Trans. Nonferrous Met. Soc. China 23(2013) 2123-2128
Preparation and oxidation resistance of B2O3-coated boron-modified carbon foams
Bin WANG, He-jun LI, Yu-lei ZHANG, Qian WANG
C/C Composites Technology Research Center, state Key Laboratory of solidification processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 30 January 2013; accepted 6 May 2013
Abstract:
To improve the oxidation resistance of boron-modified carbon foams, the B2O3 coating was prepared on boron-modified carbon foams by low-cost slurry method. The microstructures and phase compositions of the coated carbon foams were characterized by scanning electron microscopy and X-ray diffraction, respectively. Oxidation resistances of uncoated and coated boron-modified carbon foams were investigated at 873 K in air. The results showed that as-received B2O3 coating could protect boron-modified carbon foams from oxidation at 873 K. B2O3-coated carbon foam doped with 7% B2O3 (mass fraction) (BO-7) had better oxidation resistance, exhibiting mass loss of 17.40% after oxidation at 873 K for 120 min. The melting glass layer formed on the surface of BO-7 could prevent oxygen from diffusing into boron-modified carbon foams substrate during oxidation to some extent.
Key words:
carbon foam; B2O3; coating; oxidation resistance;
1 Introduction
Carbon foams as new promising materials, have caught great attention due to their low density, potential high strength and impact absorption [1-3]. They are conventionally made from coal, pitch or resin. Resin-derived carbon foams are suitable for high temperature thermal insulation materials for their low thermal conductivity [4,5]. More recently, many detailed research on the mechanical and thermal properties of resin-derived carbon foams have been reported. Several additives such as aluminosilicate [4], K2Ti6O13 whiskers [6], and hollow ceramic microspheres [7] have been introduced into carbon foams for better performance.
However, rapid oxidation of carbon-matrix materials above 773 K limits their wide applications in oxidizing atmosphere [8]. High temperature oxidation resistance is very important to carbon foams for practical use in the field of thermal insulation. Few reports on the high temperature oxidation resistance of carbon foams were published. The oxidation resistance of carbon foams doped with aluminosilicate at 673 K was investigated [4], which developed the lowest mass loss of 49.06% with aluminosilicate of 11% in mass fraction. The influence of boron content on the oxidation resistance of carbon foam was analyzed by TGA [9]. Boron-based compound could protect carbon foam from oxidation to some extent. But it may apply a fuzzy measurement to assess oxidation behavior. The specimen with the lowest mass loss presents “best oxidation resistance” based on TGA results, while appreciable macroscopic cracks, even fracture may occur under practical oxidation environment.
Coating of carbon materials is an effective route to prevent them from oxidation at high temperature [10-12]. Several coating methods, such as pack cementation [13], plasma spray [14], and slurry [10] have been developed to coat carbon material surfaces. Among these methods, slurry is considered a low-cost and short-cycle technology. The application of B2O3 glass has been extensively studied [15,16]. B2O3 can heal microcracks in carbon-matrix materials below 1273 K for higher fluidity. In the present work, B2O3 coating was prepared on resin-derived carbon foams doped with boron by slurry method, which was attempted to protect carbon foams from oxidation at 873 K. The microstructure and oxidation resistance of the coated carbon foams were investigated. The adhesive strengths of the coatings were evaluated by scratch tests. These results will be helpful for promoting the profound research on the high temperature resistance of carbon foams.
2 Experimental
2.1 Raw material
Phenolic resin was fabricated using phenol (AR grade), formaldehyde (37% aqueous solution in mass fraction) and sodium hydroxide, all supplied by Beijing Chemical Reagents Company.
Hollow phenolic microspheres (from Asian Pacific Company), H3BO3 and B2O3 (Beijing Chemical Reagents Company) were selected as additives. Ethanol was provided by Beijing Chemical Reagents Company.
2.2 Processing
Phenolic resin was synthesized by mixing proper amounts of phenol and formaldehyde homogenously in 358 K water bath, taking some sodium hydroxide as catalyst. The phenolic foams (also called the precursor of carbon foams) were produced by ultrasonic dispersion of hollow microspheres and boron additives in the resultant phenolic resin, then pre-cured at room temperature for 24 h, and subsequently cured at 398 K for another period of 24 h. The volume ratio of hollow microspheres to phenolic resin was 7:3. The boron additive contents are listed in Table 1.
Table 1 Content of boron additives
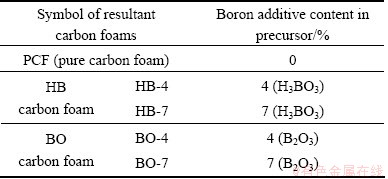
After curing, the samples were heated to 1123 K for carbonization, then cooled to room temperature slowly. The heating/cooling rate was maintained at 1 K/min, and the whole treatment process was under an argon atmosphere. Boron-modified carbon foams were obtained finally.
The slurry was synthesized by mixing B2O3 and ethanol with the solid-to-liquid mass ratio of 2:1 for 1 h, to ensure that the brush of the slurry onto the specimens was easy and uniform. After applying the slurry to the surface of boron-modified carbon foams, the samples were dried in a drier at 353 K for a sufficient time. The coating thickness was controlled artificially by the brush times. Then the samples endured a heat-treatment at 973 K for 60 min under a slight argon flow.
2.3 Characterization
The WS-2005 scratch tester (made by Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences) was employed to evaluate adhesive strength of the coatings. A diamond tip with a radius of 0.2 mm was used to slide at a scratch speed of 2 mm/min between the coatings and substrates.
The morphology of carbon foams matrix and coatings was analyzed by scanning electron microscopy (SEM, JSM-6460). The crystalline structures of carbon foam and coating were measured by X'pert Philips X-ray diffraction (XRD), with a Cu Ka radiation source (λ=0.15406 nm) at 40 kV and 35 mA. The diffraction angle 2θ was between 15° and 90° with a step width of 0.033°.
The as-coated and uncoated carbon foams were placed upon a corundum support and experimented at 873 K in air in an electrical furnace to testify the isothermal oxidation resistance. Mass of the samples was measured by an electronic balance with a sensitivity of ± 0.1 mg.
Specimens were machined to blocks of d12 mm×10 mm to testify the shrinkage by a vernier caliper with a sensitivity of ± 0.02 mm.
3 Results and discussion
3.1 Microstructure of boron-modified carbon foams
Figure 1 shows the SEM images of the boron- modified carbon foam (BO-7) and unmodified one (PCF). There seems to be no clear difference between them. They all consist of hollow carbon microspheres, resin carbon matrices and pores. The hollow carbon microspheres (40-100 μm) are uniformly distributed in the resin carbon matrices. The carbon matrices form a good interconnected network in the composites. The pores are mainly located at three positions of carbon foams: between the carbon microspheres, inside the carbon microspheres or on the resin carbon matrices; they have a size ranging from submicron to tens of micrometers. Comparing the pure carbon foam (PCF), the boron-modified carbon foam is denser due to the diffusing of boron into the holes in the composites.
Figure 2 exhibits the XRD pattern of BO-7. There are two broad peaks at 2θ of near 25° and 44°, which are attributed to planes (002) and (101) of amorphous carbon respectively [17]; the peaks of B2O3 are sharp. It indicates that the carbon foam produced in this work is amorphous, and B2O3 in the carbon foam has good crystallinity.
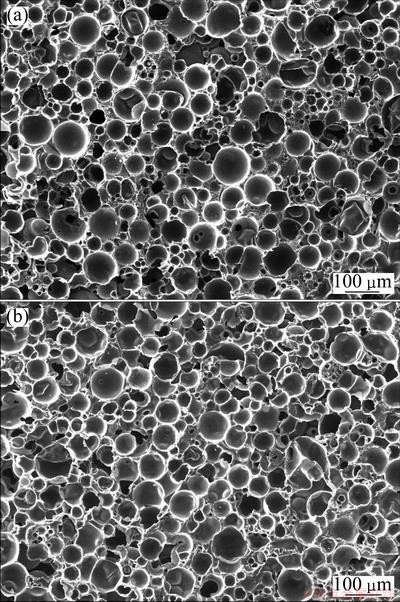
Fig. 1 SEM images of PCF (a) and BO-7 (b)
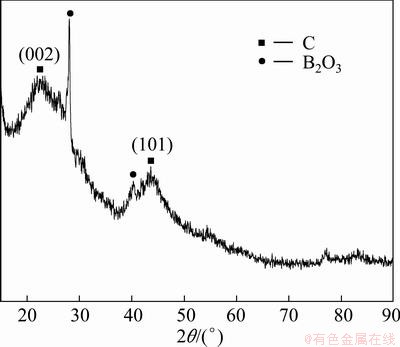
Fig. 2 XRD pattern of specimen BO-7
Figure 3 shows the shrinkag of boron-modified carbon foams during carbonization, ranging from 15.5% to 18.5%. For the sample with the same boron content, the shrinkage along axial (φh) and radial (φd) directions is almost similar. It could be inferred that the boron- modified phenolic foams had homogeneous chemical reactions during heat treatment, and contracted uniformly. Carbonization was the process of the densification of carbon foams, during which large molecules were cracked into smaller molecules, then escaped [7].
3.2 Microstructure of B2O3-coated boron-modified carbon foams
The surface and cross-section images of the coated
specimen BO-7 are exhibited in Fig. 4. Figure 4(a) shows a representative SEM image showing the morphology of coating on the surface of boron-modified carbon foams. It can be seen that a continuous and smooth layer without big hole formed on BO-7. The surface coverage on the BO-7 substrate is complete. The formation of small holes resulted from the volatilization of ethanol in slurry during heat treatment. The coating is about 45 μm in thickness, as shown in Fig. 4(b). No gaps between the boron-modified carbon foam and coating are found, which indicates good interaction between them.
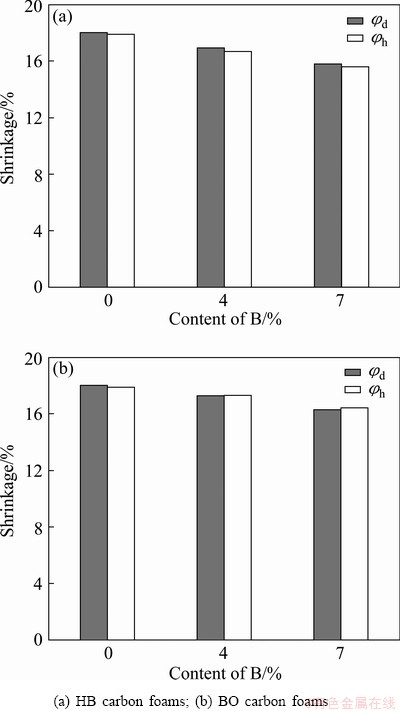
Fig. 3 Shrinkage of boron-modified carbon foams with different boron content
Figure 5 shows the XRD pattern of the coating prepared on the surface of BO-7 substrate, from which the sharp diffraction peaks of B2O3 are detected. It can be inferred that the coating consists of B2O3, showing the good crystallinity.
The adhesive strength between the B2O3 coating and BO-7 substrate is shown in Fig. 6, which is characterized by the critical load value, corresponding to the position of the first peak. The scratch test result revealed that the critical load value of the coating is 13.5 N.
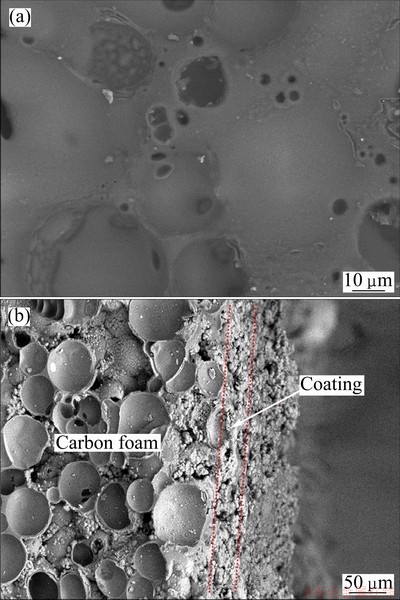
Fig. 4 Surface (a) and cross-section (b) SEM images of coated specimen BO-7
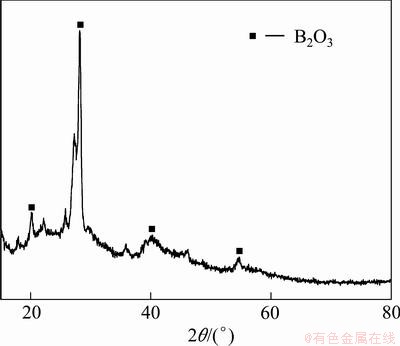
Fig. 5 XRD pattern of coated specimen BO-7

Fig. 6 Critical load value of coating on specimen BO-7
3.3 Oxidation resistance properties of uncoated and B2O3-coated boron-modified carbon foams
Figure 7 shows macrographs of PCF, uncoated BO-7 and coated BO-7 specimens after isothermal oxidation at 873 K in air for 120 min. PCF has been exhausted by exposure to air, so it is absent in Fig. 7. The uncoated BO-7 collapses completely, former porous skeleton being debris, which suggests its poor oxidation resistance; while the coated BO-7 maintains relatively integrity structure in the same oxidation environment. It can be inferred that only doping boron proves limit oxidation protection for the carbon foam. The B2O3 coating can provide good oxidation protection for the boron-modified carbon foams at 873 K in air.

Fig. 7 Macrographs of uncoated and coated BO-7 specimens after oxidation at 873 K in air for 120 min
The results of isothermal oxidation test at 873 K in air are shown in Fig. 8. All the specimens exhibit quick mass loss at the initial stage (below 60 min) due to the quick oxidation of carbon foam substrates. After oxidation for 60 min, the mass loss rates decrease. The coated PCF exhibits the worst oxidation resistance among all the specimens. Its mass loss reaches 54.29% after oxidation for 30 min. After oxidation for 120 min, the mass loss is 71.43%. The coated BO-7 specimen has the best oxidation resistance due to the high B2O3 content in the specimen; the mass loss rate is only 17.40% after oxidation for 120 min.
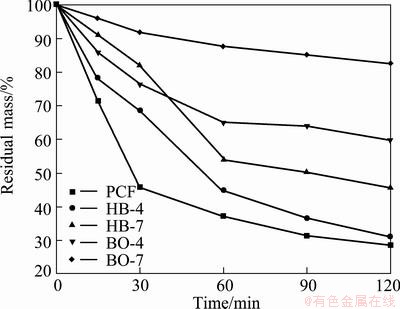
Fig. 8 Isothermal oxidation curves of coated boron-modified carbon foams at 873 K in air
Comparing the coated BO-4 specimen with the coated BO-7, the oxidation resistance increases with the increasing content of boron additive under the same oxidation condition. The similar result goes for the coated HB-4 and coated HB-7.
The oxidation resistance of the coated BO carbon foams is better than that of the coated HB carbon foams for the same boron content in precursor. The following two dehydration reactions during carbonization are assumed to be responsible for this [18]:
H3BO3→HBO2+H2O (1)
2HBO2→B2O3 +H2O (2)
H3BO3 in precursor decomposes to less amount of B2O3 after carbonization. Thus, for same boron content in precursor, HB carbon foams get less amount of boron than BO carbon foams, resulting in the oxidation resistance of HB carbon foams being worse than that of BO carbon foams.
Figure 9 shows the surface morphology of the coating on specimen BO-7 after oxidation at 873 K for 120 min. A continuous glass layer with some microcracks was found on the surface of BO-7. It consists of B2O3 and amorphous carbon, deduced by their obvious diffraction peaks in XTD pattern shown in Fig. 10. No penetrating-cross cracks are found in the coating. Hollow microsphere and pores are distributed on the surface of carbon foam substrate, deflecting the path of crack extending and absorbing energy of crack growth. Therefore, porous structure of carbon foam substrate baffles the formation of penetrating-cross cracks in the coating. The crack widths in the coating are from 15 to 40 μm.
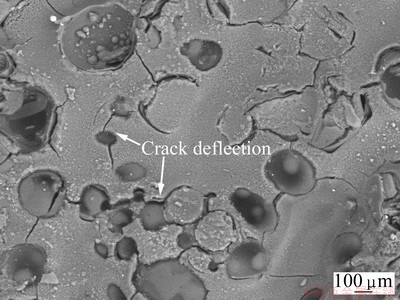
Fig. 9 Backscattering SEM image of surface morphology of B2O3-coated BO-7 after oxidation at 873 K in air for 120 min
The cracks are generated during quick cooling from 873 K to room temperature. Melting B2O3 can flow into and seal microcracks formed in the coating, even infiltrate into internal voids and wrap microspheres of the carbon foam at 873 K. So the cracks can be sealed when the sample is heated to 873 K again. Although these cracks could be self-sealed partially at high temperature, carbon foam substrate would be oxidized by oxygen diffusing through the cracks in the coating at the temperature between the crack sealing temperature and the original oxidizing temperature of carbon foams substrate. The unavoidable cracks can decrease the oxidation-protective ability of the coating. B2O3 scatters on coating surface, and shields active spots of carbon atoms. Oxygen has a lower diffusivity in B2O3 than in carbon [18,19]. Therefore, the melting glass layer could prevent oxygen from penetrating into the carbon foam substrate during oxidation to some extent.
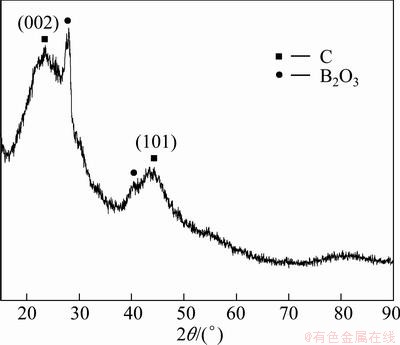
Fig. 10 XRD pattern of B2O3-coated BO-7 after oxidation at 873 K in air for 120 min
4 Conclusions
1) The B2O3 coating was prepared on the surface of boron-modified carbon foams by low-cost slurry method. It is a continuous and smooth layer without big hole.
2) The B2O3 coating can provide oxidation resistance for boron-modified carbon foams at 873 K in air.
3) B2O3-coated BO-7 specimen exhibits better oxidation resistance with mass loss of 17.40% after oxidation at 873 K in air for 120 min. There are no penetrating-cross cracks in the coating after oxidation. The melting glass layer formed on the surface of BO-7 can prevent oxygen from diffusing into boron-modified carbon foams substrate during oxidation to some extent.
References
[1] GALLEGO N C, KLETT J W. Carbon foams for thermal management [J]. Carbon, 2003, 41(7): 1461-1466.
[2] CHEN C, KENNEL E B, STILLER A H. Carbon foam derived from various precursors [J]. Carbon, 2006, 44(8): 1535-1543.
[3] XIAO Feng, ZHANG Hong-bo. Effects of foaming pressure on microstructures and properties of mesophase-pitch-derived carbon foams [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(7): 1346-1352. (in Chinese)
[4] WU Xiao-wen, LIU Yan-gai. Preparation and characterization of carbon foams derived from aluminosilicate and phenolic resin [J]. Carbon, 2011, 49(5): 1782-1786.
[5] LEI Shi-wen, GUO Quan-gui. Preparation of phenolic-based carbon foam with controllable pore structure and high compressive strength [J]. Carbon, 2010, 48(9): 2644-2646.
[6] LUO Rui-ying, NI Yong-feng. The mechanical and thermal insulating properties of resin-derived carbon foams reinforced by K2Ti6O13 whiskers [J]. Materials Science and Engineering A, 2011, 528(4): 2023-2027.
[7] WANG Shao-bo, LUO Rui-ying. Preparation and characterization of resin-derived carbon foams reinforced by hollow ceramic microspheres [J]. Materials Science and Engineering A, 2010, 527(15): 3392-3395.
[8] HIROSHI H, TAKUYA A, YASUO K. High-temperature oxidation behavior of SiC-coated carbon fiber-reinforced carbon matrix composites [J]. Composites Part A, 1999, 30(4): 515-520.
[9] ELENA R, ROBERTO G. Characterisation of boron-doped coal-derived carbon foams and their oxidation behaviour [J]. Fuel, 2012, 93(1): 288-297.
[10] HUANG Min, LI Ke-zhi. Double-layer oxidation protective SiC/Cr-Al-Si coating for carbon-carbon composites [J]. Surface & Coatings Technology, 2007, 201(18): 7842-7846.
[11] FU Qian-gang, XUE Hui. MoSi2-improved SiC coating to protect carbon/carbon composites against oxidation [J]. Surface Review and Letters, 2007, 14(4): 795-799.
[12] ZHANG Wu-zhuang, ZENG Yi. Preparation and oxidation property of ZrB2-MoSi2/SiC coating on carbon/carbon composites [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(7): 1538-1544.
[13] ZHANG Yu-lei, LI He-jun. Oxidation protective C/SiC/Si-SiC multilayer coating for carbon/carbon composites applying at 1873 K [J]. Journal of Material Science and Technology, 2010, 26(12): 1139-1142.
[14] WU Heng, LI He-jun. Microstructures and ablation resistance of ZrC coating for SiC-coated carbon/carbon composites prepared by supersonic plasma spraying [J]. Journal of Thermal Spray Technology, 2011, 20(6): 1286-1291.
[15] HU Zhi-biao, LI He-jun. Fabrication and tribological properties of B2O3 as friction reducing coatings for carbon-carbon composites [J]. New Carbon Materials, 2007, 22(2): 131-134.
[16] FU Qian-gang, ZOU Xu. A multilayer MoSi2-SiC-B coating to protect SiC-coated carbon/carbon composites against oxidation [J]. Vacuum, 2012, 86(12): 1960-1963.
[17] NARASIMMANR,PRABHAKARAN K. Preparation of low density carbon foams by foaming molten sucrose using an aluminium nitrate blowing agent [J]. Carbon, 2012, 50(5):1999-2009.
[18] HU Xing-hua, ZHA Qing-fang. Research on improving the antioxidative ability of porous carbon by solid boron-doping [J]. Journal of Fuel Chemistry and Technology, 2007, 35(3): 349-353. (in Chinese)
[19] ZHONG D H, SANO H. Effect of low-level boron doping on oxidation behavior of polyimide-derived carbon films [J]. Carbon, 2000, 38(8): 1199-1206.
B2O3涂覆硼改性炭泡沫的制备及其抗氧化性能
王 斌,李贺军,张雨雷,王 茜
西北工业大学 凝固技术国家重点实验室,C/C复合材料工程技术研究中心,西安 710072
摘 要:为了改善硼改性炭泡沫的抗氧化性能,采用低成本的料浆涂刷技术,在其表面制备B2O3涂层。采用SEM和XRD分析涂覆B2O3的改性炭泡沫的微观形貌与相组成;并研究无涂层与涂覆B2O3的硼改性炭泡沫的抗氧化性能。结果表明,在873 K下 B2O3涂层可有效保护硼改性炭泡沫不受氧化。涂覆B2O3后,含7% B2O3(质量分数)的炭泡沫(BO-7)的抗氧化性能最好,氧化120 min后质量损失为17.40%;该试样表面出现一层熔融玻璃层,在一定程度上阻挡了外来氧渗入到硼改性炭泡沫基体。
关键词:炭泡沫;氧化硼;涂层;抗氧化
(Edited by Hua YANG)
Foundation item: Projects (51072107, 51272213, 51221001) supported by the National Natural Science Foundation of China; Project (B08040) supported by the Program of Introducing Talents of Discipline to Universities of China (“111”Project)
Corresponding author: He-jun LI; Tel: +86-29-88495764; E-mail: lihejun@nwpu.edu.cn
DOI: 10.1016/S1003-6326(13)62706-5
Abstract: To improve the oxidation resistance of boron-modified carbon foams, the B2O3 coating was prepared on boron-modified carbon foams by low-cost slurry method. The microstructures and phase compositions of the coated carbon foams were characterized by scanning electron microscopy and X-ray diffraction, respectively. Oxidation resistances of uncoated and coated boron-modified carbon foams were investigated at 873 K in air. The results showed that as-received B2O3 coating could protect boron-modified carbon foams from oxidation at 873 K. B2O3-coated carbon foam doped with 7% B2O3 (mass fraction) (BO-7) had better oxidation resistance, exhibiting mass loss of 17.40% after oxidation at 873 K for 120 min. The melting glass layer formed on the surface of BO-7 could prevent oxygen from diffusing into boron-modified carbon foams substrate during oxidation to some extent.


