Trans. Nonferrous Met. Soc. China 27(2017) 377-381
Synthesis and characterization of ε-VOPO4 nanosheets for secondary lithium-ion battery cathode
Ze-hua CHEN1, Yi-zhu MA2, Peng-cheng MA1, Jian-liang CAO1, Yan WANG1, Guang SUN1, Xiao-dong WANG1, Hari BALA1, Chuan-xiang ZHANG1, Zhan-ying ZHANG1
1. College of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo 454000, China;
2. National Center for Quality National Center for Quality Supervision and Inspection of Building Decoration Materials, Zhengzhou 450004, China
Received 24 August 2015; accepted 8 December 2015
Abstract:
Vanadium (III) phosphate monoclinic VPO4·H2O was synthesized hydrothermally. The ε-VOPO4 nanosheets, formed by the oxidative de-intercalation of protons from monoclinic VPO4·H2O, can reversibly react with more than 1 mol lithium atoms in two steps. Crystal XRD analysis revealed that the structure of the ε-VOPO4 nanosheets is monoclinic with lattice parameters of α=7.2588(4) 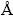 , b=6.8633(2)
, b=6.8633(2) 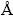 and c=7.2667(4)
and c=7.2667(4) 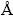 . The results show that the ε-VOPO4 nanosheets have a thickness of 200 nm and uniform crystallinity. Electrochemical characterization of the ε-VOPO4 monoclinic nanosheets reveals that they have good electrochemical properties at high current density, and deliver high initial capacity of 230.3 mA·h/g at a current density of 0.09 mA/cm2. Following the first charge cycle, reversible electrochemical lithium extraction/insertion at current density of 0.6 mA/cm2 affords a capacity retention rate of 73.6% (2.0-4.3 V window) that is stable for at least 1000 cycles.
. The results show that the ε-VOPO4 nanosheets have a thickness of 200 nm and uniform crystallinity. Electrochemical characterization of the ε-VOPO4 monoclinic nanosheets reveals that they have good electrochemical properties at high current density, and deliver high initial capacity of 230.3 mA·h/g at a current density of 0.09 mA/cm2. Following the first charge cycle, reversible electrochemical lithium extraction/insertion at current density of 0.6 mA/cm2 affords a capacity retention rate of 73.6% (2.0-4.3 V window) that is stable for at least 1000 cycles.
Key words:
lithium-ion battery; nanosheet; vanadium phosphate; cathode material; synthesis; characterization;
1 Introduction
During the past decade, the energy shortage and the environment pollution have become serious due to the development of economy and the increase of the population. As a result, the intermittence of the renewable energies such as wind, wave and solar outputs, better energy storage and assistance systems have been the main focuses. However, these renewable energies are dependent on time and season [1]. Undoubtedly, energy storage systems should be developed and used to the reality, which is of vital importance to the social development in the future. Among various storage technologies, the development of battery systems with high energy density as well as long-term cycling stability is a crucial step on the path toward broad implementation of hybrid or electric cars and is a key element for broad adoption of renewable energy technologies [2-4].
Nowadays, lithium-ion batteries have been widely used in many fields as power suppliers for mobile equipment. Most of the present lithium batteries used in electronic devices or hybrid electric vehicles employ transition metal oxides such as LiCoO2, LiMn2O4 or mixed metal analogs such as Li(Ni,Mn,Co)O2, olivine LiFePO4 as the active cathode materials [5-8]. The present commercial cathode material, LiCoO2, possesses some problems in large-scale utilization, due to its high cost and low safety [9-11]. The oxide-based materials (LiCoO2, LiNiO2), the spinel LiMn2O4, and all their substituted variations have been extensively studied as positive electrode materials [12-17]. Vanadium phosphates have been extensively studied because of their importance in selective catalysis of hydrocarbon oxidation reactions. Vanadium phosphates are also attractive due to their higher free energy of reaction, and the greater possible change of oxidation state.
Seven phases of VPO4 were synthesized and their lithium intercalation properties were investigated as cathode materials [15,17-19]. Several vanadyl phosphates VOPO4 were investigated as cathode materials [20,21]. Till now, the polymorphs have not satisfactory electrochemical performance, and more efforts are needed to make use of the high operating voltage and reasonable theoretical capacity of VOPO4. The ε-VOPO4 compound has particularly interesting properties, such as an approximately 4 V flat discharge potential, which is about 0.5 V higher than that of LiFePO4, and higher electronic conductivity, leading to the possibility of attaining higher power systems and theoretical energy density compared with LiFePO4 [8]. This material adopts a stable 3D tunneling structure with the theoretical specific capacity of ~168 mA·h/g (α-LiVOPO4). Compared with LiFePO4, ε-VOPO4 has a much higher conductivity (1×10-6 S/cm vs 1×10-10 S/cm of LiFePO4) [22,23]. Furthermore, similar to LiFePO4, the rate capability of VOPO4 can be improved by decreasing the particle size [19]. For ε-VOPO4, we might intercalate two lithium ions into the structure, which may deliver larger capacity if both lithium ions participate in electrochemistry. Overall, all these advantages make ε-VOPO4 a great candidate for next generation of high energy density lithium-ion batteries. Although various preparation methods have been reported for ε-VOPO4, there is a possibility that ε-VOPO4 with larger capacity and better cycle stability can be obtained by changing the preparation method and crystal structure size. In Ref. [21], we reported the electrochemical behavior of the β-VOPO4/ε-VOPO4 composite and nanostrucutral ε-VOPO4 formed from the monoclinic and tetragonal forms of VPO4·H2O. In this study, ε-VOPO4 nanosheets were synthesized and their electrochemical performances as the cathode materials were investigated.
2 Experimental
The ε-VOPO4 was prepared using the method as described by SONG et al [24]. The monoclinic VPO4·H2O precursor was synthesized in the aqueous solution, the reactants were 16.7 mL standardized H3PO4 solution (2.39 mol/L), 1.60 g VCl3, and 20 mL deionized water, with the pH adjusted by triethylmethylammonium hydroxide (20% in water) to 3 under stirring for 7 d. The monoclinic VPO4·H2O precursors were obtained in corresponding solutions in 125 mL PTFE-lined Parr autoclaves at 180 °C for 3 d, then ramp-cooled to room temperature at 0.5 °C/min. The ε-VOPO4 from the monoclinic phase was obtained by heating the precursor in oxygen at 500 °C for 3 h.
The electrochemical properties of these samples were evaluated in 2325-type coin cells. The cathodes were prepared by grinding 80% (mass fraction) active material with 10% (mass fraction) conductive carbon and 10% (mass fraction) PVDF binder in a mortar and pestle. NMP (1-methyl-2-pyrrolidinone, Aldrich) was added to create a paste, which was laminated onto aluminum foil and vacuum dried at 90 °C before use. The electrolyte was 1 mol/L LiPF6 (lithium hexafluorophosphate) dissolved in a solution of ethylene carbonate (EC) and dimethyl carbonate (DMC) (LP30 from EM Industries) with a volume ratio of 1:1; a Celgard 2400 separator (Hoechst Celanese) was used. The coin cells were assembled in a glove box filled with pure helium. A Macpile potentiostat was used to cycle the cells at 0.09 mA/cm2 between 2 and 4.3 V at room temperature.
3 Results and discussion
The diffraction data were collected for the prepared material over a 12 h period on a Scintag XDS 2000 powder diffractometer using Cu Kα radiation (λ=1.5418 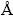 ) operating in a θ-θ geometry between 2θ values of 15° and 75° with a step size of 0.02° and a collection time of 8 s per point. The XRD pattern of the prepared material is shown in Fig. 1, which agrees with those previously reported [20,24]. The XRD pattern can be indexed as a monoclinic structure with a space group Cc. Rietveld refinement was carried out using GSAS + EXPGUI [25]. Cell parameters were refined to a=7.2588(4)
) operating in a θ-θ geometry between 2θ values of 15° and 75° with a step size of 0.02° and a collection time of 8 s per point. The XRD pattern of the prepared material is shown in Fig. 1, which agrees with those previously reported [20,24]. The XRD pattern can be indexed as a monoclinic structure with a space group Cc. Rietveld refinement was carried out using GSAS + EXPGUI [25]. Cell parameters were refined to a=7.2588(4) 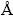 ; b=6.8633(2)
; b=6.8633(2) 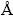 ; c=7.2667(4)
; c=7.2667(4) 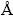 ; β=115.391(3)
; β=115.391(3) 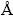 ; V=328.26(2)
; V=328.26(2) 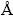 3 (space group Cc). The XRD data revealed that the sample was a single-phase material. The two main diffraction peaks were observed at 26° and 29°. This agreed with our previous report [20].
3 (space group Cc). The XRD data revealed that the sample was a single-phase material. The two main diffraction peaks were observed at 26° and 29°. This agreed with our previous report [20].
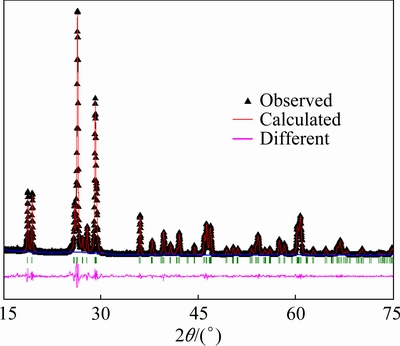
Fig. 1 XRD pattern and Rietveld refinement of ε-VOPO4 nanosheets
The ε-VOPO4 nanosheets have been derived from monoclinic VPO4·H2O. The crystal structure of ε-VOPO4 consists of corner sharing VO6 octahedra and PO4 tetrahedra [26], as shown in Fig. 2. The easy inversion of vanadyl bonds offers a mechanism which allows the VOPO4 host lattice to fine-adjust for the accommodation of the lithium ions. The VOPO4 and corresponding LiVOPO4 polymorphs were characterized. Every second V=O···V chain has to be inverted upon intercalation of one equivalent lithium into ε-VOPO4 [26]. In the LiPF6 solution, the reaction is ε-VOPO4→α-LiVOPO4 [23]. Vanadium oxide is a typical intercalation compound due to its layered structure. For lithium-ion intercalation applications, vanadium oxide offers the essential advantages of low cost, abundant source, easy synthesis, and high energy densities [27]. VOXO4 compounds (X=S, P, As) present well-open 2D or 3D frameworks which facilitate lithium intercalation process [28].
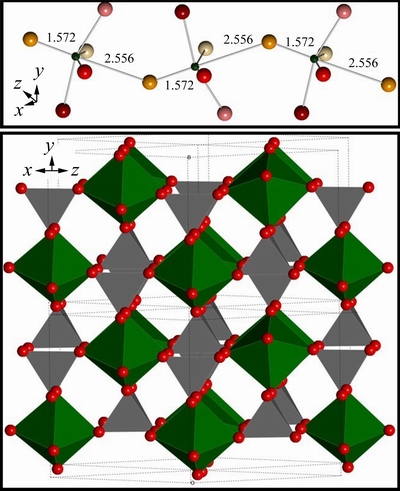
Fig. 2 Crystal structures of ε-VOPO4
As the particle size has a significant impact on the electrochemical performance of a material, we characterized the nanosheets of the ε-VOPO4 phases. The micrographs are shown in Fig. 3. The nanosheets of the ε-VOPO4 consist of single crystals with thickness up to 200 nm. It is confirmed that most of the prepared ε-VOPO4 materials are nanosheets. The synthetic product has high crystallinity and purity.
The first charge-discharge curve was selected, as shown in Fig. 4. A pair of potential plateaus is observed in the charge–discharge curve of each polymorph, which can be attributed to the redox reaction of the V5+/V4+ couple. These data were collected at 25 °C and a current density of 0.09 mA/cm2 in voltage range of 2.0-4.3 V (vs Li+/Li). The initial oxidation process equates to a material specific capacity of 230.2 mA·h/g during this lithium extraction. The theoretical specific capacities for inserted lithium ions 1 and 2 of VOPO4 are 165 and 331 mA·h/g, respectively. Based on a theoretical specific capacity for the inserted lithium ion 1 of 165 mA·h/g and assuming no side reactions, the fully charged material reaches 69.48% of the theoretical specific capacity. Subsequent testing indicated that this coulombic inefficiency was restricted primarily to the first cycle because subsequent constant current cycles showed significantly improved reversibility.
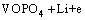
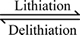
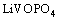 (1)
(1)
Therefore, the lithiation/delithiation process proceeds via a two-phase reaction. The lithium extraction/insertion behavior for the LiVOPO4 active material relies on the reversibility of the V4+/V5+ redox couple. This agrees with the results in Ref. [22].
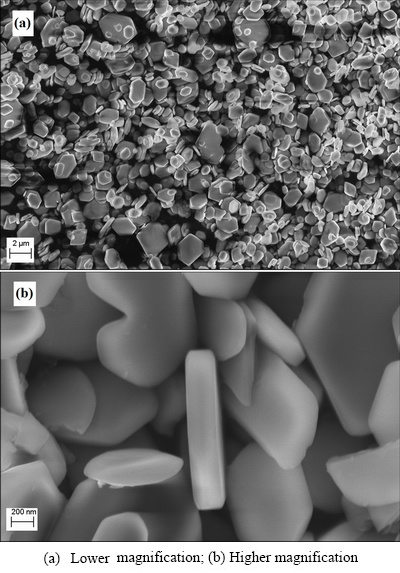
Fig. 3 SEM images of ε-VOPO4 nanosheets
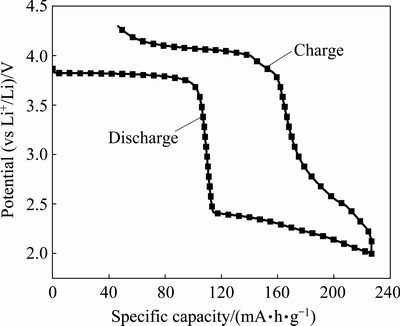
Fig. 4 Initial charge-discharge curves of specific ε-VOPO4 nanosheets composite at current density of 0.09 mA/cm2
To establish the stability of the lithium insertion reaction, we conducted current cycling trial by using metallic lithium coin cells. The result of the trial is shown in Fig. 5. The test was undertaken in voltage range of 2.0-4.1 V at 25 °C. In summary, stabilized reversible capacity of 110 mA·h/g was measured at 1000 cycles and the current density of 0.6 mA/cm2. Reversibility is a concern; however, as capacity fading occurs during cycling, especially at faster rates. This may be due to the lithium ions becoming trapped within the structure, perhaps at grain boundary generated during the intercalation/deintercalation process, or as a result of limitations on transport in the material. Overall, the measured specific capacity at high current density is somewhat higher than what we expected based on the results in Refs. [20,21].
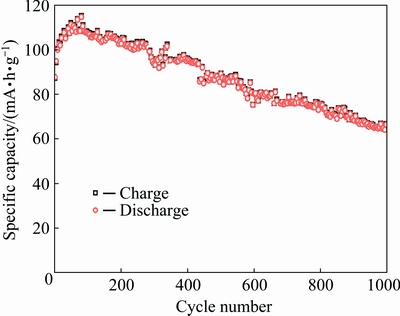
Fig. 5 Charge-discharge capacity of ε-VOPO4 nanosheet composite at current density of 0.6 mA/cm2
This good rate capability was achieved without harsh control of particle size. The ε-VOPO4 nanosheets show greatly improved rate capability at high current density due to the largely enhanced electronic transport and the smaller particle size [29]. It can be found that the cyclic performance at high current density is obviously stable and the specific capacity is high.
In order to find out whether the variation trend of the charge transfer resistance is consistent with that of the capacity, the electrochemical impedance spectroscopy (EIS) spectra were recorded, as shown in Fig. 6. Impedance spectra were recorded to evaluate the electrochemical kinetic of the ε-VOPO4 nanosheets after different charge–discharge cycles [1]. The semicircle in high frequency region represented the charge transfer resistance (Rct) between the electrolyte and active material, involving SEI film resistance [30]. The sloping line in the low frequency region corresponds to the diffusion of Li+ ion in the electrode bulk namely Warburg impedance [31]. As seen in Fig. 6(a), the semicircle diameter of the 30th cycle is much smaller than that of the initial cycle in the high-frequency region, indicating that the charge transfer resistance of the ε-VOPO4 nanosheets becomes lower while the charge- discharge processing is carried out [32]. The lower charge transfer resistance is due to the nanostructure of the ε-VOPO4 nanosheets which can provide shorter diffusion distance for Li+ ion and the pretreated ε-VOPO4 nanosheets have good electric conductivity [33].
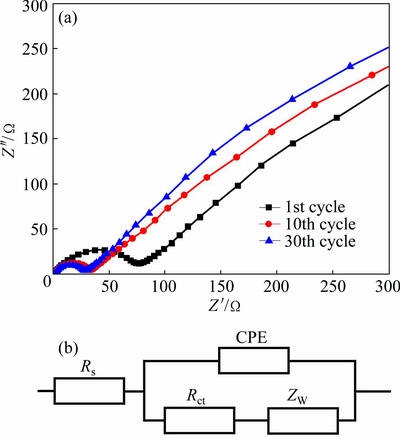
Fig. 6 Impedance spectra of different cycles at current of 0.6 mA/cm2 (a), and equivalent circuit used for fitting experimental parameters of EIS (b)
4 Conclusions
1) The ε-VOPO4 nanosheets with smaller size and uniform shape were synthesized via the thermal decomposition of VPO4·H2O. From the structural point of view, we used XRD to characterize the prepared ε-VOPO4 nanosheets material.
2) From the electrochemical point of view, the cycle stability of the ε-VOPO4 nanosheets is enhanced at high current density through obtaining nanostructural particles. When the ε-VOPO4 nanosheets are discharged to 2 V more than one lithium atom per formula can be reversibly inserted/extracted. The crystal size is smaller and the initial specific capacity can reach 230.2 mA·h/g at a current density of 0.09 mA/cm2. Furthermore, the capacity retention rate is 73.6% at a current density of 0.6 mA/cm2 after 1000 cycles.
References
[1] PEREIRA N, AMATUCCI G G, WHITTINGHAM M S, HAMLEN R. Lithium–titanium disulfide rechargeable cell performance after 35 years of storage [J]. Journal of Power Sources, 2015, 280: 18-22.
[2] VAUGHEY J T, LIU G, ZHANG J G. Stabilizing the surface of lithium metal [J]. MRS Bulletin, 2014, 39: 429-435.
[3] WHITTINGHAM M S. Ultimate limits to intercalation reactions for lithium batteries [J]. Chemical Reviews, 2014, 114: 11414-11443.
[4] YAMADA A. Iron-based materials strategies [J]. MRS Bulletin, 2014, 39: 423-428.
[5] CHEN Ze-hua, HUANG Ke-long, LIU Su-qin. Preparation and characterization of spinel LiMn2O4 nanorods as lithium-ion battery cathodes [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 2309-2313.
[6] LIN Meng-chang, GONG Ming, LU Bin-gan, WANG Di-yan. An ultrafast rechargeable aluminium-ion battery [J]. Nature Letter, 2015, 10: 14340-14342.
[7] WHITTINGHAM M S. Lithium batteries and cathode materials [J]. Chemical Reviews, 2004, 104: 4271-4302.
[8] WHITTINGHAM M S, SONG Y N, LUTTA S. Some transition metal (oxy) phosphates and vanadium oxides for lithium batteries [J]. Journal of Materials Chemistry, 2005, 15: 3362-3379.
[9] NAZAR L F, CUISINIER M, PANG Q. Lithium-sulfur batteries [J]. MRS Bulletin, 2014, 39: 436-442.
[10] OMENYA F, MILLER J K, FANG J, WEN B H, ZHANG R B, WANG Q, CHERNOVA N A, WHITTINGHAM M S. Single-phase lithiation and delithiation of simferite compounds Li(Mg,Mn,Fe)PO4 [J]. Chemistry of Materials, 2014, 26: 6206-6212.
[11] SHTERENBERG, SALAMA M, GOFER Y, LEVI E, AURBACH D. The challenge of developing rechargeable magnesium batteries [J]. MRS Bulletin, 2014, 39: 453-460.
[12] KIM Y S, LEE H D, KANG S H. First-principles and experimental investigation of the morphology of layer-structured LiNiO2 and LiCoO2 [J]. Journal of Materials Chemistry, 2012, 22: 12874-12881.
[13] MIZUNO Y, HOSONO E, SAITO T, OKUBO M, NISHIO-HAMANE D, HO-ISHI K, KUDO T, ZHOU H S. Electrospinning synthesis of wire-structured LiCoO2 for electrode materials of high-power Li-ion batteries [J]. Journal of Physical Chemistry C, 2012, 116: 10774-10780.
[14] NOLIS G M, OMENYA F, ZHANG R B, FANG B, UPRETI S, CHERNOVA N A, WANG F , GRAETZ J, HU Y Y, GREY C P, WHITTINGHAM M S. Structure, defects and thermal stability of delithiated olivine phosphates [J]. Journal of Materials Chemistry, 2012, 22: 20482-20489.
[15] AZMI B M, ISHIHARA T, NISHIGUCHI H, TAKITA Y. Vanadyl phosphates of VOPO4 as a cathode of Li-ion rechargeable batteries [J]. Journal of Power Sources, 2003, 119: 273-277.
[16] BELHAROUAK I, JOHNSON C, AMINE K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4 [J]. Electrochemistry Communications, 2005, 7: 983-988.
[17] GAUBICHER J, MERCIER T L, CHABRE Y, ANGENAULT J, QUARTON M. Li/β-VOPO4: A new 4 V system for lithium batteries [J]. Journal of the Electrochemical Society, 1999, 146: 4375-4379.
[18] AZMI B M, ISHIHARA T, NISHIGUCHI H, TAKITA Y. Cathodic performance of VOPO4 with various crystal phases for Li ion rechargeable battery [J]. Electrochimica Acta, 2002, 48: 165-170.
[19] DUPRE N, GAUBICHER J, ANGENAULT J, WALLEZ G. Electrochemical performance of different Li-VOPO4 systems [J]. Journal of Power Sources, 2001, 97: 532-534.
[20] CHEN Ze-hua, CHEN Qi-yuan, CHEN Li-quan, ZHANG Rui-bo, ZHOU Hu, CHERNOVA N A, WHITTINGHAM M S. Electrochemical behavior of nanostructured ε-VOPO4 over two redox plateaus [J]. Journal of the Electrochemical Society A, 2013, 160: 1777-1780.
[21] CHEN Ze-hua, CHEN Qi-yuan, WANG Hai-yan, ZHANG Rui-bo, ZHOU Hui, CHEN Li-quan, WHITTINGHAM M S. A β-VOPO4/ε-VOPO4 composite Li-ion battery cathode [J]. Electrochemistry Communications, 2014, 46: 67-70.
[22] CHEN L, ZHANG R G, MIZUNO F. Phase stability and its impact on the electrochemical performance of VOPO4 and LiVOPO4 [J]. Journal of Materials Chemistry A, 2014, 2: 12330-12339.
[23] ZHANG M, ZHANG S, GAO H, MENG F L, DENG C. Exploring high performance VOPO4 for lithium batteries: A comparison between β and δ polymorphs [J]. Journal of Electroanalytical Chemistry, 2014, 713: 119-124.
[24] SONG Y N, ZAVALIJ P Y, WHITTINGHAM M S. ε-VOPO4: Electrochemical synthesis and enhanced cathode behavior [J]. Journal of the Electrochemical Society A, 2005, 152: 721-728.
[25] XIAO P F, LAI M O, LU L. Transport and electrochemical properties of high potential tavorite LiVPO4F [J]. Solid State Ionics, 2013, 242: 10-19.
[26] GIRGSDIES F, DONG W S, BARTLEY J K. The crystal structure of ε-VOPO4 [J]. Solid State Sciences, 2006, 8: 807-812.
[27] CHENG F Y, LIANG J, TAO Z L. Functional materials for rechargeable batteries [J]. Advanced Materials, 2011, 23: 1695-1715.
[28] LI Y H, ZHANG J P, YANG F M, SUN H, TANG S W, WANG R S. Morphology and surface properties of LiVOPO4: A first principles study [J]. Physical Chemistry Chemical Physics, 2014, 16: 24604-24609.
[29] KWABI D G, ORTIZ-VITORIANO N, FREUNBERGER S A. Materials challenges in rechargeable lithium-air batteries [J]. MRS Bulletin, 2014, 39: 443-452.
[30] KIM H Y, HONG J H, PARK K Y, KIM H S, KIM S W, KANG K S. Aqueous rechargeable Li and Na ion batteries [J]. Chemical Reviews, 2014, 114: 11788-11827.
[31] KUBOTA K, YABUUCHI N, YOSHIDA H, DAHBI M, KOMABA S. Layered oxides as positive electrode materials for Na-ion batteries [J]. MRS Bulletin, 2014, 39: 416-422.
[32] NGUYEN V H, WANG W L, GU H B. Enhanced electrochemical performance of Li3V2(PO4)3 structurally converted from LiVOPO4 by graphite nanofiber addition [J]. Ceramics International, 2015, 41: 5403-5413.
[33] LUO Yun-ze, HE Li-hua, LIU Xu-heng. Effect of Mg doping on electrochemical performance of Li3V2(PO4)3/C cathode material for lithium ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(7): 2266-2271.
锂离子二次电池正极材料ε-VOPO4纳米片的制备与表征
陈泽华1,马亿珠2,麻鹏程1,曹建亮1,王 燕1,孙 广1,王晓冬1,哈日巴拉1,张传祥1,张战营1
1. 河南理工大学 化学化工学院,焦作 454000;2. 国家建筑装修材料质量监督检验中心,郑州 450004
摘 要:通过水热法合成钒系磷酸盐单斜系VPO4·H2O,通过氧化作用得到ε-VOPO4 纳米片。ε-VOPO4纳米片在嵌锂两个过程中与超过1 mol的锂原子发生反应。对制备的ε-VOPO4 纳米片进行XRD测试,得到晶胞参数为α=7.2588(4) 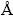 ; b=6.8633(2)
; b=6.8633(2) 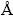 ; c=7.2667(4)
; c=7.2667(4) 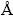 。结果表明,制备的ε-VOPO4纳米片厚度为200 nm且结晶度完美。电化学性能表征结果表明,制备的单斜系ε-VOPO4 纳米片在高电流密度下表现出优越的电化学性能,在电流密度为0.09 mA/cm2情况下的初始放电容量为230.3 mA·h/g。初始放电后,在电流密度为0.6 mA/cm2下经过1000次循环后其容量保持率为73.6% (放电窗口为2.0~4.3 V)。
。结果表明,制备的ε-VOPO4纳米片厚度为200 nm且结晶度完美。电化学性能表征结果表明,制备的单斜系ε-VOPO4 纳米片在高电流密度下表现出优越的电化学性能,在电流密度为0.09 mA/cm2情况下的初始放电容量为230.3 mA·h/g。初始放电后,在电流密度为0.6 mA/cm2下经过1000次循环后其容量保持率为73.6% (放电窗口为2.0~4.3 V)。
关键词:锂离子电池;纳米片;钒系磷酸盐;正极材料;合成;表征
(Edited by Wei-ping CHEN)
Foundation item: Projects (51172065, 51404097, 51504083, U1404613) supported by the National Natural Science Foundation of China; Project (16A150009) supported by the Key Scientific Research Project for Higher Education of Henan Province, China; Project (16A150009) supported by the Natural Science Foundation of Henan Province (General Program), China; Project (166115) supported by the Postdoctoral Science Foundation of Henan Province, China
Corresponding author: Ze-hua CHEN; Tel: +86-18839155118; E-mail: chen1861@hpu.edu.cn
DOI: 10.1016/S1003-6326(17)60042-6
Abstract: Vanadium (III) phosphate monoclinic VPO4·H2O was synthesized hydrothermally. The ε-VOPO4 nanosheets, formed by the oxidative de-intercalation of protons from monoclinic VPO4·H2O, can reversibly react with more than 1 mol lithium atoms in two steps. Crystal XRD analysis revealed that the structure of the ε-VOPO4 nanosheets is monoclinic with lattice parameters of α=7.2588(4) 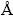 , b=6.8633(2)
, b=6.8633(2) 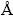 and c=7.2667(4)
and c=7.2667(4) 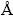 . The results show that the ε-VOPO4 nanosheets have a thickness of 200 nm and uniform crystallinity. Electrochemical characterization of the ε-VOPO4 monoclinic nanosheets reveals that they have good electrochemical properties at high current density, and deliver high initial capacity of 230.3 mA·h/g at a current density of 0.09 mA/cm2. Following the first charge cycle, reversible electrochemical lithium extraction/insertion at current density of 0.6 mA/cm2 affords a capacity retention rate of 73.6% (2.0-4.3 V window) that is stable for at least 1000 cycles.
. The results show that the ε-VOPO4 nanosheets have a thickness of 200 nm and uniform crystallinity. Electrochemical characterization of the ε-VOPO4 monoclinic nanosheets reveals that they have good electrochemical properties at high current density, and deliver high initial capacity of 230.3 mA·h/g at a current density of 0.09 mA/cm2. Following the first charge cycle, reversible electrochemical lithium extraction/insertion at current density of 0.6 mA/cm2 affords a capacity retention rate of 73.6% (2.0-4.3 V window) that is stable for at least 1000 cycles.


