- Abstract:
- 1 Introduction▲
- 2 Experimental ▲
- 3 Results and discus...▲
- 4 Conclusions▲
- References
- Figure
- Fig. 1 XRD patterns of ZnO:La3+,Li+ prepared by different methods:
- Fig. 2 XRD patterns of samples prepared by co-precipitation and solid-state method at different calcination temperatures:
- Fig. 3 SEM images of samples prepared by different methods:
- Fig. 4 Room-temperature excitation spectra of ZnO: La3+, Li+ prepared by different methods:
- Fig. 5 Luminescence emission spectra of samples derived from different methods:
- Fig. 6 Photoluminescence emission spectra of samples prepared by co-precipitation and solid-state method with different calcination temperatures:
J. Cent. South Univ. (2013) 20: 332–336
DOI: 10.1007/s11771-013-1492-0
precipitation processes and luminescence properties of ZnO: La3+, Li+ nanoparticles
GU Ying-ying(古映莹), LI Lu-ke(李陆柯), ZHANG Wen-wen(张稳稳), LIU Ying(刘英), LU Zhou-guang(卢周广)
1. College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Resources Chemistry of Nonferrous Metals, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Abstract:
ZnO:La3+,Li+ nanoparticles were successfully prepared by co-precipitation, citric acid-assisted co-precipitation, co-precipitation combined solid-state reaction and thermal decomposition method. X-ray diffraction (XRD), scanning electron microscopy (SEM) and luminescence spectrophotometry were employed to characterize the crystal phases, particle sizes and luminescence properties of the as-prepared nanopowders. The results indicate that all the prepared samples crystallize in a hexagonal wurtzite structure. The ZnO:La3+,Li+ prepared by citric acid-assisted co-precipitation method has a particle size of about 80 nm, which is the smallest among all the samples. Fluorescence (FL) spectra of all samples exhibit three typical emissions: a violet one centered at around 400 nm, blue around 450 nm and 466 nm, and weak green near 520 nm. But the samples prepared by co-precipitation method show a strong and wide green light emission located at about 500 nm. The ZnO:La3+,Li+ nanoparticles synthesized by the co-precipitation method demonstrate relatively the strongest emission intensity.
Key words:
ZnO:La3+,Li+nanoparticles; doping; co-precipitation method; luminescence properties;
1 Introduction
Recently, semiconductor materials doped with metal ions have received increasing attention due to their much improved light, electrical and other properties. A lot of doping systems of semiconductor materials have appeared, such as ZnS [1], SnO2 [2], In2O3 [3], and ZnO [4–5] doping systems. Especially, industry departments attach great importance to the research and development of nano ZnO, because it owns the advantage of both semiconductor oxide and nanometer materials. At present, scientific researchers have taken more interest in optical and electrical research of nano ZnO. In order to realize the applications of ZnO in light-emitting devices, more effort should be put on solving the problems of luminous efficiency and color purity. Many experimental results have already demonstrated that adding new elements and making the sample nanorization are the two effective ways to solve the above problems. La3+ and Li+ were chosen as the doping elements in this work. ZnO is an oxide semiconductor material with a wide band gap (3.37 eV at 300 K), which has been widely applied to the surface acoustic equipment, gas sensor, piezoelectric, flat panel display, transparent electrodes and solar battery window due to its excellent photoelectric, piezoelectric, gas-sensitive features [6–8].. Rare earth elements have special 4f layer electronic structure, which can produce a lot of absorption and fluorescence information when electrons jump in f–f or f–d shells. These special magnetic and fluorescence information can lead to special physical and chemical properties of rare earth elements, providing important guarantees in synthesis of new materials with special functions [9].
ZnO:Li+ thin film has been fabricated by Zhu et al [10] by frequency magnetron sputtering method and La3+ doped ZnO nanopowders have been reported quite recently [11–13]. But there are no reports on the synthesis of La3+,Li+ co-doped ZnO. The transmission of energy between La3+ and ZnO is weak because the ionic radius of La3+ (106 pm) is much larger than that of Zn2+ (74 pm). While the radius of Li+ is 68 pm, which makes it a good sensitization agent [10–14]. Furthermore, it can also function as charge compensator. So it was extraordinarily useful and constructive, not just for the preparation of the La3+,Li+ co-doped ZnO nanopowders, but for studying the structure and optical properties. In this work, ZnO:La3+,Li+ nanopowders were prepared by different routes, including co-precipitation, citric acid-assisted co-precipitation, co-precipitation combined solid-state reaction and thermal decomposition method. Moreover, the structure and optical properties of the samples prepared by different methods are also discussed in details.
2 Experimental
2.1 Chemicals
Chemical reagents, Zn(NO3)2·6H2O (Sinopharm Chemical Reagent Co. Ltd), La(NO3)3·nH2O (Sinopharm Chemical Reagent Co. Ltd), Na2CO3(Chemical Co. Ltd, Taishan), C6H5Na3O7·2H2O (Sinopharm Chemical Reagent Co. Ltd), CH3CH2OH (Organic Reagents Factory, Changsha), LiNO3 (Chemical Plant, Nanhui Peng), Li2CO3 (Chemical Plant, Taishan, Guangdong), NaOH (Chemical Co. Ltd, Xilong) etc. reagents were of analytical grade and used without further purification.
2.2 Synthesis
2.2.1 Co-precipitation method
1% La(NO3)3 and 4% LiNO3 were dissolved into 50 mL 0.5 mol/L Zn(NO3)2 solution. after fully dissolved, 60 mL 0.5 mol/L Na2CO3 solution was slowly dropped into the above mixture solution under vigorous stirring. After dropping completely, the mixture was continued stirring for 1 h, and then aged for 2 h. White precipitate was filtered, washed with deionized water and alcohol for three times, and dried at 80°C for 3 h. ZnO:La3+,Li+ nanopowders were obtained after calcining the precursors in muffle furnace at 650°C for 3 h.
2.2.2 Citric acid-assisted co-precipitation method
0.025 mol C6H5Na3O7·2H2O, 1% La(NO3)3, 4% LiNO3 (mass fraction) were dissolved in 50 mL 0.5mol/L Zn(NO3)2 solution. after fully dissolved, 60 mL 0.5 mol/L Na2CO3 solution was slowly dropped into the above mixture solution, then using the same treat method as section 2.2.1, ZnO:La3+,Li+ nanopowders were obtained.
2.2.3 Co-precipitation combined solid-state reaction method
2% Li2CO3 was added into the precursors of ZnO: La3+ prepared in section 2.2.1, and ground in agate mortar for 0.5 h, ZnO:La3+,Li+ nanopowders were obtained after calcining in muffle furnace.
2.2.4 Thermal decomposition method
A certain molar fractions of Zn(NO3)2, La(NO3)2, LiNO3 (1:0.01:0.04) were dissolved in 30 mL solution with the liquid composed of water and ethanol by volume ratio of 1:2. Hydrolytic precursors were obtained after hydrolyzing at 105°C for 2 h. ZnO:La3+,Li+ nanopowders were obtained after calcining the precursors in muffle furnace.
2.3 Characterization
The phase structures of the samples prepared in this work were analyzed by X-ray diffraction, using D/Max 2500 diffractometer with a Cu Kα radiation source (l=0.154 06 nm), voltage of 40 kV, current of 250 mA, and a scanning rate of 8.0 (°)/min in 2 θ ranging from 10° to 85°. Morphology was tested by scanning electron microscope, using hitachi X650 with the voltage of 20 kV. The doping amounts of La3+ and Li+ were detected by ICP, using Perkin Elemer 5300DV. The optical properties were tested by F-4500 fluorescence photometric with the following parameters: scanning speed 1 200 nm/min, delay time 0 s, arouse unit slit 5.0 nm, transmission unit slit 5.0 nm, phototubes load voltage 700 V, and response speed 0.01 s.
3 Results and discussion
3.1 XRD analysis
Figure 1 shows the XRD patterns of ZnO: La3+,Li+ samples prepared by different methods. All samples were calcined at 650°C for 3 h. The structures of four samples all belonged to hexagonal wurtzite phase. Reflections due to La (OH)3 were also detected in Fig. 2 (d). During the process of heating and evaporating, Zn2+, La3+ and Li+ were hydrolyzed into Zn(OH)2, La(OH)3 and LiOH, respectively. Decomposing temperature of La(OH)3 is 750°C, but it can reduce when the mixture of Zn(OH)2, La(OH)3 and LiOH are calcined together with LiOH as cosolvent. So some La(OH)3 was decomposed into La3+, and some existed in the form of La(OH)3 when calcinated at 650°C, which is consistent with the result reported by YAO et al [15]. The diffraction peaks of La2O3 were detected in Figs. 1(b) and (c) and no diffraction peaks of impurity phase were detected. The particle size of sample prepared by citric acid-assisted co-precipitation method is the smallest due to its relatively wider half-peak.
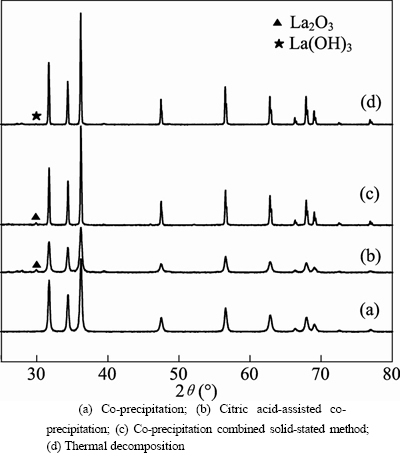
Fig. 1 XRD patterns of ZnO:La3+,Li+ prepared by different methods:
Figure 2 shows the XRD patterns of ZnO: La3+,Li+ prepared by co-precipitation and solid-stated method and calcined at different calcination temperatures for 3 h. All the reflections can be indexed to hexagonal wurtzite phase. Secondary phase of La2O3 appears in the 950 oC sample. The intensity of diffraction peaks increases first and then decreases with the calcination temperature increasing.
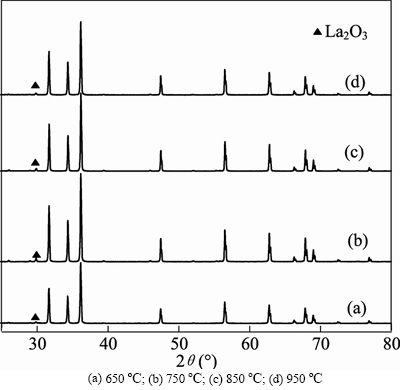
Fig. 2 XRD patterns of samples prepared by co-precipitation and solid-state method at different calcination temperatures:
The intensity of peaks reaches the maximum when calcination temperature is 750°C. The intensities of Figs. 2(c) and (d) are almost the same, which indicates that there is almost no effect on crystal formation when the temperature attains to a certain value, so the calcining temperature was chosen as 750°C.
3.2 SEM analysis
SEM images of ZnO: La3+,Li+ prepared by different methods are shown in Fig. 3. The particle size (about 90 nm) of the sample prepared by citric acid-assisted co-precipitation method is the smallest compared with the other three methods, which is consistent with the analysis result of Fig. 2, but it appears to be agglomerated to a certain degree. The distribution of ZnO:La3+,Li+ particle prepared by thermal decomposition- solid-state method is nonuniform and the diameter (about 600 nm) is comparatively bigger.
3.3 Luminescence analysis
3.3.1 Excitation spectrum
Figure 4 presents the excitation spectra of ZnO: La3+,Li+ prepared by co-precipitation, citric acid-assisted co-precipitation, co-precipitation combined solid-state reaction and thermal decomposition-solid-state processes. As we can see, the sample from the citric acid-assisted co-precipitation method has the highest intensity of excitation wavelength, while the weakest is from thermal decomposition-solid-state process, and the most important of all is the intensity of excitation wave-length of all the samples reach maximum at 326 nm. So excitation wavelength was chosen at 326 nm all through the experiment processes.

Fig. 3 SEM images of samples prepared by different methods:
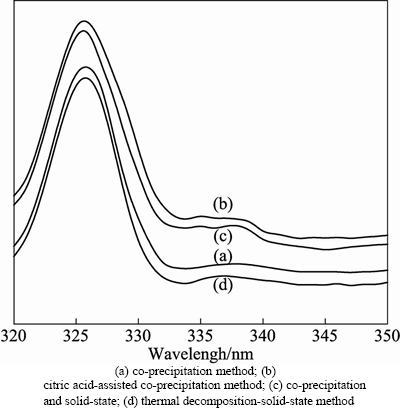
Fig. 4 Room-temperature excitation spectra of ZnO: La3+, Li+ prepared by different methods:
3.3.2 Emission spectra of ZnO:La3+,Li+ prepared by different methods
In order to compare with emission spectra of ZnO: La3+,Li+ prepared by different methods, the samples were all prepared in the following conditions: the calcination temperature was 650°C, calcining time was 3 h, the doping amounts of La3+ and Li+ were 1% and 4%, respectively. The fluorescence (FL) properties of four samples were tested under 326 nm as excitation peak. The results are shown in Fig. 5.
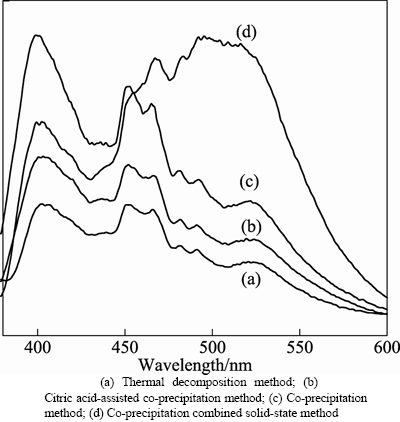
Fig. 5 Luminescence emission spectra of samples derived from different methods:
The results in Fig. 5 show that the emission peak shapes of ZnO: La3+,Li+ prepared by thermal decomposition- solid-state, citric acid-assisted co-precipitation method, co-precipitation method and co-precipitation and solid-state method are almost the same, and all samples mainly have three emission peaks: a violet one centered at around 400 nm, blue around 450 nm and 466 nm, and weak green near 520 nm. While the emission peak shape of the sample prepared by co-precipitation and solid-state method is different from the others. It has three main emission peaks: a violet one centered at around 400 nm, blue one around 466 nm, and weak green near 520 nm.
The mechanism of violet emission peak is from excitons recombination formed by electrons in the conduction band and holes in valence band [16–18], and blue emission peaks near the center of 450 and 466 nm are from the transition between shallow donor level formed by V0 and top Valence band level [19]. The green light emission is related to the concentration of V0 [20].
The existence of La(OH)3 in ZnO:La3+,Li+ prepared by thermal decomposition-solid-state method may be the cause of the weakest intensity of emission peak in visible area. The intensity of emission peak in visible area of ZnO:La3+,Li+ prepared by citric acid-assisted co-precipitation method is weaker than that of the samples prepared by co-precipitation method. The particle size of ZnO:La3+,Li+ prepared by citric acid-assisted co-precipitation method was the smallest compared with the other samples prepared by the other three methods. So the surface area is relatively greater, which leads to the farther distance of transition, that is, the transition probability is correspondingly less and the intensity of emission peak in visible area of the ZnO: La3+,Li+ decreases.
3.3.3 Emission spectra of ZnO:La3+,Li+ samples prepared by co-precipitation and solid-state method under different calcination temperatures
The samples calcined at different temperatures (650°C, 750°C, 850°C, 950°C) were prepared by co-precipitation and solid-state method, considering that the intensity of visible light emission peak was comparatively stronger. The emission spectra of the samples are shown in Fig. 6.
The results in Fig. 6 show that the emission peaks of the samples prepared by different calcination temperatures are almost the same. The intensity of blue light centered at around 466 nm and green light about 500 nm presents the trend of first increasing and then decreasing with the rising temperatures. The sample shows the relatively most intense emission peaks when the calcination temperature is 750°C. Meanwhile, in combination with Fig. 3, the better the crystal morphology, the stronger the emission intensity is presented.
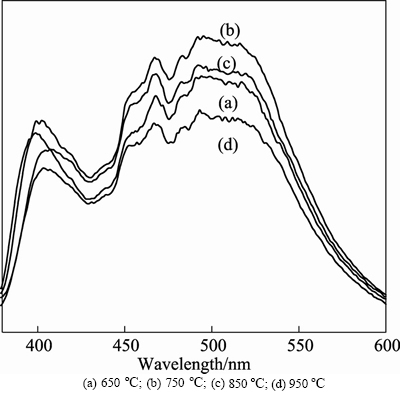
Fig. 6 Photoluminescence emission spectra of samples prepared by co-precipitation and solid-state method with different calcination temperatures:
4 Conclusions
1) The structures of ZnO:La3+,Li+ prepared by co-precipitation method, citric acid-assisted co- precipitation method, co-precipitation and solid-state method and thermal decomposition-solid-state method, all belonged to hexagonal wurtzite phase, and the particle size of the sample prepared by citric acid-assisted co-precipitation method is the smallest in the four methods.
2) All samples mainly have three emission peaks, a violet one centered at around 400 nm, blue around 450 nm and 466 nm, and weak green near 520 nm, except the samples prepared by co-precipitation and solid-state method, which appears a strong and wide green light emission peak locating at about 500 nm, but the blue light emission near 450 nm disappears.
3) The as-prepared ZnO:La3+,Li+ nanoparticles demonstrate the most intensive FL emission intensity at 402.4 nm, 467.2 nm and 494.6 nm,when using the method of co-precipitation and solid-state. The doping amounts of La3+and Li+ are approaching 1% and 4% separately with the heat treatment of these precursors at 750°C for 3 h.
References
[1] LIU Ling-yun, YANG Lin, PU Yun-ti, XIAO Ding-quan, ZHU Jian-guo. Optical properties of water-soluble Co(2+): ZnS semiconductor nanocrystals synthesized by a hydrothermal process [J]. Materials Letters, 2012, 66(1): 121–124.
[2] DEL-CASTILLO J, YANES A C, VELAZQUEZ J J, MENDEZ-RAMOS J, RODRIGUEZ V D. Luminescent properties of Eu3+-Tb3+-doped SiO2-SnO2-based nano-glass-ceramics prepared by sol-gel method [J]. Journal of Alloys and Compounds, 2009, 473(1/2): 571–575.
[3] ZHU Yun-qing, CHEN Yi-qing. Sn-doped polyhedral In2O3 particles: Synthesis, characterization, and origins of luminous emission in wide visible range [J]. Journal of Solid State Chemistry, 2012, 186: 182–188.
[4] HE Rong-liang, Hocking R K, Tsuzuki T. Local structure and photocatalytic property of sol-gel synthesized ZnO doped with transition metal oxides [J]. Journal of Materials Science, 2012, 47(7): 3150–3158.
[5] THAT C T, FOLEY M, PHILLIPS M R, TSUZUKI T, SMITH Z. Correlation between the structural and optical properties of Mn-doped ZnO nanoparticles [J]. Journal of Alloys and Compounds, 2012, 522: 114–117.
[6] SHARMA D, SHARMA S, KAITH B S, RAJPUT J, KAUR M. Synthesis of ZnO nanoparticles using surfactant free in-air and microwave method [J]. Applied Surface Science, 2011, 257(22): 9661–9672.
[7] FANG Xiao-sheng, Yoshio B, Ujjal K G, ZHAI Tian-you, ZENG Hai-bo, XU Xi-jin, LIAO Mei-yong, Dmitri G. ZnO and ZnS nanostructures: Ultraviolet-light emitters, lasers, and sensors [J]. Critical Reviews in Solid State and Materials Sciences, 2009, 34(3/4): 190–223.
[8] KHAN S B, FAISAL M, RAHMAN M M, JAMAL A. Low-temperature growth of ZnO nannoparticles: Photocatalyst and acetone sensor [J]. Talanta, 2011, 85(2): 943–949.
[9] WEN Jun. Investigation of structure of Nd-doped ZnO and La-doped ZnO thin films [J]. Journal of Hebei Normal University Journal: Natural Science Edition, 2009, 33(5): 616–619. (in Chinese)
[10] ZHU Xing-wen, LI Yong-qiang, LU Ye. Optical properties of Li:ZnO thin films with [101] orientation [J]. Journal of Inorganic Materials, 2007, 22(2): 359–362.
[11] FAN Xue-yun, WANG Yan-xiang, ZHANG Yi-lai, ZENG Ling-ke. Study on preparation and optical properties of rare earth doped nano-sized zinc oxide power [J]. Journal of Synthetic Crystals, 2008, 37(5): 1166–1172. (in Chinese)
[12] NIU Xin-shu, CHEN Xiao-li, RU Xiang-li, WANG Xue-li, WEI Ping-tao. preparation of La3+-doped ZnO nanoparticles and its photocatalytic properties [J]. Chemical Industry Environmental Protection, 2009, 29(1): 67–70. (in Chinese)
[13] WEN Jun, CHEN Chang-le. Investigation of structure and luminescence properties of RE-doped ZnO thin films grown by RF magnetron sputtering [J]. Opto-electronic Engineering, 2008, 35(8): 124–127. (in Chinese)
[14] KANG Ming, LU Zhong-yuan, YIN Guang-fu, SUN Rong, TANG Jin. The study on the preparation of the ZnO-based red luminescence material [J]. Materials Review, 2006, 20(12): 129–131. (in Chinese)
[15] YAO Cao, MA Jiang-quan, LIN Xi-ping, WANG Xin. The preparation of nanosized lanthanum oxide [J]. Journal of Chemical Engineering of Chinese Universities, 2003, 17(6): 685–688. (in Chinese)
[16] HSIEH P T, CHEN Y C, KAO K S, LEE M S, CHENG C C. The ultraviolet emissionmechanism of ZnO thin film fabricated by sol-gel technology [J]. Journal of the European Ceramic Society, 2007, 27(13/14/15): 3815–3818.
[17] KURBANO S S, PANIN G N, KIN T W, KANG T W. Strong violet luminescence from ZnO nanocrystals grown by the lowtemperature chemical solution deposition [J]. Journal of Luminescence, 2009, 129(9): 1099–1104.
[18] Lü Jian-guo, LIU Chang-long, GONG Wan-bing, ZI Zhen-fa, CHEN Xiao-shuang, HUANG Kai, WANG Tao, HE Gang, SONG Xue-ping, SUN Zhao-qi. Effect of solution concentration on crystal structure, surface topographies and photoluminescence properties of ZnO thin films [J]. Superlattices and Microstructures, 2012, 51(6): 886–892.
[19] LIANG Zhi-wen, YU Xiang, LEI Bing-fu, LIU Peng-yi, MAI Wen-jie. Novel blue-violet photoluminescence from sputtered ZnO thin films [J]. Journal of Alloys and Compounds, 2011, 509(17): 5437–5440.
[20] HSIEH P T, CHIN H S, CHANG P K, WANG C M, CHEN Y C, HOUNG M P. Effects of the annealing environment on green luminescence of ZnO thin films [J]. Physica B: Condensed Matter, 2010, 405(11): 2526–2529.
(Edited by HE Yun-bin)
Foundation item: Project(50972166) supported by the national natural science Foundation of China
Received date: 2011–12–18; Accepted date: 2012–04–12
Corresponding author: GU Ying-ying; professor; Tel: +86–13467517387; E-mail: guyy02@163.com
Abstract: ZnO:La3+,Li+ nanoparticles were successfully prepared by co-precipitation, citric acid-assisted co-precipitation, co-precipitation combined solid-state reaction and thermal decomposition method. X-ray diffraction (XRD), scanning electron microscopy (SEM) and luminescence spectrophotometry were employed to characterize the crystal phases, particle sizes and luminescence properties of the as-prepared nanopowders. The results indicate that all the prepared samples crystallize in a hexagonal wurtzite structure. The ZnO:La3+,Li+ prepared by citric acid-assisted co-precipitation method has a particle size of about 80 nm, which is the smallest among all the samples. Fluorescence (FL) spectra of all samples exhibit three typical emissions: a violet one centered at around 400 nm, blue around 450 nm and 466 nm, and weak green near 520 nm. But the samples prepared by co-precipitation method show a strong and wide green light emission located at about 500 nm. The ZnO:La3+,Li+ nanoparticles synthesized by the co-precipitation method demonstrate relatively the strongest emission intensity.
- precipitation processes and luminescence properties of ZnO: La3+, Li+ nanoparticles


