10): 44-48.
HU Ya-chen. How to prevent and control antimony pollution in River Basin?[J]. Environmental Economy, 2013(10): 44-48.
[5] 杨秀贞, 周腾智, 任伯帜. 矿区锑污染及其控制技术研究现状[J]. 枣庄学院学报, 2018, 35(5): 16-23.
YANG Xiu-zhen, ZHOU Teng-zhi, REN Bo-zhi. Research status of antimony pollution and its control technology in mining area[J]. Journal of Zaozhuang University, 2018, 35(5): 16-23.
[6] 朱 静, 王立英, 郭建阳, 等. 锑的环境地球化学研究进展概述[J]. 地球与环境, 2010(1):112-119.
ZHU Jing, GUO Jian-yang, WANG Li-ying, et al. An overview of environmental geochemistry of antimony[J]. Earth and Environment, 2010(1):112-119.
[7] 黄艳超, 武雪芳, 周羽化, 等. 水环境中锑污染及其修复技术研究进展[J]. 南京师大学报(自然科学版), 2015, 38(4): 122-128.
HUANG Yan-chao, WU Xue-fang, ZHOU Yu-hua, et al. Research progress of antimony pollution and remediation technology in water[J]. Journal of Nanjing Normal University(Natural Science Edition), 2015, 38(4): 122-128.
[8] SIEWERS U. The geochemical atlas of Finland—Part 2: Till: KOLJONEN T, ed. Geological Survey of Finland, Espoo, 1992, 218 pp., ISBN 951-690-379-7 (hardcover)[J]. Chemical Geology, 1994, 113(3/4): 377-378.
[9] SALMINEN R. Antimony in the environment: Lessons from geochemical mapping[J]. Applied Geochemistry, 2010, 25(2): 175-198.
[10] BIRKE M, RAUCH U, RASCHKA H, et al. 2006. Geochemical Atlas of the Federal Republic of Germany. Distribution of inorganic and organic parameters in surface waters and brook segments. Examples[DS]. Hannover: Federal Institute for Geosciences and Raw Materials (unpublished data), 2006: 214-217.
[11] MARIUSSEN E, LJONES M, STROMSENG A E. Use of sorbents for purification of lead, copper and antimony in runoff water from small arms shooting ranges[J]. Journal of Hazardous Materials, 2012, 243: 95-104.
[12] HILLER E, LALINSKA B, CHOVAN M. Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia[J]. Applied Geochemistry, 2012, 27(3): 594-614.
[13] CICMANOVA S. Environmental and health risk assessment in abandoned mining area, Zlata Idka, Slovakia[J]. Environmental Geology, 2006, 51(3): 387-397.
[14] ASHLEY P M, CRAW D, GRAHAM B P. Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand[J]. Journal of Geochemical Exploration, 2003, 77(1): 1-14.
[15] WILSON J N. Antimony distribution and environmental mobility at an historic antimony smelter site, New Zealand[J]. Environment Pollution, 2004, 129(2): 257-266.
[16] CASIOT C, UJEVIC M, MUNOZ M. Antimony and arsenic mobility in a creek draining an antimony mine abandoned 85 years ago (upper Orb basin, France)[J]. Applied Geochemistry, 2007, 22(4): 788-798.
[17] CHRISTOPHE MIGON C M A O. Arsenic and antimony contamination in a riverine environment affected by an abandoned realgar mine[J]. Toxicological & Environmental Chemistry, 1995, 52: 221-230.
[18] MIGON C M C. Arsenic and antimony release from sediments in a Mediterranean estuary[J]. Hydrobiologia, 1999, 392(1): 81-88.
[19] HARTMANN L M, CRAIG P J, JENKINS R O. Influence of arsenic on antimony methylation by the aerobic yeast Cryptococcus humicolus[J]. Archives of Microbiology, 2003, 180(5): 347-352.
[20] RESONGLES E, CASIOT C, ELBAZ-POULICHET F, et al. Fate of Sb(Ⅴ) and Sb(Ⅲ) species along a gradient of pH and oxygen concentration in the Carnoules mine waters(Southern France)[J]. Environmental Science Processes & Impacts, 2013, 15: 1536-1544.
[21] RESONGLES E, CASIOT C, FREYDIER R. Variation of dissolved and particulate metal(loid) (As, Cd, Pb, Sb, Tl, Zn) concentrations under varying discharge during a Mediterranean flood in a former mining watershed, the Gardon River (France)[J]. Journal of Geochemical Exploration, 2015, 158: 132-142.
[22] QUENTEL F. Particle size and mineralogical composition of inorganic colloids in waters draining the adit of an abandoned mine, Goesdorf, Luxembourg[J]. Applied Geochemistry, 2009, 24(1): 52-61.
[23] LITTLEJOHN D. Speciation of antimony in natural waters: the determination of Sb(Ⅲ) and Sb(Ⅴ) by continuous flow hydride generation-atomic absorption spectrometry[J]. Chemical Speciation & Bioavailability, 1990, 2(3): 117-122.
[24] GUNDUZ O. Groundwater contamination mechanism in a geothermal field: A case study of Balcova, Turkey[J]. Journal of Contaminant Hydrology, 2009, 103(1/2): 13-28.
[25] BORCINOVA RADKOVA A, JAMIESON H E, et al. Antimony mobility during the early stages of stibnite weathering in tailings at the Beaver Brook Sb deposit, Newfoundland[J]. Applied Geochemistry, 2020, 115: 104528.
[26] WAI C M. Distribution and mobilization of arsenic and antimony species in the Coeur d'Alene River, Idaho[J]. Environ Sci Technol, 1990, 24(1): 102-108.
[27] ROUTH J, IKRAMUDDIN M. Trace-element geochemistry of Onion Creek near Van Stone lead-zinc mine (Washington, USA)—Chemical analysis and geochemical modeling[J]. Chemical Analysis and Geochemical, 1996, 133(1/4): 211-224.
[28] ASAOKA S, TAKAHASHI Y, ARAKI Y, et al. Comparison of antimony and arsenic behavior in an Ichinokawa River water-sediment system[J]. Chemical Geology, 2012, 334: 1-8. DOI: 10.1016/j.chemgeo.2012.09.047
[29] RAVENGAI S, LOVE D, MABVIRA-MECK M. Water quality in an abandoned gold mining belt, Beatrice, Sanyati Valley, Zimbabwe[J]. Physics & Chemistry of the Earth, 2005, 30(11/16): 826-831.
[30] 曹秀红. 长江流域、黄河口及其邻近海域溶解态砷、硒、锑的分布、季节变化及影响因素[D]. 青岛: 中国海洋大学, 2012.
CAO Xiu-hong. Distribution, seasonal variation and influencing factors of dissolved arsenic, selenium and antimony in the Yangtze River Basin, the Yellow River Estuary and its adjacent waters[D]. Qingdao: Ocean University of China, 2012.
[31] LIAO H. Health risk associated with dietary co-exposure to high levels of antimony and arsenic in the world's largest antimony mine area[J]. Science of the Total Environment, 2011, 409(18): 3344-3351.
[32] LI N. Behaviors of dissolved antimony in the Yangtze River Estuary and its adjacent waters[J]. Journal of Environmental Monitoring Jem, 2011, 13(8): 22-92.
[33] HE M, WANG X, WU F. Antimony pollution in China[J]. Science of The Total Environment, 2012, 421/422: 41-50.
[34] GUO W, FU Z, HAO W. Environmental geochemical and spatial/temporal behavior of total and speciation of antimony in typical contaminated aquatic environment from Xikuangshan, China[J]. Microchemical Journal, 2018, 137: 181-189.
[35] LI S, ZHANG Q. Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China[J]. Journal of Hazardous Materials, 2010, 181(1/3): 1051-1058.
[36] 张晓军, 胡明安, 赵颖虹, 等. 大冶铁山地区重金属污染分析[J]. 环境科学与技术, 2005, 28(1): 40-43.
ZHANG Xiao-jun, HU Ming-an, ZHAO Ying-hong, et al. Analysis of heavy metal pollution in Tieshan area of Daye[J]. Environmental Science and Technology, 2005, 28(1): 42-45.
[37] 李雪华. 锑矿区沉积物生态风险评价及修复技术研究[D]. 北京: 北京林业大学, 2013.
LI Xue-hua. Study on ecological risk assessment and remediation technology of sediment in Antimony Mine Area[D]. Beijing: Beijing Forestry University, 2013.
[38] 周建伟, 温 冰, 郝春明, 等. 水环境中锑(Sb)污染研究进展[C]//中国地质学会2015学术年会论文摘要汇编(下册). 西安: 中国地质学会, 2015: 708-710.
ZHOU Jian-wei, WEN Bing,HAO Chun-ming, et al. Research progress of antimony pollution in the water environment[C]//Abstract Summary of Papers of 2015 Annual Meeting of Chinese Geological Society (Volume II). Xi'an: Chinese Geological Society, 2015: 708-710.
[39] 吴 思. 贵州某锑矿区水体污染特征及生态风险研究[D]. 衡阳: 南华大学, 2017.
WU Si. Study on water pollution characteristics and ecological risk of a antimony mining area in Guizhou Province[D]. Hengyang: Nanhua University, 2017.
[40] 宁增平, 肖唐付, 杨 菲, 等. 锑矿区水体水环境锑污染及硫同位素示踪研究[J]. 矿物岩石地球化学通报, 2011, 30(2): 135-141.
NING Zeng-ping, XIAO Tang-fu, YANG Fei, et al. Antimony pollution and sulfur isotope study in the waters of an antimony mine area [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2011, 30(2): 135-141.
[41] 王 其. 都柳江水体-沉积物间锑的迁移转化规律[J]. 贵州大学学报(自然科学版), 2013, 30(3): 131-136.
WANG Qi. Antimony mobility in water-sediment of Duliujiang River[J]. Journal of Guizhou University(Natural Science Edition), 2013, 30(3): 131-136.
[42] 张国平. 贵州典型矿山的水环境地球化学特征[D]. 北京: 中国科学院研究生院(地球化学研究所), 2005.
ZHANG Guo-ping. Geochemical characteristics of water environment of typical mines in Guizhou Province[D]. Beijing: Graduate School of Chinese Academy of Science (Institute of Geochemistry), 2005.
[43] GOLDFARB R J. Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska[J]. Chemical Geology, 2013, 335: 172-188.
[44] 张润宇. 湖南锡矿山周边水体的环境特征[J]. 环境科学学报, 2009, 29(3): 655-661.
ZHANG Run-yu. Environmental characteristics of water bodies around Xikuangshan in Hunan Province[J]. Journal of Environmental Science, 2009, 29(3): 655-661.
[45] FILELLA M, BELZILE N, CHEN Y. Antimony in the environment: a review focused on natural waters: II. Relevant solution chemistry[J]. Earth Science Reviews, 2002, 59(1): 265-285.
[46] LEUZ A, JOHNSON C A. Oxidation of Sb(Ⅲ) to Sb(Ⅴ) by O2 and H2O2 in aqueous solutions[J]. Geochimica et Cosmochimica Acta, 2005, 69(5): 1165-1172.
[47] QUENTEL F O, FILELLA M, ELLEOUET C. Sb(Ⅲ) oxidation by iodate in seawater: A cautionary tale[J]. Science of the Total Environment, 2006, 355(1/3): 259-263.
[48] ELLEOUET C, QUENTEL F O, MADEC C L. The effect of the presence of trace metals on the oxidation of Sb(Ⅲ) by hydrogen peroxide in aqueous solution[J]. Journal of Environmental Monitoring Jem, 2005, 7(12): 1220-1225.
[49] LU H, ZHANG W, TAO L. Enhanced removal of antimony by acid birnessite with doped iron ions: Companied by the structural transformation[J]. Chemosphere, 2019, 226: 834-840.
[50] BAI Y, Jefferson W A, LIANG J. Antimony oxidation and adsorption by in-situ formed biogenic Mn oxide and Fe-Mn oxides[J]. Journal of Environmental Sciences, 2017, 54: 126-134.
[51] BUSCHMANN J, SIGG L. Antimony(Ⅲ) binding to humic substances: Influence of pH and type of humic acid[J]. Environmental Science & Technology, 2004, 38(17): 4535-4541.
[52] BUSCHMANN J, CANONICA S, SIGG L. Photoinduced oxidation of antimony(Ⅲ) in the presence of humic acid[J]. Environmental Science & Technology, 2005, 39(14): 5335-5341.
[53] WU T L. Mechanisms of Sb(Ⅲ) oxidation mediated by low molecular weight[J]. Chemical Engineering Journal, 2019, 365(15): 190-198.
[54] DOS SANTOS FERREIRA C, SILVEIRA MARTINS P, DEMICHELI C. Thiol-induced reduction of antimony(Ⅴ) into antimony(Ⅲ): A comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione[J]. BioMetals, 2003, 16(3): 441-446.
[55] ILGEN A G, TRAINOR T P. Homogeneous oxidation of Sb(Ⅲ) by aqueous O2: The effect of ionic strength, Pb2+ and EDTA[J]. Environmental Chemistry, 2016, 13(6): 936-944.
[56] MITSUNOBU S, TAKAHASHI Y, SAKAI Y. Abiotic reduction of antimony(Ⅴ) by green rust (Fe4(Ⅱ)-Fe2(Ⅲ)- (OH)12SO4·3H2O)[J]. Chemosphere, 2008, 70(5): 942-947.
[57] ZHAO D C. Efficient recovery of Sb(Ⅴ) by hydrated electron reduction followed by cathodic deposition in a photoeletrochemical process[J]. Chemical Engineering Journal, 2020, 395: 124153.
[58] LI J, WANG Q, OREMLAND R S. Microbial antimony biogeochemistry: Enzymes, regulation, and related metabolic pathways[J]. Applied and Environmental Microbiology, 2016, 82(18): 5482-5495.
[59] PRAKASH C, LONI M W W W. Mechanism of microbial dissolution and oxidation of antimony in stibnite under[J]. Journal of Hazardous Materials, 2019, 385: 1-8.
[60] KANTIN R. Chemical speciation of antimony in marine algae[J]. Limnology and Oceanography, 1983, 28(1): 165-168.
[61] ZHANG H K. Bioadsorption and microbe-mediated reduction of Sb(Ⅴ) by a marine bacterium in the presence of sulfite/thiosulfate and the mechanism study[J]. Chemical Engineering Journal, 2019, 359: 755-764.
[62] WANG L, YE L, YU Y. Antimony redox biotransformation in the subsurface: effect of indigenous Sb(Ⅴ) respiring microbiota[J]. Environmental Science & Technology, 2018, 52(3): 1200-1207.
[63] TELLA M, POKROVSKI G S. Stability and structure of pentavalent antimony complexes with aqueous organic ligands[J]. Chemical Geology, 2012, 292/293: 57-68.
[64] HERATH I, VITHANAGE M, BUNDSCHUH J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport[J]. Environmental Pollution, 2017, 223: 545-559.
[65] SAITO. Solvent extraction studies of the hydrolysis of antimony(Ⅲ) in tracer concentrations[J]. Bulletin of the Chemical Society of Japan, 1974, 47(10): 2502-2507.
[66] Pauling, Linus. The formulas of antimonic acid and the antimonates[J]. J Am Chem Soc, 1933, 55(5): 1895-1900.
[67] OZTURK I, FILIMONOVA S, HADJIKAKOU S K. Structural motifs and biological studies of new antimony(Ⅲ) iodide complexes with thiones[J]. Inorganic Chemistry, 2010, 49(2): 488-501.
[68] WADE R J. Radio-tracer methods for studying speciation in natural waters[J]. Journal of Radioanalytical Chemistry, 1979, 48(1/2): 9-16.
[69] TELLA M, POKROVSKI G S. Antimony(Ⅴ) complexing with O-bearing organic ligands in aqueous solution: An X-ray absorption fine structure spectroscopy and potentiometric study[J]. Mineralogical Magazine, 2008, 72(1): 205-209.
[70] 李飒英. 锑配合物的合成与结构研究[J]. 科协论坛(下半月), 2010(4): 95-96.
LI Sa-ying. Synthesis and structure of antimony complex[J]. Science & Technology Association Forum, 2010(4): 95-96.
[71] TELLA M, POKROVSKI G S. Antimony(Ⅲ) complexing with O-bearing organic ligands in aqueous solution: An X-ray absorption fine structure spectroscopy and solubility study[J]. Geochimica et Cosmochimica Acta, 2009, 73(2): 268-290.
[72] 王 利. 铁修饰好氧颗粒污泥对含锑废水中重金属的吸附研究[D]. 上海: 复旦大学, 2014.
WANG Li. Adsorption of heavy metals in antimony bearing wastewater by iron modified aerobic granular sludge[D]. Shanghai: Fudan University, 2004.
[73] LI Y, JIA Y. Insights into antimony adsorption on {001} TiO2: XAFS and DFT study[J]. Environmental Science & Technology, 2017, 51(11): 6335-6341.
[74] WANG X, HE M, LIN C. Antimony(Ⅲ) oxidation and antimony(Ⅴ) adsorption reactions on synthetic manganite[J]. Geochemistry, 2012, 72: 41-47.
[75] LIU R, XU W, HE Z. Adsorption of antimony(Ⅴ) onto Mn(Ⅱ)-enriched surfaces of manganese-oxide and Fe Mn binary oxide[J]. Chemosphere, 2015, 138: 616-624.
[76] ILGEN A G, TRAINOR T P. Sb(Ⅲ) and Sb(Ⅴ) sorption onto Al-rich phases: Hydrous Al oxide and the clay minerals kaolinite KGa-1b and oxidized and reduced nontronite NAu-1[J]. Environmental Science & Technology, 2011, 46(2): 843-851.
[77] LUO J, HU C, MENG X. Antimony removal from aqueous solution using novel α-MnO2 nanofibers: Equilibrium, kinetic, and density functional theory studies[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(3): 2255-2264.
[78] REN S C, AI Y J, ZHANG X Y, et al. Recycling antimony(III) by magnetic carbon nanospheres: Turning waste to recoverable catalytic for synthesis of esters and triazoles[J]. ACS Sustainable Chemistry & Engineering, 2020, 8: 469-477.
[79] WANG J T, CHEN Y X, ZHANG Z Q. Microwell confined iron oxide nanoparticles in the honeycomb-like carbon spheres for the adsorption of Sb(Ⅲ) and sequential utilization as a catalyst[J]. ACS Sustainable Chemistry & Engineering, 2018, 6: 12925-12934.
[80] LI J, LI X D, Tasawar hayat. screening of zirconium-based metal-organic frameworks for efficiently simultaneous removal of antimonite (Sb(Ⅲ)) and antimonate (Sb(Ⅴ)) from aqueous solution[J]. ACS Sustainable Chemistry & Engineering, 2017, 5: 11496-11503.
[81] HE X, MIN X, LUO X. Efficient removal of antimony (Ⅲ, Ⅴ) from contaminated water by amino modification of a zirconium metal-organic framework with mechanism study[J]. Journal of Chemical & Engineering Data, 2017, 62(4): 1519-1529.
[82] TU Y H. Adsorption of antimonite and antimonate from aqueous solution using modified[J]. Journal of Hazardous Materials, 2020, 388(15): 1-42.
[83] BIVER M, SHOTYK W. Stibnite (Sb2S3) oxidative dissolution kinetics from pH 1 to 11[J]. Geochimica et Cosmochimica Acta, 2012, 79: 127-139.
[84] 温 冰. 湖南锡矿山水环境中锑来源及迁移转化的多元同位素解析[D]. 北京: 中国地质大学, 2017.
WEN Bing. Multi isotope analysis of antimony source, migration and transformation in the landscape environment of Hunan Tin Mine[D]. Beijing: China University of Geosciences, 2017.
[85] WEN B, ZHOU J, ZHOU A. Sources, migration and transformation of antimony contamination in the water environment of Xikuangshan, China: Evidence from geochemical and stable isotope (S, Sr) signatures[J]. Science of the Total Environment, 2016, 569/570: 114-122.
[86] HU X, HE M, KONG L. Photopromoted oxidative dissolution of stibnite[J]. Applied Geochemistry, 2015, 61: 53-61.
[87] YAN L, CHAN T, JING C. Mechanistic study for stibnite oxidative dissolution and sequestration on pyrite[J]. Environmental Pollution, 2020, 262: 114-309.
[88] JOHNSON C A, MOENCH H, WERSIN P. Solubility of antimony and other elements in samples taken from shooting ranges[J]. J Environ Qual, 2005, 34(1): 248-254.
[89] RENARD F, PUTNIS C V, MONTES-HERNANDEZ G. Sequestration of Antimony on calcite observed by time-resolved nanoscale imaging[J]. Environmental Science & Technology, 2017, 52(1): 107-113.
[90] ANEZKA BORCINOVA RADKOVA H E J K. Antimony mobility during the early stages of stibnite weathering in tailings at the Beaver Brook Sb deposit, Newfoundland[J]. Applied Geochemistry, 2020, 115: 140-528.
[91] JOHN C. Competitive sorption and transport of heavy metals in soils and geological media[J]. Soil Science Society of America Journal, 2013, 77(6): 2216-2216.
[92] DIEMAR G A, FILELLA M, LEVERETT P. Dispersion of antimony from oxidizing ore deposits[J]. Pure and Applied Chemistry, 2009, 81(9): 1547-1553.
[93] OKKENHAUG G, ZHU Y, LUO L. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area[J]. Environmental Pollution, 2011, 159(10): 2427-2434.
Research progress on speciation of antimony in natural water
LIU Xiao-yun1, LIU Jing-jing1, 3, KE Yong1, 2, 4, WANG Qing-wei1, 2, 4, YAN Xu1, 2, 4
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control & Treatment of Heavy Metal Pollution, Central South University, Changsha 410083, China;
3. Changsha Environmental Protection College, Changsha 410004, China;
4. Water Pollution Control Technology Key Lab of Hunan Province, Changsha 410004, China)
Abstract: Antimony(Sb), as a kind of hazardous contamination, has potential toxicity and carcinogenicity. With the wide application of Sb-containing compounds, a large number of Sb-containing pollutants has been distributed throughout the environment as a result of mining and smelting, which brings risks to the ecological environment and human health. The toxicity and content of Sb in water are strongly associated with its species. The Sb speciation in water and its migration and transformation in complex environment are the theoretical basis of Sb contamination control. This paper reviewed the research progress of the speciation, oxidation and reduction, hydrolysis and coordination, adsorption and desorption, dissolution and precipitation of Sb in water. Based on above, Sb pollution control in water was prospected.
Key words: antimony; pollution characteristics; chemical speciation; migration and transformation
Foundation item: Projects(2019SK2281, 2019SK2291) supported by the Key R&D Program of Hunan Province, China
Received date: 2020-06-17; Accepted date: 2021-02-20
Corresponding author: YAN Xu; Tel: +86-13574198657; E-mail: yanxu1202@csu.edu.cn
(编辑 何学锋)
基金项目:湖南省重点领域研发计划项目(2019SK2281,2019SK2291)
收稿日期:2020-06-17;修订日期:2021-02-20
通信作者:颜 旭,副教授,博士;电话:13574198657;E-mail:yanxu1202@csu.edu.cn
摘 要:锑是一种具有潜在毒性和致癌性的有色金属元素,随着锑及其化合物的广泛应用,大量含锑污染物进入水体中,给生态环境和人类健康带来风险。锑的毒性及其在水中的含量与形态结构密切相关,锑在复杂水体环境中的迁移转化规律是锑污染控制的理论基础。综述了近年来国内外对水中锑的形态及其氧化和还原、水解和配位、吸附和解吸、溶解和沉淀等迁移转化规律的研究进展,基于此对水体中锑的污染防治及未来研究重点作了展望。
[1] 客绍英, 石洪凌, 刘冬莲. 锑的污染及其毒性效应和生物有效性[J]. 化学世界, 2005(6): 382-384.
[2] 《冶金常识》编写组. 十种常用有色金属 锡锑汞(修订本)[M]. 北京: 冶金工业出版社, 1972.
[4] 胡亚琛. 流域锑污染如何综合防治?[J]. 环境经济, 2013(10): 44-48.
[5] 杨秀贞, 周腾智, 任伯帜. 矿区锑污染及其控制技术研究现状[J]. 枣庄学院学报, 2018, 35(5): 16-23.
[6] 朱 静, 王立英, 郭建阳, 等. 锑的环境地球化学研究进展概述[J]. 地球与环境, 2010(1):112-119.
[7] 黄艳超, 武雪芳, 周羽化, 等. 水环境中锑污染及其修复技术研究进展[J]. 南京师大学报(自然科学版), 2015, 38(4): 122-128.
[30] 曹秀红. 长江流域、黄河口及其邻近海域溶解态砷、硒、锑的分布、季节变化及影响因素[D]. 青岛: 中国海洋大学, 2012.
[36] 张晓军, 胡明安, 赵颖虹, 等. 大冶铁山地区重金属污染分析[J]. 环境科学与技术, 2005, 28(1): 40-43.
[37] 李雪华. 锑矿区沉积物生态风险评价及修复技术研究[D]. 北京: 北京林业大学, 2013.
[38] 周建伟, 温 冰, 郝春明, 等. 水环境中锑(Sb)污染研究进展[C]//中国地质学会2015学术年会论文摘要汇编(下册). 西安: 中国地质学会, 2015: 708-710.
[39] 吴 思. 贵州某锑矿区水体污染特征及生态风险研究[D]. 衡阳: 南华大学, 2017.
[40] 宁增平, 肖唐付, 杨 菲, 等. 锑矿区水体水环境锑污染及硫同位素示踪研究[J]. 矿物岩石地球化学通报, 2011, 30(2): 135-141.
[41] 王 其. 都柳江水体-沉积物间锑的迁移转化规律[J]. 贵州大学学报(自然科学版), 2013, 30(3): 131-136.
[42] 张国平. 贵州典型矿山的水环境地球化学特征[D]. 北京: 中国科学院研究生院(地球化学研究所), 2005.
[44] 张润宇. 湖南锡矿山周边水体的环境特征[J]. 环境科学学报, 2009, 29(3): 655-661.
[70] 李飒英. 锑配合物的合成与结构研究[J]. 科协论坛(下半月), 2010(4): 95-96.
[72] 王 利. 铁修饰好氧颗粒污泥对含锑废水中重金属的吸附研究[D]. 上海: 复旦大学, 2014.


