
Initial corrosion behavior of AZ91 magnesium alloy in
simulating acid rain under wet-dry cyclic condition
ZHOU Wan-qiu(周婉秋)1, 2, SHAN Da-yong(单大勇)2,
HAN En-hou(韩恩厚)2, KE Wei(柯 伟) 2
1. College of Chemistry and Life Science, Shenyang Normal University, Shenyang 110034, China;
2. State Key Laboratory for Corrosion and Protection, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 12 June 2008; accepted 5 September 2008
Abstract:
Corrosion behavior of AZ91 magnesium alloy in simulating acid rain under wet-dry cyclic condition was investigated. The results show that corrosion potential shifts positively and the corrosion current density decreases at low wet-dry cyclic time. Further increase of the cyclic time results in the negative movement of corrosion potential and the increase of current density. SEM observation indicates that corrosion occurs only in α phase, β phase is inert in corrosive medium, and the corrosion of AZ91 magnesium appears in uniform characteristic. XPS analysis suggests that the corrosion product is mainly composed of oxide and hydroxide of magnesium and aluminum, and a small amount of sulfate is also contained in the film.
Key words:
magnesium alloy; atmospheric corrosion; simulating acid rain; wet-dry cycle;
1 Introduction
The great potential of magnesium alloys in a variety of structural application such as automobile and aero-space industry is well known[1-3]. One of the most commonly used magnesium alloys is AZ91. The use of magnesium alloys is restricted due to their susceptibility to corrosion. The corrosion behavior of magnesium alloys has mainly been studied using immersion in aqueous salt solution and salt-spray test[2-4]. These tests are not applicable to outdoor exposure as they accelerate the corrosion too much and may induced different corrosion mechanism. Recently, the atmospheric corrosion of magnesium alloys has received much attention. Several field and laboratory studies have established that the corrosion of magnesium alloys in atmosphere is influenced by chloride deposition and by gaseous dioxide such as CO2 and SO2[5-12]. It was found that the combination of high humidity and NaCl was very corrosive towards magnesium alloys[5, 9]. The NaCl induced corrosion was inhibited in the presence of CO2 because of the formation of a protective layer of hydrated magnesium hydroxy carbonate[5, 10]. However,the attack was accelerated in the presence of SO2 due to the dissolution effect of SO2 to the initial formed corrosion product containing MgO and Mg(OH)2[12].
Rain is one of the transmitting ways for air pollutant to deposition on metal surface. Gaseous dioxide in the air dissolved in the rain and formed the acid rain. Corrosion induced by acid rain is a wet-dry alternate process in atmospheric environments. Some researchers studied the corrosion behavior of aluminum alloys, bronze, mild steel and zinc in acid rain condition using EIS, electrochemical noise, polarization resistance, SEM, Raman and X-ray photoelectron spectroscopy[13-16]. However, few investigations deal with the corrosion of magnesium alloys in the acid rain environment.
An understanding of corrosion behavior of magnesium alloys in the acid rain environment is needed. In this work, an attempt to investigate the atmospheric corrosion of magnesium alloy under cyclic wet-dry conditions in the early stage was conducted. A corrosive medium containing 0.01 mol/L NaHSO3 and 0.001 mol/L NaCl was used to simulate the acid rain formed in industrial environment. Special attention has been paid to the influence of time of wet-dry cycle on the corrosion of AZ91 magnesium alloy.
2 Experimental
2.1 Materials and specimen preparation
AZ91D magnesium alloy was used with the composition of 8.3%-9.7% aluminum, 0.35%-1.0% zinc, 0.15%-0.5% manganese, <0.1% silicon, <0.03% copper, <0.002% nickel, <0.005% iron, and balance magnesium. Specimens were polished with 1 200# grit SiC paper and 1 μm diamond paste. The samples for SEM observation were etched for about 2 s in 3% nital solution before wet-dry cycle exposure.
2.2 Wet-dry cycle experiment
The wet-dry cycle experiment was conducted by exposing the sample to an alternate condition of 4 h immersion in a solution of 0.01 mol/L NaHSO3 and 0.001 mol/L NaCl, and 20 h drying at 298 K and 60% relative humidity(RH) in the air. The solution was prepared from analytical grade chemical of NaHSO3 and NaCl and distilled water.
2.3 Electrochemical measurement
The polarization curves were measured at a scan rate of 0.5 mV/s using EG&G Model 273 potentiostat. A saturated calomel electrode was used as reference electrode and a platinum flake was used as auxiliary electrode. The testing specimen was used as working electrode covered with cold setting resin with only 1 cm2 surface exposed. The specimen was polished with 1 200# grit SiC paper, followed by washing with acetone and distilled water.
2.4 Surface morphology and composition analysis
Morphology was observed by environmental scanning electron microscope(ESEM)(Model XL30 FEG). Composition of the coating was analyzed by EDX facility attached to ESEM.
2.5 XPS analysis
The XPS instrument employed was a Thermo VG ESCALAB250 surface analysis system which uses a mono-chromatic Al Kα (1 486.6 eV) X-ray source with a spot size of 500 μm.
3 Results
3.1 Polarization curves
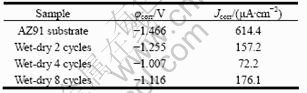
Fig.1 shows the polarization curves of AZ91D substrate and samples exposed in simulating acid rain for different wet-dry cyclic time, and the electrochemical data are listed in Table 1. For the sample after 2 wet-dry cycles exposure, the corrosion potential increased and the current density decreased compared with AZ91 substrate. After 4 wet-dry cycles exposure, corrosion potential shifted positively to -1.007 V (SCE), and the current density further decreased to 72.2 μA/cm2. With the increase of wet-dry cycle time, corrosion potential decreased and the current density increased compared with that of 4 wet-dry cycles sample.
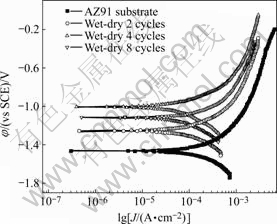
Fig.1 Polarization curves in simulating acid rain for AZ91D after wet-dry cycle exposure
Table 1 Fitting results of polarization curves
3.2 Corrosion morphology
The surface morphology for 2 wet-dry cycles exposure is shown in Fig.2 (a). Flaky corrosion product was deposited in α phase, micro-cracks existed in the precipitation, and some flake dropped off from the substrate.
EDX analysis demonstrated the existence of magnesium, aluminum, oxygen and sulfur in corrosion product. At the position where the flake dropped off, only magnesium and aluminum were found, which implied the formation of a thin film, and the metal substrate was exposed after the film dropping off.
At β phase, no corrosion product could be found. EDX identified the existence of magnesium, aluminum and a small amount of oxygen at β phase, and no sulfur could be detected, which supplied that corrosion attack did not take place at β phase.
After 4 wet-dry cycle exposure, corrosion extended in α phase interior, and plenty of micro-cracks existed in the surface film. The film was broken because of the inner stress, and the new attack occurred, which resulted in the further corrosion in deep direction, as shown in Fig.2 (b).
Corrosion spread all over α phase after 8 cycles
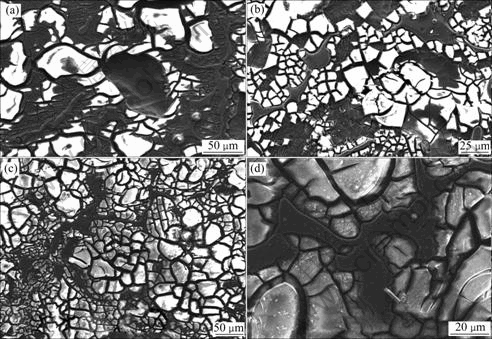
Fig.2 Morphologies of AZ91 magnesium alloys after wet-dry cyclic exposure: (a) 2 cycles; (b) 4 cycles; (c), (d) 8 cycles
exposure, β distributed along grain boundary in network feature and did not suffer corrosion, and the corrosion form presented in uniform appearance, as shown in Figs.2(c) and 2(d).
3.3 XPS analysis
XPS spectra of sample after 8 wet-dry cycles exposure were detected, and the general survey spectra contained relatively strong Mg 1s and O 1s lines, whereas the lines corresponding to Al 2p and S 2p were relatively weak.
Multiplex scanning provided more detailed spectra for the above elements. There was a clear Mg 1s peak in Fig.3(a) and a relatively weak Al 2p peak in Fig.3(b). The S 2p signal was very weak with relative strong back noise shown in Fig.3(c), and the O 1s spectrum indicated the presence of OH- and O2- as well as SO42- presented in Fig.3 (d).
The peaks of Mg 1s and Al 2p, characterized by the binding energies of 1 304.1eV and 72.4 eV, corresponded to Mg2+ and Al3+ respectively. The XPS O 1s could be deconvoluted into three distinct peaks, which suggested the presence of both oxide and hydroxide of magnesium and aluminum, as well as a small amount of sulfate of magnesium and aluminum.
4 Discussion
In aqueous environment, the corrosion of magnesium alloys was conducted according to the following reactions:
Anodic reaction: Mg=Mg2++2e; Al=Al3++3e
Cathodic reaction: 2H2O+2e=H2+2OH-
Anodic reaction involved in the dissolution of magnesium and aluminum, and the cathodic reaction proceeded by the reduction of water, which resulted in the produce of OH- and hydrogen gas.
Anodic dissolution took place professionally in α phase because of its low aluminum content and high chemical activity. Mg2+ and Al3+ produced in anodic reaction combined with the OH- produced in cathodic reaction, and the hydroxide of magnesium and aluminum was deposited on the surface of α phase.
The film of oxide of magnesium and aluminum could be formed in the air. In each wet-dry cycle, the sample was immersed in simulating acid rain for 4 h and then exposed in air for 20 h. The oxygen in the air could transport through the cracks on the surface of the film and reached to the metal substrate, which resulted in the oxidization of metal substrate and the formation of oxide film at the film/substrate interface.
HSO3- ion was produced when NaHSO3 was dissolved in water. The HSO3- ion could be oxidized by O2 in the air, which resulted in the produce of SO42- and H+. H+ reacted with oxide, which led to the dissolution of oxide film and metal substrate. Mg2+ and Al3+ combined with SO42- and the sulfate was formed. The corrosion product was constituted with oxide and hydroxide as well as sulfate. Magnesium and aluminum sulfate dissolved in water easily, which suggested the small amount of sulfate deposition in the film.
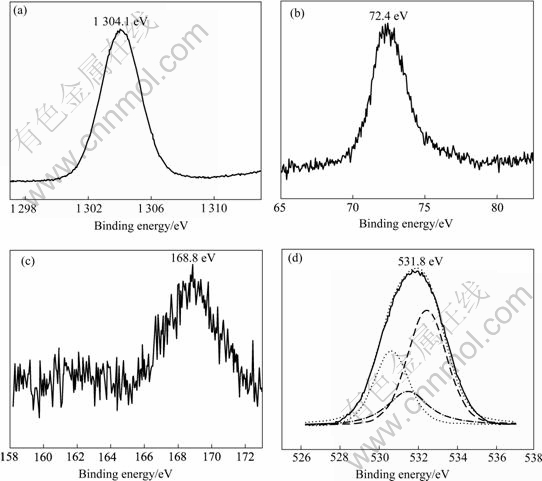
Fig.3 Typical multiplex XPS spectra of corrosion product: (a) Mg 1s; (b) Al 2p; (c) S 2p; (d) O 1s
With the increase of wet-dry cycle, the potential shifted positively and the corrosion current density decreased, owing to the preferential dissolution of α phase and the increase of β phase fraction on sample surface. The metal matrix was covered because of the deposition of corrosion product on α phase. The film blocked the diffusion of corrosion medium to metal substrate and prevented the subsequent attack, and corrosion rate was decreased. The abundant cracks in the surface film provided the transport way for corrosive medium and oxygen, and further attack could not been stopped completely.
In simulating acid rain, corrosion occurred in α phase, and the inert β phase distributed uniformly in alloys, which accounted for the uniform corrosion morphology characteristic of AZ91D under wet-dry cycles exposure condition.
5 Conclusions
1) When AZ91D magnesium alloys is exposed in simulating acid rain under wet-dry cyclic condition, corrosion occurs in α phase and flaky corrosion product is precipitated on metal matrix.
2) The β phase is inert and attack does not take place on β phase.
3) The further attack could not be inhibited completely because of the existence of large amount of crack in the film.
4) The corrosion product is composed of oxide and hydroxide as well as small amount of sulfate of magnesium and aluminum.
References
[1] DECKER R F. The renaissance in magnesium [J]. Advanced Materials & Processes, 1998(9): 31-33.
[2] MAKAR G L, KRUGER J. Corrosion of magnesium [J]. International Materials Reviews, 1993, 38(3): 138-153.
[3] SONG Guang-ling, ATRENS A. Corrosion mechanisms of magnesium alloys [J]. Advanced Engineering Materials, 1999(1): 11-33.
[4] SONG Guang-ling, ATRENS A, WU Xian-liang. Corrosion behavior of AZ21, AZ501 and AZ91 in sodium chloride [J]. Corrosion Science, 1998, 40(10): 1769-1791.
[5] JONSSON M, PERSSON D, LEYGRAF C. Atmospheric corrosion of field-exposed magnesium alloy AZ91D [J]. Corrosion Science,2008, 50(5): 1406-1413.
[6] SONG Guang-ling, SARATH H, DAVID S J. Degradation of the surface appearance of magnesium and its alloys in simulated atmospheric environment [J]. Corrosion Science,2007, 49(3): 1245- 1265.
[7] XIAO K, DONG C F, LI J Q, LI X G, WEI D. Research on atmospheric galvanic corrosion evaluation of magnesium alloy [J]. Rare Metal Materials and Engineering,2007,36(2): 201-207.
[8] FOTEA C, CALLAWAY J, ALEXANDER M R. Characterization of the surface chemistry of magnesium exposed to the ambient atmosphere [J]. Surface and Interface Analysis, 2006, 38(10): 1363-1371.
[9] LEBOZEC N, JONSSON M, THIERRY D. Atmospheric corrosion of magnesium alloys: Influence of temperature, relative humidity, and chloride deposition [J]. Corrosion, 2004, 60(4): 356-361.
[10] LINDSTROM R, SVENSSON J E, JOHANSSON L G. The Influence of carbon dioxide on the atmospheric corrosion of some magnesium alloys in the presence of NaCl [J]. Journal of the Electrochemical Society, 2002, 149(4): B103-107.
[11] BLUCHER B D, SVENSSON J E, JOHANSSON L G, ROHWERDER M, STRATMANN M. Scanning Kelvin probe force microscopy: A useful tool for studying atmospheric corrosion of MgAl alloys in situ [J]. Journal of The Electrochemical Society, 2004, 151(12): B621-626.
[12] LIN Cui, LI Xiao-gang. Initial corrosion of AZ91D magnesium alloy in atmosphere containing SO2 [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1658-1665. (in Chinese)
[13] SHI Y Y, ZHANG Z, SU J X, CAO F H, ZHANG J Q. . Electrochemical noise study on 2024-T3 aluminum alloy corrosion in simulated acid rain under cyclic wet-dry condition [J]. Electrochimica Acta, 2006,51(23): 4977-4986.
[14] BERNARDI E, CHIAVARI C, MARTINI C, MORSELLI L. The atmospheric corrosion of quaternary bronzes: An evaluation of the dissolution rate of the alloying elements [J]. Applied Physics A-Materials Science & Processing, 2008, 92(1): 83-89.
[15] BAGHNI I M, LYON S B. Inhibition of mild steel by strontium chromate in artificial acid rain solution [J]. Corrosion Engineering Science and Technology, 2005, 40(2): 165-170.
[16] EI-MAHDY G A, KIM K B. Monitoring of initial stages of atmospheric zinc corrosion in simulated acid rain solution under wet-dry cyclic conditions [J]. Corrosion, 2005, 61(5):420-427.
(Edited by YANG You-ping)
Foundation item: Project(50671005) supported by the National Natural Science Foundation of China; Project(2007CB613705) supported by National Basic Research Program of China
Corresponding author: ZHOU Wan-qiu; Tel: +86-24-86593312; E-mail: wqzhou@imr.ac.cn
[1] DECKER R F. The renaissance in magnesium [J]. Advanced Materials & Processes, 1998(9): 31-33.
SHI Y Y, ZHANG Z, SU J X, CAO F H, ZHANG J Q. " target="blank">[13] SHI Y Y, ZHANG Z, SU J X, CAO F H, ZHANG J Q.
MORSELLI L." target="blank">[14] BERNARDI E, CHIAVARI C, MARTINI C, MORSELLI L.
BAGHNI I M, LYON S B." target="blank">[15] BAGHNI I M, LYON S B.
EI-MAHDY G A, KIM K B. " target="blank">[16] EI-MAHDY G A, KIM K B.


