Trans. Nonferrous Met. Soc. China 24(2014) 449-454
Effects of slurry pack cementation temperature on microstructure and wear resistance of Ti-Al co-deposited coating on copper plated nickel layer
Hong-xing WANG, Yan ZHANG, Shao-feng YANG, Bing-yi LIU
School of Materials Engineering, Nanjing Institute of Technology, Nanjing 211167, China
Received 2 November 2012; accepted 20 December 2013
Abstract:
In order to improve the wear resistance properties of copper substrate, a layer of electroplated nickel was firstly deposited on copper substrate, subsequently these electroplated specimens were treated by slurry pack cementation process with a slurry pack cementation mixture composed of TiO2 as titanizing source, pure Al powder as aluminzing source and also a reducer for titanizing, an activator of NH4Cl and albumen (egg white) as cohesive agent. The Ti-Al coating was fabricated on the surface of electro-deposited nickel layer on copper matrix followed by the slurry pack cementation process. The effects of slurry pack cementation temperature on the microstructures and wear resistance of Ti-Al coating were studied. The results show that the microstructure of the coating changed from NiAl+Ni3(Ti,Al) to NiAl +Ni3(Ti,Al)+Ni4Ti3 to Ni4Ti3+NiAl, and to NiAl+Ni3(Ti,Al)+NiTi with slurry pack cementation temperature ranging from 800 °C to 950 °C in 12 h. The friction coefficient of Ti-Al coating decreased and the hardness increased with increasing the slurry pack cementation temperature. The minimum friction coefficient was 1/3 and the minimum hardness was 5 times larger than that of pure copper.
Key words:
Ti-Al coating; Ni-Ti intermetallic compound; Ni-Al intermetallic compound; slurry pack cementation temperature; wear resistance;
1 Introduction
Copper is widely used because of its superior electrical and thermal conductivity. Moreover, the characteristics of copper make this metal suitable for a great variety of metallurgical applications, such as continuous casting moulds, oxygen nozzles of steelmaking converters and slag hole in blast furnaces [1,2]. Due to its low hardness and poor wear resistance, it cannot be applied to areas needing excellent wear resistance or good resistance oxidation [3].
In order to enhance wear resistance and oxidation resistance of pure copper, it is often surface treated and alloyed. Surface treatment, as opposed to bulk alloying, provides an opportunity to improve the properties of pure copper while leaving the bulk characteristics relatively unchanged. Hence, a number of techniques have been studied in the past decade to develop an efficient process to deposit coatings on copper or modify the chemical composition of its surface [4,5]. The diffusion coating processes have been used widely to deposit high-temperature oxidation and wear resistance coatings, such as aluminizing [6,7], titanizing [8,9] and siliconizing [10-16]. However, few studies have been performed on employing the pack cementation process to fabricate the Ti-Al layer on the surface of copper. In comparison with other deposition techniques of coating on metal substrates, the pack cementation process has the following distinctive advantages: 1) high volume and economical deposition of diffusion coating with easily controllable thickness; 2) simultaneous deposition of multiple elements such as Al, Si, Cr and Ti; 3) excellent adhesion between the coating and the substrate; 4) being applicable for a wide range of shapes and size and not subjected to line-of-sight restrictions; 5) low environmental impact and low capital investment and low operation cost.
In this work, the intermetallic Ni-Ti(Al) compounds were prepared by the slurry pack cementation process, and the effects of the slurry pack cementation temperature on the microstructure and wear resistance property of the coatings were discussed.
2 Experimental
The plating electrolyte was a nickel sulfamate bath of which the composition and the range of experimental process parameters are shown in Table 1. The commercial nickel plate of 120 mm × 80 mm × 10 mm was used as the anode, and a copper plate of 12 mm × 10 mm × 2 mm was used as the cathode. The electrolyte temperature was maintained at room temperature. Cathode surface was ground with a series of Al2O3 papers up to 800-grit, and ultrasonically cleaned in water bath and then dipped in a water solution of 5% H2SO4 for 3 min followed by cleaning in water and drying in air.
Table 1 Compositions of electrolyte and plating condition

After electroplating, copper coated with nickel plating was rinsed with water and then cleaned ultrasonically in acetone. In the previous studies [10], the slurry pack cementation powder mixtures mainly consisted of 32% TiO2 as titanizing source, 32% pure Al powder as aluminzing source and as a reducer for titanizing, 8% NH4Cl as an activator, albumen (egg white) as cohesive agent, and 28% Al2O3 powder as an anti-sintering reagent. Al2O3 powder is inert and does not take part in the process reactions. It is used to balance heat transfer and temperature distribution. After weighing and mixing, the slurry pack powder mixture was thoroughly ground using an alumina mortar and pestle and then mixed with the cohesive agent albumen into slurry. The slurry was thoroughly spread on the nickel coating by hand and then dried in vacuum at 80 °C for 1 h. The samples in an alumina crucible, filled with alumina powder, were placed into the vacuum firing furnace which was purged three times with argon and then filled with argon at 1.25 MPa to avoid oxidation. The furnace temperature was raised to a final depositing temperature of 800, 850, 900 and 950 °C at a rate of 10 °C/min and the holding time was 12 h. The furnace was then cooled to room temperature at its natural rate by switching off its power supply while maintaining the argon pressure. After slurry process, the treated surface was cleaned to remove adhering powders.
The microstructure was characterized with a Sirion field-emission scanning electron microscope SEM. Phase was identified by an XD-3A X-ray diffractometer with Cu Kα radiation, λ=1.5804 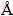 and scan speed of 5 (°)/min. Hardness of the coating was measured using HV-1000 Vickers micro-hardness tester at a constant load of 0.98 N(100 g) and dwelling time of 10 s.
and scan speed of 5 (°)/min. Hardness of the coating was measured using HV-1000 Vickers micro-hardness tester at a constant load of 0.98 N(100 g) and dwelling time of 10 s.
A WTM-2E type ball-on-disk wear testing machine was employed to evaluate wear resistance of copper substrate and the coatings. Wear tests were conducted at room temperature, and a Si3N4 ball (HV300 1590) was employed as the counterface. Wear test was carried out under a load of 7.5 N and a velocity of 0.2 m/s. Tribological behaviors of copper substrate and the coatings were studied through measurement of coefficient of friction versus sliding time.
3 Results and discussion
3.1 Effect of slurry pack cementation temperature on morphology of coatings
Morphologies of the coatings made by the slurry pack cementation process with different pack temperatures for 12 h are shown in Fig. 1. It can be seen that the coating is dense with no macro-voids or cracks after slurry pack cementation at 800 °C, as shown in Fig. 1(a); on the surface of coating, a lot of pinholes and pits appear after slurry pack cementation reaches 850 °C, as shown in Fig. 1(b); when the slurry pack cementation reaches 900 °C, the surface of coating is coarse with pinholes formed at low temperature, as shown in Fig. 1(c); when the slurry pack temperature further reaches 950 °C, the size of the holes of coating becomes more big and surface of coating is uneven. The reason is that gaseous halides decomposed from the activator have reacted with the surface of nickel layer, and then the gaseous nickel chlorides have formed. Due to the concentration gradient, the gaseous nickel chlorides diffused into the pack cementation system, nickel atoms reacted with the gaseous halides leaving the vacancies on the surface of nickel layer and forming micromoles. Micro-holes obtain more energy and grow greatly with the increase of the slurry pack cementation temperature.
3.2 Effect of pack cementation temperature on structure of coatings
The results of X-ray diffraction of the coatings prepared at different slurry pack cementation temperatures for 12 h are shown in Fig. 2. As can be seen from Fig. 2, the phase structure of the coatings is mainly composed of the mixture of NiAl and Ni-Ti intermetallic compounds, and the content of Ti atoms in the Ni-Ti intermetallic compounds increases with increasing the slurry pack cementation temperature. It is also revealed that the diffraction peaks of Ni appear with the slurry pack temperature ranging from 800 °C to 850 °C.

Fig. 1 SEM images of coating prepared at different packing temperatures
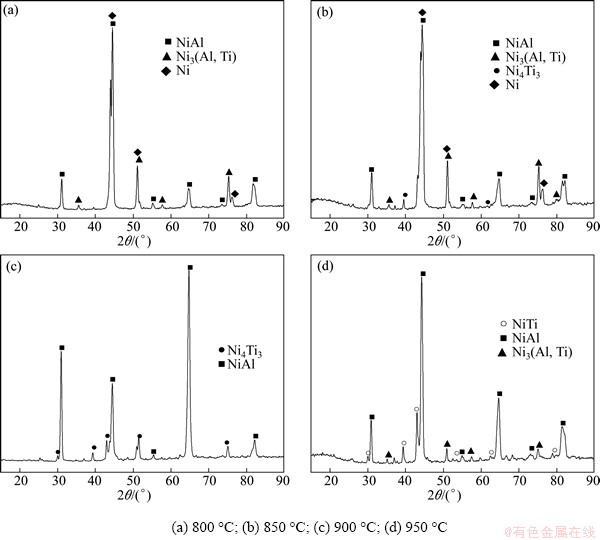
Fig. 2 XRD patterns of surface of coating after slurry pack cementation at different packing temperature
Perhaps the reason is that the thickness of coating is too thin and the X-ray penetrates into the coatings.
The pack cementation process can be divided into four interrelated steps [17]: 1) a thermodynamic equilibrium state between the activator and the master alloy, which determines the vapor pressure of the active gaseous species in the pack; 2) the gaseous diffusion of the metal halides from the pack to the substrate surface, driven by the chemical potential gradients in the gas phase; 3) surface reactions at the substrate to deposit the coating elements and form the vapor product; and 4) solid-state diffusion of the coating elements into the substrate.
In the slurry pack cementation process, NH4Cl firstly dissociates into NH3 and HCl at temperature higher than 340 °C, as shown in reaction (1):
NH4Cl(s)=NH3(g)+HCl(g) (1)
With the increase of the slurry pack cementation temperature, Al2O3+Ti system is more thermo- dynamically stable than TiO2-Al system, and the element Al begins to reduce TiO2 into Ti via the following conversion reaction:
3TiO2+4Al=3Ti+2Al2O3 (2)
The element Al in the mixture powders performs two roles: the one is that the element Al reacts with HCl to form the gaseous aluminum chlorides (AlClx, x=1-3) [18], and then the active Al atoms diffuse into the nickel plating before depositing Ti atoms; the other is that the element Al is used as reducer, reducing TiO2 to Ti. At the slurry pack cementation temperature of 800 °C, the content of Ti atoms reduced by reaction (2) is lower and consequently the content of gaseous titanium chlorides is also lower, the fraction of Al atoms in Ni3Al intermetallic was replaced by Ti atoms, and then intermetallic compound Ni3(Ti,Al) was formed, as shown in Fig. 2(a). With the increase of temperature, the content of Ti atoms previously reduced by reaction (2) got increase and as a result, the content of gaseous titanium chlorides also increased. This is favor of the thermodynamics for Ti atoms depositing and diffusion. When the slurry pack cementation temperature reaches 850 °C, the content of Ti atoms reduced by reaction (2) increased, the content of gaseous titanium chlorides also increased, which favors for the thermodynamics of Ti elements depositing and diffusion. As the concentration of Ti increased, in surface layer, the intermetallic Ni4Ti3 phase is formed, as shown in Fig. 2(b). When the slurry pack temperature further reaches 950 °C, subsequently further supply of Ti reduced by Al from the pack medium will result in the formation of NiTi phase, as shown in Fig. 2(d).
3.3 Effect of pack cementation temperature on wear resistance of coatings
Figure 3 shows the hardness of coatings and the friction coefficient curves versus sliding time, respectively. Figure 3(a) illustrates that the hardness of coatings increases with increasing the packing temperature. The minimum hardness of coating made at 800 °C is 5 times larger than that of copper substrate (HV 70). This is attributed to formation of the Ni-Ti(Al) intermetallic coating during the slurry pack cementation process.

Fig. 3 Effect of packing temperature on microhardness (a) and friction coefficient (b) of Ti-Al coating
Figure 3(b) shows the friction coefficient of coatings and copper matrix under a load of 7.5 N for 0.5 h. It can be seen that the coefficient of the coating decreases with increasing the slurry pack cementation temperature. The coating packed at 800 °C shows a relatively constant value of 0.50; the friction coefficient of coating decreases from 0.42 to 0.30; when the pack cementation temperature increases from 850 °C to 950 °C, the maximum value is still significantly lower than that of copper matrix, around 0.83. The lower coefficient of friction in coatings can be explained by the formation of Ni-Ti(Al) intermetallic compound coatings having a higher hardness and a lower friction inherent characteristic.
Figure 4 shows the worn surface of coatings made at different slurry pack cementation temperatures. It can be seen that the worn surface of coatings is smooth and no cracks or pits are observed when the pack temperature ranges from 800 °C to 900 °C; the worn surface is coarse with a lot of micro-holes at 950 °C, which is agreement with the result of surface morphology analysis (Fig. 1(d)).

Fig. 4 SEM images showing worn surface on Ti-Al coatings prepared at different slurry pack temperatures
4 Conclusions
1) Ti-Al intermetallic compound coating is formed on the surface of copper plated nickel layer and followed by the slurry pack cementation process, and the coating becomes loose with increasing the slurry pack temperature.
2) When the slurry pack temperature increases from 800 °C to 950 °C, the structure of the coatings changes from NiAl+Ni3(Ti,Al) to NiAl+Ni3(Ti,Al)+Ni4Ti3 to Ni4Ti3+NiAl to NiAl +Ni3(Ti,Al)+NiTi, and the NiAl intermetallic compound is the main phase.
3) The friction coefficient of the coating decreases from 0.50 to 0.30 with increasing the slurry pack cementation temperature from 800 to 950 °C. The minimum friction coefficient of coating is 1/3 and the minimum hardness is 5 times larger than that of pure copper.
References
[1] ZHANG Yong-zhong, TU Yi, XI Ming-zhe, SHI Li-kai. Characterization on laser clad nickel based alloy coating on pure copper [J]. Surface and Coatings Technology, 2008, 202(24): 5924-5928.
[2] YAN Hua, WANG Ai-hua, XU Kai-dong, WANG Wen-yan, HUANG Zao-wen. Microstructure and interfacial evaluation of Co-based alloy coating on copper by pulsed Nd: YAG multilayer laser cladding [J]. Journal of Alloys Compounds, 2010, 505(2): 645-653.
[3] XIANG J H, GESMUNDO F, NIU Y. The oxidation of two Ni-Cu-10at% Al alloy in 1 atm of pure O2 at 800-900 °C [J]. Corrosion Science, 2004, 46(8): 2025-2039.
[4] SONG Wen-ming, YANG Gui-rong, LU Jin-jun, HAO Yuan, MA Ying. Microstructure and wear behaviour of Ni-based surface coating on copper substrate [J]. Wear, 2007, 262(7-8): 868-875.
[5] BAHROLOLOOM M E, SANI R. The influence of pulse plating parameters on the hardness and wear resistance of nickel–alumina composite coatings [J]. Surface and Coatings Technology, 2005, 192(2-3): 154-163.
[6] WANG Hong-xing, SHENG Xiao-bo, CHU Cheng-lin, LIN Ping-hua. DONG Ying-sheng. Study on high temperature oxidation behavior and microstructure of Ni2Al3 coating on copper [J]. Transactions of Materials and Heat Treatment, 2008, 29(2): 166-170. (in Chinese)
[7] RAFIEE H, ARABI H, RASTEGARI S. Microstructure and interfacial evaluation of Co-based alloy coating on copper by pulsed Nd:YAG multilayer laser cladding [J]. Journal of Alloys and Compounds, 2010, 505(2): 206-212.
[8] GUO Chun, ZHUO Jian-song, YU You-jun, WANG Ling-qian, ZHUO Hui-di, CHEN Jian-min. Microstructure and tribological properties of Ti-Cu intermetallic compound coating [J]. Materials and Design, 2012, 36: 482-489.
[9] WANG Hong-xing, CHU Cheng-lin, SHENG Xiao-bo, LIN Ping-hua, DONG Ying-sheng. Effect Al content on microstructure and properties of an intermetallic Ni-Ti(Al)/Ni graded coating deposited on copper substrate [J]. International Journal of Modern Physics B, 2009, 23(6-7): 1916-1923.
[10] KUBATIK T, JAGLOVA M, KALABISOVA E, CILHAL V. Improvement of oxidation resistance of TiAl6V4 alloy by siliconizing from liquid phase using melts with high silicon content [J]. Journal of Alloys Compounds, 2011, 509(18): 5493-5499.
[11] WANG Hong-xing, CHU Cheng-lin, SHENG Xiao-bo, LIN Ping-hua, DONG Ying-sheng. Siliconizing formation mechanism and its property by slurry pack cementation on electro-depositied nickel layer into copper matrix [J]. Journal of Wuhan University of Technology: Materials Science Edition, 2009, 24(6): 883-887.
[12] TAO Shun-yan, YIN Zhi-jian, ZHUO Xia-ming, DING Wen-jiang. Sliding wear characteristics of plasma-sprayed Al2O3 and Cr2O3 coatings against copper alloy under severe conditions [J]. Tribology International, 2010, 43(1-2): 69-75.
[13] ZHANG Yong-zhong, TU Yi, XI Ming-zhe, SHI Li-kai. Characterization on laser clad nickel based alloy coating on pure copper [J]. Surface and Coatings Technology, 2008, 202(1-2): 5924-5928.
[14] LI Ming-yu, CHAO Ming-ju, LIANG Er-jun, YU Ju-mei, ZHANG Jun-ji, LI De-chuan. Improving wear resistance of pure copper by laser surface modification [J]. Applied Surface Science, 2011, 258(4): 1599-1604.
[15] MANGAM V, BHATTACHARA S, DAS K, DAS S. Friction and wear behavior of Cu-CeO2 nanocomposite coatings synthesized by pulsed electrodeposition [J]. Surface and Coatings Technology, 2010, 205(3): 801-805.
[16] HUA Yan, ZHANG Pei-lei, YU Zhi-shui, LI Chong-gui, LI Rui-di. Development and characterization of laser surface cladding (Ti,W)C reinforced Ni–30Cu alloy composite coating on copper [J]. Optics & Laser Technology, 2012, 44(5): 1351-1358.
[17] WANG Hong-xing, SHENG Xiao-bo, CHU Cheng-lin, LIN Ping-hua, DONG Ying-sheng. Aluminizing microstructure and its formation mechanism on electro-deposited nickel layer on copper matrix [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(10): 1616-1621. (in Chinese)
[18] ZHAN Zhao-lin, HE Ye-dong, WANG De-ren, GAO Wei. Low-temperature processing of Fe-Al intermetallic coatings assisted by ball milling [J]. Intermetallics, 2006, 14(1): 75-81.
包渗温度对Ti-Al渗层组织和耐磨性能的影响
王红星,张 炎,杨少锋,柳秉毅
南京工程学院 材料工程学院,南京 211167
摘 要:为了提高纯铜表面的耐磨性能,采用电镀/浆料包渗相结合的方法,以TiO2粉为渗 Ti 源,纯 Al 粉为还原剂,在 Cu 表面预镀 Ni 随后表面浆料包渗Ti-Al,制备Ti-Al 共渗层。研究了包渗温度对 Ti-Al渗层组织和耐磨性能的影响。采用SEM和XRD分析了渗层表面形貌和结构。结果表明:在800~950 °C共渗12 h时,随着温度的升高,渗层组织变化过程为NiAl+Ni3(Ti,Al)→NiAl+Ni3(Ti,Al)+Ni4Ti3→Ni4Ti3+NiAl→NiAl+Ni3(Ti,Al)+ NiTi;Ti-Al 渗层的摩擦因数随着包渗温度的升高而降低,最小摩擦因数约为纯铜的1/3,最小硬度为纯铜的5倍。
关键词:Ti-Al 共渗层;Ni-Ti 金属间化合物;Ni-Al 金属间化合物;料浆包渗温度;耐磨性能
(Edited by Hua YANG)
Foundation item: Projects (YKJ201203, CKJB201205) supported by the Nanjing Institute of Technology, China
Corresponding author: Hong-xing WANG; Tel: +86-25-86118287; E-mail: wangzhao2000922@163.com
DOI: 10.1016/S1003-6326(14)63081-8
Abstract: In order to improve the wear resistance properties of copper substrate, a layer of electroplated nickel was firstly deposited on copper substrate, subsequently these electroplated specimens were treated by slurry pack cementation process with a slurry pack cementation mixture composed of TiO2 as titanizing source, pure Al powder as aluminzing source and also a reducer for titanizing, an activator of NH4Cl and albumen (egg white) as cohesive agent. The Ti-Al coating was fabricated on the surface of electro-deposited nickel layer on copper matrix followed by the slurry pack cementation process. The effects of slurry pack cementation temperature on the microstructures and wear resistance of Ti-Al coating were studied. The results show that the microstructure of the coating changed from NiAl+Ni3(Ti,Al) to NiAl +Ni3(Ti,Al)+Ni4Ti3 to Ni4Ti3+NiAl, and to NiAl+Ni3(Ti,Al)+NiTi with slurry pack cementation temperature ranging from 800 °C to 950 °C in 12 h. The friction coefficient of Ti-Al coating decreased and the hardness increased with increasing the slurry pack cementation temperature. The minimum friction coefficient was 1/3 and the minimum hardness was 5 times larger than that of pure copper.


