
J. Cent. South Univ. (2020) 27: 1395-1403
DOI: https://doi.org/10.1007/s11771-020-4375-1
Catalytic effect of silver-bearing solid waste on chalcopyrite bioleaching: A kinetic study
LIAO Rui(廖蕤)1, 2, WANG Xing-xing(王星星)1, 2, YANG Bao-jun(杨宝军)1, 2,
HONG Mao-xing(洪茂鑫)1, 2, ZHAO Hong-bo(赵红波)1, 2, WANG Jun(王军)1, 2, QIU Guan-zhou(邱冠周)1, 2
1. Key Laboratory of Biohydrometallurgy of Ministry of Education, Central South University,Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Silver ion can be useful in improving chalcopyrite bioleaching efficiency. In this work, leaching kinetics of this process was investigated using silver-bearing solid waste under different chalcopyrite/solid waste ratios. Bioleaching behavior indicates that silver-bearing solid waste can enhance the bioleaching process, and the redox potential is much higher than the proposed appropriate range (380-480 mV vs Ag/AgCl) with the solid waste added. There is a positive correlation between temperature and copper extraction rate. The kinetics data fit well with the shrinking-core model. Under these leaching conditions, the bioleaching of chalcopyrite is controlled by internal diffusion with calculated apparent activation energy (Ea) of 28.24 kJ/mol. This work is possible benificial to promote the industrial application of silver catalyst in leaching of chalcopyrite.
Key words:
chalcopyrite; silver-bearing solid waste; bioleaching; kinetics;
Cite this article as:
LIAO Rui, WANG Xing-xing, YANG Bao-jun, HONG Mao-xing, ZHAO Hong-bo, WANG Jun, QIU Guan-zhou. Catalytic effect of silver-bearing solid waste on chalcopyrite bioleaching: A kinetic study [J]. Journal of Central South University, 2020, 27(5): 1395-1403.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4375-11 Introduction
Bioleaching is a low energy consumption and environment-friendly technology that can be used for abstracting metal from low-grade ores. By comparison with the pyrometallurgy method that expends vast quantity of energy and produces plenty of contamination, bioleaching process gets lots of advantages [1-8]. However, the application of bioleaching has been hindered for its slow kinetics and low extraction rate. Chalcopyrite (CuFeS2) is the most widespread ore containing copper [9-14]. During its bioleaching process, there are secondary reaction products formed on chalcopyrite surface. These products, also regarded as passivation layers, are thick and dense, and have very low conductivity, making it impossible for ions to pass through the surface of chalcopyrite [15-17]. As a result, the leaching kinetics of chalcopyrite is slow, hindering its further applications in industry [18-21]. Therefore, people are working on finding ways to improve the bioleaching efficiency of chalcopyrite [11, 22-27].
It is of particular interest that certain amount of catalyst could effectively enhance bioleaching reaction rate, and silver ion is reported to be one of the most effective catalysts [3, 28, 29]. When silver ion was added in the leaching system, the copper extraction was close to 80%. While with no extra silver added, copper extraction was nearly 40% [30]. Silver is very expensive and it is to date impossible to recycle the silver added; therefore, the utilization of silver in industrial application is uneconomical [31]. Later, researchers have discovered silver-containing concentrate and tailing also can enhance the bioleaching process [12, 24, 32, 33].
There has been a great controversy over the mechanism of silver catalysis in chalcopyrite bioleaching, and all the proposed viewpoints could be categorized into three types [34, 35], namely, direct catalysis of silver ions on leaching of chalcopyrite [36], indirect catalysis of silver ions by reducing the concentration of hydrogen sulfide in the leaching medium near the chalcopyrite surface [37] and silver sulfide that incorporated in the chalcopyrite structure improving the electrical conductivity of the chalcopyrite passivation film [35, 38].
Meanwhile, the kinetics of silver catalysis leaching of chalcopyrite is still under debate by researchers. MILLER et al [36] proposed that the process is controlled by the intermediate electrochemical reaction of Fe3+ with Ag2S crystal (as Eq. (1)). They put forward this viewpoint by the fact that the reaction rate was related to the concentration of Fe3+, Fe2+ and Ag+, but independent of the Cu2+ concentration. Besides, the rate has been unrelated to the initial chalcopyrite particle size because the reaction occurs on the surface of the Ag2S crystal. The activation energy calculated was 65.7 kJ/mol, which was consistent with the rate control by an electrochemical reaction.
Ag2S+2Fe3+=2Ag++S0+2Fe2+ (1)
WANG et al [39] established a modified kinetic model for chalcopyrite bioleaching catalyzed by silver ions on the basis of the shrinking core model. They proposed that silver ions substitute for metal ions in the chalcopyrite, which limits the leaching rate, and both the theoretical studies and experimental results fit the proposal well.
CORDOBA et al [38, 40] used the simplified shrinking core model to plot the linear curves and the activation energy calculated was 29.25 kJ/mol (with catalyst silver ions) and 130.7 kJ/mol (without catalyst), respectively. According to the remarkable decrease of activation energy, they believed that silver improved the electrical conductivity of the chalcopyrite surface and proposed that the leaching of silver-catalyzed chalcopyrite was controlled by electrochemical processes, chemical reaction processes, and diffusion processes.
In the present work, the rate-controlling step and the corresponding kinetic model of chalcopyrite silver-catalyzed bioleaching system were studied using the silver-bearing wastes. This work is possible benificial to provide a point of view for studying the kinetics of silver-catalyzed chalcopyrite bioleaching process, finding out the rate-limiting step of the process and promoting the industrial application of silver catalysis in chalcopyrite bioleaching.
2 Materials and methods
2.1 Mineral samples
Chalcopyrite mineral samples used in the experiments were obtained from a copper flotation plant. The silver-bearing solid waste was acquired from Chenzhou Smelting Plant, Hunan Province, China. The X-ray diffraction analyses (XRD) of chalcopyrite sample and silver-bearing solid waste are given in Figure 1, and the elements analyses are given in Tables 1 and 2. The main mineralogical components of chalcopyrite were chalcopyrite, quartz and mica, containing 29.45% Cu, 16.76% Fe and 24.49% S. The percentages of silver and copper in solid waste were merely 0.004% and 0.6379%, respectively, and the mineralogical components were magnesium and magnesiochromite. All minerals samples used in this work were ground and sieved to particles size less than 0.074 mm.
2.2 Bioleaching experiments
The microorganisms used were obtained from the Key Laboratory of Biohydrometallurgy of Ministry of Education, China. Mixed culture consisting of Acidithiobacillus caldus (A. caldus) and Leptospirillum ferriphilum (L. ferriphilum) was used. A. caldus and L. ferriphilum were separately cultured and domesticated. The microorganisms were then sub-cultured into the sterile 9K medium. The 9K medium solution consisted of 3.0 g/L (NH4)2SO4, 0.1 g/L KCl, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O, and 0.01 g/L Ca(NO3)2. 44.7 g/L of FeSO4·7H2O was added to L. ferriphilum and 10 g/L of sulfur was added to A. caldus system as energy source. AgNO3 solution was employed in both systems to domesticate the microorganisms.
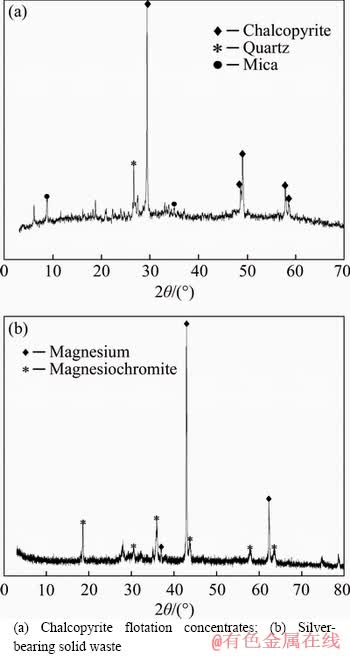
Figure 1 XRD analyses of mineral samples:
Table 1 Elements analysis of chalcopyrite (mass fraction, %)
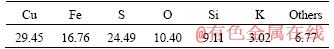
Table 2 Elements analysis of silver-bearing solid waste (mass fraction, %)
As the microbial cultures grew to the exponential growth phase and meanwhile, the concentrations exceeded 1.0×107 cell/mL, L. ferriphilum and A. caldus were centrifuged and mixed. 10 mL of the culture medium was inoculated into 250 mL Erlenmeyer flask containing 90 mL of sterile 9K solution, with 1 g of chalcopyrite sample and a set number of silver-bearing solid waste (0.1, 0.5, 1.0, 2.0, 3.0, 6.0 g). The shake flasks were then placed into an orbital shaker, setting the speed at 170 r/min with a certain temperature (308, 313, 318, 323 K). Sulfuric acid and sodium hydroxide were used to regulate the pH value maintained in the range of 1.7-1.8, and deionized water was supplemented regularly to fill in the water lost through evaporation.
2.3 Analysis methods
In bioleaching period, the concentration of Cu2+ was measured regularly with an inductively coupled plasma-atomic emission spectrometer (ICP-AES) (America Baird Co. PS-6). A pH meter (PHSJ-4A) and a Pt electrode with reference to an Ag/AgCl electrode (3.0 mol/L KCl) (BPP-922) were used to detect the pH and ORP values of the leaching solutions respectively. The bacterial concentration was monitored with a microscope (CX31).
2.4 Leaching kinetic model
The leaching process in hydrometallurgical system can be divided into two types: with or without the formation of a solid product layer during reaction. For the reaction in which a solid product layer is formed, the type can be expressed by the nuclear shrinkage model, and the internal diffusion control (as shown in Eq. (2)) and chemical reaction control equation (as shown in Eq. (3)) [41] were used during the research process:
1-2α/3-(1-α)2/3=kt (2)
1-(1-α)1/3=kt (3)
where t represents reaction time; α means copper extraction rate; k shows the reaction rate constant.
3 Result and discussion
3.1 Effect of silver concentrations and temperature on bioleaching behaviors
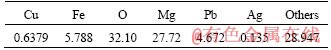
Figure 2 shows the copper extractions and oxidation-reduction potential of chalcopyrite bioleaching with different catalyst concentrations. In the sole chalcopyrite bioleaching system, the final copper extraction was 26%. While with a small amount of silver-bearing solid waste added (0.1 g compared to 1 g of chalcopyrite), the copper extraction doubled. By increasing the quantity of solid waste, a highest extraction rate of 87% was achieved with a mass ratio of 1:6 (chalcopyrite/silver-bearing solid waste). It has been mentioned that ORP plays a key role in chalcopyrite bioleaching, and many researchers proposed that an appropriate redox potential range of 380-480 mV (vs Ag/AgCl) was beneficial to promote chalcopyrite bioleaching [42-45]. In the sole chalcopyrite system, chalcopyrite dissolved comparatively fast in the first five days, during which the redox potential was at the optimal range. The bioleaching rate became slower on the 6th day, and almost stagnated on the 8th day when the ORP was higher than 600 mV. However, in the system with catalysis (e.g. chalcopyrite/solid waste of 1:1 g/g), the ORP rose from 375 mV to 475 mV in the first four day, and reached 650 mV on the 5th day; while chalcopyrite kept dissolving even when the ORP was beyond the optimal range. As for the system with 6 g solid waste, the redox potential was kept between 325 mV and 450 mV, far lower than the other six systems. It can be speculated that the addition of silver may change the previous bioleaching dissolution mechanism of chalcopyrite [12].
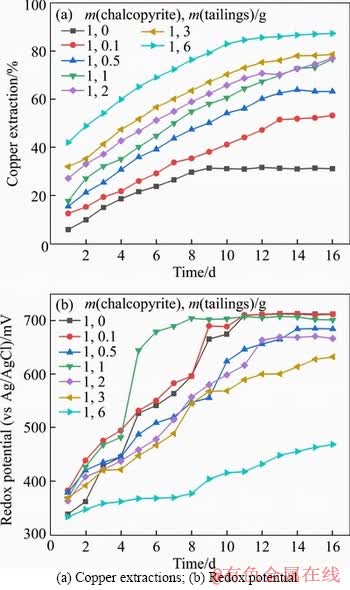
Figure 2 Bioleaching of chalcopyrite under different catalyst concentrations:
Figures 3(a) and (b) show the copper extractions and oxidation-reduction potential of chalcopyrite bioleaching under different temperatures. There was a positive correlation between temperature and copper extraction rate. In the system under the temperature of 308 K, the final copper extraction was around 65%. With the increase of temperature, the bioleaching rate of copper reached 83% at 323 K. Meanwhile, the redox potential of this system was also lower than that of the other three systems. Figure 4 shows the growth curve of microorganisms in the solutions at different temperatures. When the temperature was 318 K, the microorganisms got the optimum condition for growth. Combined with the figures mentioned above, the copper extraction rate reached the highest at 323 K, at which the microorganism concentration stayed at the lowest position. It has been reported that microorganism is not the only condition determining the bioleaching efficiency of chalcopyrite. Under certain circumstances, the oxidation ability of microbe needs to be controlled to make the ORP maintain in an appropriate range [46, 47].
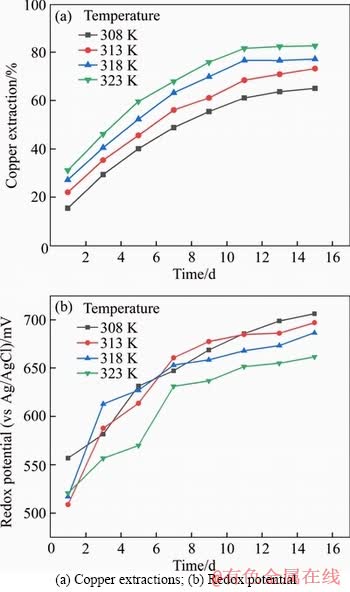
Figure 3 Bioleaching of chalcopyrite under different temperatures:
Figure 4 Microbial growth curve under different temperatures
The bioleaching of chalcopyrite is a liquid- solid phase reaction in which a layer of insoluble solid product is produced on the surface of mineral [48, 49], so the process can be considered to fit the shrinking core model.
3.2 Kinetics study
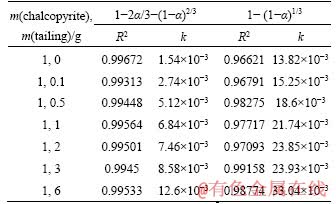
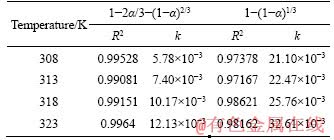
The kinetics of chalcopyrite bioleaching under different catalyst concentrations (1:0, 1:0.1, 1:0.5, 1:1, 1:2, 1:3, 1:6) and different temperatures (308, 313, 318, 323 K) was studied. In the leaching system, the shrinking-core model is often used to analyze the kinetic data for the mineral particles are usually regarded as spherical particles. According to different rate-controlling steps theories, the copper extraction and reaction time were fitted using the internal diffusion controlling reaction equation (as Eq. (2)) and the chemical reaction controlling reaction equation (as Eq.(3)). The fitting results are given in Figures 5 and 6, separately. By processing the data according to Eqs. (2) and (3), the relevant kinetic parameters (reaction rate constant, linear correlation coefficient) were obtained as given in Tables 3 and 4. CORDOBA et al [38] proposed that some last experimental data should be removed from the linear regression because the kinetic curves of the data showed a zero slope.
Apparently, by comparing the linear correlation coefficient (R2), the chalcopyrite bioleaching was controlled by internal-diffusion process. It can be proposed that with the reaction proceeding, the secondary reaction products formed on the surface of chalcopyrite, such as jarosite, polysulfide, and metal-deficient sulphides, resulting in the internal-diffusion leaching as rate-limiting step. The apparent rate constant of system without silver added was 1.54×10-3 d-1. Even a slight amount of silver-bearing solid waste can make the constant almost double (increase from 1.54×10-3 to 2.74×10-3 d-1). When the mass ratio turned to 1:6 (chalcopyrite/silver-bearing solid waste), the apparent rate constant was 12.6×10-3 d-1, increased by 8 times, in another way to prove that the addition of silver-bearing waste can effectively accelerate the bioleaching process.
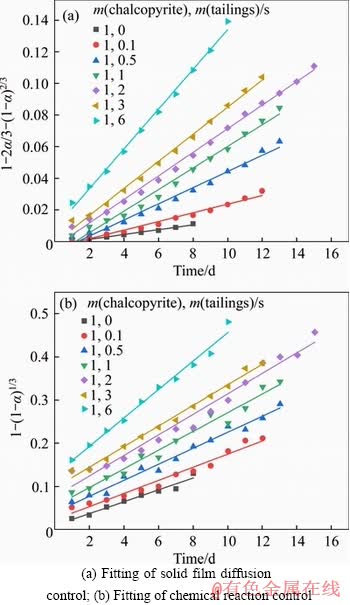
Figure 5 Fitting of leaching rate and time under different catalyst concentration:
With all the temperatures that we chose, the chalcopyrite had a good linear relationship with the time using the internal diffusion control reaction equation, as seen in previous section, indicating that the bioleaching was controlled by solid film diffusion. Arrhenius equation was employed to evaluate the effect of temperature. According to the Arrhenius formula [41] as given in Eq. (4), where A represents the frequency factor, Ea stands for the apparent activation energy, R means the gas constant and T shows the thermodynamic temperature. The equation was treated with logarithmic processing, and we got Eq. (5) [50]:
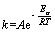 (4)
(4)
lnk=-Ea/(RT)+lnA (5)
The liner fitting curve according to Arrhenius equation is shown in Figure 7, and the activation energy of the reaction (Ea) calculated was 28.24 kJ/mol, which is less than 41.8 kJ/mol. This was further illustrated that the bioleaching process is controlled by internal diffusion. WANG et al [39] showed that the key step during bioleaching is silver ions diffusing into the chalcopyrite particle, and silver ions taking place of metallic ions is the rate-limiting step. The kinetic equation of the process is given as Eq. (6).
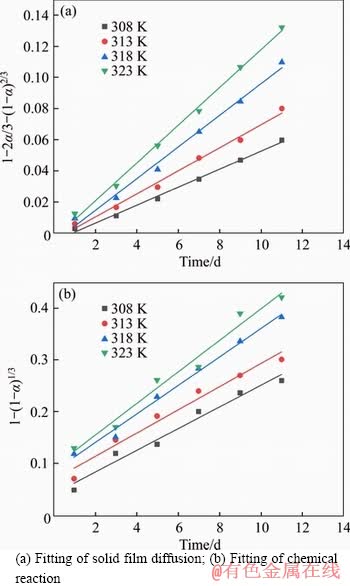
Figure 6 Fitting line under different temperatures:
Table 3 Correlation coefficients of two dynamic models with different concentrations of silver
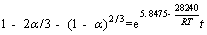 (6)
(6)
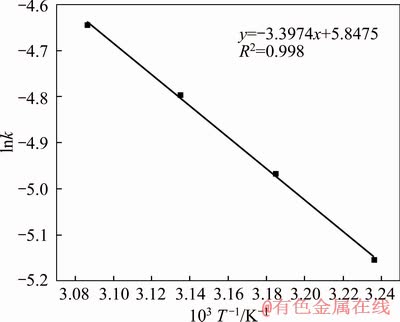
Figure 7 Arrhenius plot of effect of temperature
Table 4 Correlation coefficients of two dynamic models at different temperatures
4 Conclusions
1) The addition of silver-bearing solid waste as catalysis can effectively increase the bioleaching efficiency of chalcopyrite from 26% to 87%. There was a positive correlation between temperature and copper extraction rate.
2) The redox potential of silver added systems was beyond the proposed optimal range of 380 mV to 480 mV. The addition of silver has changed the widely recognized dissolving mechanism of chalcopyrite.
3) The bioleaching of chalcopyrite is controlled by internal diffusion, with an apparent energy Ea of 28.24 kJ/mol. Silver-bearing solid increased the apparent rate constant and the kinetic equation of the process has been given.
References
[1] WANG Xing-xing, LIAO Rui, ZHAO Hong-bo, HONG Mao-xing, HUANG Xiao-tao, PENG Hong, WEN Wen, QIN Wen-qing, QIU Guan-zhou, HUANG Cao-ming, WANG Jun. Synergetic effect of pyrite on strengthening bornite bioleaching by Leptospirillum ferriphilum [J]. Hydrometallurgy, 2018, 176: 9-16. DOI: 10.1016/ j.hydromet.2017.12.003.
[2] ZHAO Hong-bo, ZHANG Yi-sheng, ZHANG Xian, QIAN Lu, SUN Meng-lin, YANG Yu, ZHANG Yan-sheng, WANG Jun, KIM Hyun-jung, QIU Guan-zhou. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview [J]. Minerals Engineering, 2019, 136: 140-154. DOI: 10.1016/j.mineng.2019.03.014.
[3] YANG Bao-jun, ZHAO Chun-xiao, LUO Wen, LIAO Rui, GAN Min, WANG Jun, LIU Xue-duan, QIU Guan-zhou. Catalytic effect of silver on copper release from chalcopyrite mediated by Acidithiobacillus ferrooxidans [J]. Journal of Hazardous Materials, 2020, 392: 122290. DOI: 10.1016/j.jhazmat.2020.122290.
[4] WANG Jun, TAO Lang, ZHAO Hong-bo, HU Ming-hao, ZHENG Xi-hua, PENG Hong, GAN Xiao-wen, XIAO Wei, CAO Pan, QIN Wen-qing, QIU Guan-zhou, WANG Dian-zuo. Cooperative effect of chalcopyrite and bornite interactions during bioleaching by mixed moderately thermophilic culture [J]. Minerals Engineering, 2016, 95: 116-123. DOI:10.1016/j.mineng.2016.06.006.
[5] HONG Mao-xing, WANG Xing-xing, WU Ling-bo, FANG Chao-jun, HUANG Xiao-tao, LIAO Rui, ZHAO Hong-bo, QIU Guan-zhou, WANG Jun. Intermediates transformation of bornite bioleaching by Leptospirillum ferriphilum and Acidithiobacillus caldus [J]. Minerals, 2019, 9: 159. DOI: 10.3390/min9030159.
[6] FANG Chao-jun, YU Shi-chao, WANG Xing-xing, ZHAO Hong-bo, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Synchrotron radiation XRD investigation of the fine phase transformation during synthetic chalcocite acidic ferric sulfate leaching [J]. Minerals, 2018, 8: 461. DOI: 10.3390/min8100461.
[7] ZHAO Hong-bo, HUANG Xiao-tao, HU Ming-hao, ZHANG Chen-yang, ZHANG Yi-sheng, WANG Jun, QIN Wen-qing, QIU Guan-zhou. Insights into the surface transformation and electrochemical dissolution process of bornite in bioleaching [J]. Minerals, 2018, 8: 173. DOI:10.3390/min8040173.
[8] WANG Jun, ZHU Shan, ZHANG Yan-sheng, ZHAO Hong-bo, HU Ming-hao, YANG Cong-ren, QIN Wen-qing, QIU Guan-zhou. Bioleaching of low-grade copper sulfide ores by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Journal of Central South University, 2014, 21: 728-734. DOI: 10.1007/s11771-014-1995-3.
[9] ZHAO Hong-bo, WANG Jun, QIN Wen-qing, HU Ming-hao, ZHU Shan, QIU Guan-zhou. Electrochemical dissolution process of chalcopyrite in the presence of mesophilic microorganisms [J]. Minerals Engineering, 2015, 71: 159-169. DOI: 10.1016/j.mineng.2014.10.025.
[10] YANG Bao-jun, LUO Wen, WANG Xing-xing, YU Shi-chao, GAN Min, WANG Jun, LIU Xue-duan, QIU Guan-zhou. The use of biochar for controlling acid mine drainage through the inhibition of chalcopyrite biodissolution [J]. Science of the Total Environment, 2020: 139485. DOI: 10.1016/j.scitotenv. 2020.139485.
[11] YANG Bao-jun, LIN Mo, FANG Jing-hua, ZHANG Rui-yong, LUO Wen, WANG Xing-xing, LIAO Rui, WU Ling-bo, WANG Jun, GAN Min, LIU Bin, ZHANG Yi, LIU Xue-duan, QIN Wen-qing, QIU Guan-zhou. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans [J]. Science of the Total Environment, 2020, 698: 134175. DOI: 10.1016/j.scitotenv.2019.134175.
[12] ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, HU Ming-hao, ZHANG Er-xing, QIN Wen-qing, QIU Guan- zhou. Cooperative bioleaching of chalcopyrite and silver- bearing tailing by mixed moderately thermophilic culture: An emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis [J]. Minerals Engineering, 2015, 81: 29-39. DOI: 10.1016/j.mineng.2015.07.015.
[13] WANG Jun, QIN Wen-qing, ZHANG Yan-sheng, YANG Cong-ren, ZHANG Jian-wen, NAI Shao-shi, SHANG He, QIU Guan-zhou. Bacterial leaching of chalcopyrite and bornite with native bioleaching microorganism [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 1468-1472. DOI: 10.1016/S1003-6326(09)60027-3.
[14] WANG Jun, ZHAO Hong-bo, QIN Wen-qing, YANG Cong-ren, QIU Guan-zhou. Investigation of interface reactions and electrochemical behaviors of chalcopyrite dissolution in different leaching mediums [J]. International Journal of Electrochemical Science, 2013, 12(8): 12590- 12599.
[15] ZHAO Hong-bo, WANG Jun, QIN Wen-qing, ZHENG Xi-hua, TAO Lang, GAN Xiao-wen, QIU Guan-zhou. Surface species of chalcopyrite during bioleaching by moderately thermophilic bacteria [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 2725-2733. DOI: 10.1016/S1003-6326(15)63897-3.
[16] ZHAO Hong-bo, HU Ming-hao, LI Yi-ni, ZHU Shan, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Comparison of electrochemical dissolution of chalcopyrite and bornite in acid culture medium [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 303-313. DOI: 10.1016/S1003- 6326(15)63605-6.
[17] FANG Jing-hua, LIU Yong, HE Wan-li, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Transformation of iron in pure culture process of extremely acidophilic microorganisms [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1150-1155. DOI: 10.1016/S1003-6326(17)60134-1.
[18] WATLING H R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate– chloride and sulfate–nitrate process options [J]. Hydrometallurgy, 2013, 140: 163-180. DOI: 10.1016/ j.hydromet.2013.09.013
[19] DABNEY B L, CLEMENTS W H, WILLIAMSON J L, RANVILLE J F. Influence of metal contamination and sediment deposition on benthic invertebrate colonization at the north Fork Clear Creek Superfund Site, Colorado, USA [J]. Environmental Science Technology, 2018, 52: 7072-7080. DOI: 10.1021/acs.est.7b06556.
[20] MA Li-yuan, WANG Xing-jie, LIU Xue-duan, WANG Shan-quan, WANG Hong-mei. Intensified bioleaching of chalcopyrite by communities with enriched ferrous or sulfur oxidizers [J]. Bioresource Technology, 2018, 268: 415-423. DOI: 10.1016/j.biortech.2018.08.019.
[21] WANG Jun, GAN Xiao-wen, ZHAO Hong-bo, HU Ming-hao, LI Kai-yun, QIN Wen-qing, QIU Guan-zhou. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis [J]. Minerals Engineering, 2016, 98: 264-278. DOI: 10.1016/j.mineng.2016.09.008.
[22] ZHAO Hong-bo, WANG Jun, HU Ming-hao, QIN Wen-qing, ZHANG Yan-sheng, QIU Guan-zhou. Synergistic bioleaching of chalcopyrite and bornite in the presence of Acidithiobacillus ferrooxidans [J]. Bioresource Technology, 2013, 149: 71-76. DOI: 10.1016/j.biortech.2013.09.035.
[23] LI Yu-biao, YAO Yi-lun, WANG Bing, QIAN Gu-jie, LI Zhi-ming, ZHU Yang-ge. New insights into chalcopyrite leaching enhanced by mechanical activation [J]. Hydrometallurgy, 2019, 189: 105131. DOI: 10.1016/ j.hydromet.2019.105131.
[24] ZHAO Hong-bo, HUANG Xiao-tao, WANG Jun, LI Yi-ni, LIAO Rui, WANG Xing-xing, QIU Xiao, XIONG Yu-ming, QIN Wen-qing, QIU Guan-zhou. Comparison of bioleaching and dissolution process of p-type and n-type chalcopyrite [J]. Minerals Engineering, 2017, 109: 153-161. DOI: 10.1016/ j.mineng.2017.03.013.
[25] WANG Jun, HU Ming-hao, ZHAO Hong-bo, TAO Lang, GAN Xiao-wen, QIN Wen-qing, QIU Guan-zhou. Well- controlled column bioleaching of a low-grade copper ore by a novel equipment [J]. Journal of Central South University, 2015, 22: 3318-3325. DOI: 10.1007/s11771-015-2872-4.
[26] HUANG X, ZHAO H, ZHANG Y, LIAO R, WANG J, QIN W, QIU G. A strategy to accelerate the bioleaching of chalcopyrite through the goethite process [J]. Minerals & Metallurgical Processing, 2018, 35: 171-175. DOI: 10.19150/mmp.8593.
[27] ZHAO Chun-xiao, YANG Bao-jun, WANG Xing-xing, ZHAO Hong-bo, GAN Min, QIU Guan-zhou, WANG Jun. Catalytic effect of visible light and Cd2+ on chalcopyrite bioleaching [J]. Transactions of Nonferrous Metals Society of China, 2020, 30(4): 1078-1090. DOI: 10.1016/s1003- 6326(20)65279-7.
[28] NIE Zhen-yuan, ZHANG Wei-wei, LIU Hong-chang, XIA Jin-lan, ZHU Wei, ZHANG Duo-rui, ZHENG Lei, MA Chen-yan, ZHAO Yi-dong, WEN We. Synchrotron radiation based study of the catalytic mechanism of Ag+ to chalcopyrite bioleaching by mesophilic and thermophilic cultures [J]. Minerals, 2018, 8: 382. DOI: 10.3390/ min8090382.
[29] NIKOLOSKI A N, OMALLEY G P, BAGAS S J. The effect of silver on the acidic ferric sulfate leaching of primary copper sulfides under recycle solution conditions observed in heap leaching. Part 1: Kinetics and reaction mechanisms [J]. Hydrometallurgy, 2017, 173: 258-270. DOI: 10.1016/ j.hydromet.2017.08.020.
[30] ZHAO Hong-bo, ZHANG Yi-sheng, SUN Meng-lin, OU Peng-fei, ZHANG Yan-jun, LIAO Rui, QIU Guan-zhou. Catalytic mechanism of silver in the oxidative dissolution process of chalcopyrite: Experiment and DFT calculation [J]. Hydrometallurgy, 2019, 187: 18-29. DOI: /10.1016/ j.hydromet.2019.05.002.
[31] GOMEZ E, BALLESTER A, BLAZQUEZ M L, GONZALEZ F. Silver-catalysed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms [J]. Hydrometallurgy, 1999, 51: 37-46. DOI: 10.1016/S0304-386X(98)00070-X.
[32] MUNOZ J A, DRESINGER D B, COOPER W C, YOUNG S K. Silver catalyzed bioleaching of low-grade copper ores. Part III: Column reactors [J]. Hydrometallurgy, 2007, 88: 35-51. DOI: 10.1016/j.hydromet.2007.04.003.
[33] WANG Jun, LIAO Rui, TAO Lang, ZHAO Hong-bo, ZHAI Rui, QIN Wen-qing, QIU Guan-zhou. A comprehensive utilization of silver-bearing solid wastes in chalcopyrite bioleaching [J]. Hydrometallurgy, 2017, 169: 152-157. DOI: 10.1016/j.hydromet.2017.01.006.
[34] ZHAO Hong-bo, GAN Xiao-wen, WANG Jun, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Stepwise bioleaching of Cu-Zn mixed ores with comprehensive utilization of silver-bearing solid waste through a new technique process [J]. Hydrometallurgy, 2017, 171: 374-386. DOI: 10.1016/ j.hydromet.2017.06.002.
[35] GHAHREMANINEZHAD A, RADZINSKI R, GHEORGHIU T, DIXON D G, ASSELIN E. A model for silver ion catalysis of chalcopyrite (CuFeS2) dissolution [J]. Hydrometallurgy, 2015, 155: 95-104. DOI: 10.1016/ j.hydromet.2015.04.011.
[36] MILLER J, PORTILLO H. In silver catalysis in ferric sulphate leaching of chalcopyrite [C]// 13th International Mineral Processing Congress: Part A. Amsterdam: Elsevier, 1979: 851-901.
[37] HIROYOSHI N, ARAI M, MIKI H, TSUNEKAWA M, HIRAJIMA T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2002, 63: 257-267. DOI: 10.1016/S0304-386X(01)00228-6.
[38] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part III: Effect of redox potential on the silver-catalyzed process [J]. Hydrometallurgy, 2008, 93: 97-105. DOI: 10.1016/j.hydromet.2007.11.006.
[39] WANG M, ZHANG Y, DENG T, WANG K. Kinetic modeling for the bacterial leaching of chalcopyrite catalyzed by silver ions [J]. Minerals Engineering, 2004, 17: 943-947. DOI: 10.1016/j.mineng.2003.11.021.
[40] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential [J]. Hydrometallurgy, 2008, 93: 88-96. DOI: 10.1016/j.hydromet. 2008.04.016.
[41] LEVENSPIEL O. Chemical reaction engineering [M]. 3rd edition. New York: John Wiley & Sons Incorporation, 1999.
[42] BEVILAQUA D, LAHTI-TOMMILA H, GARCIA O Jr, PUHAKKA J A, TUOVINEN O H. Bacterial and chemical leaching of chalcopyrite concentrates as affected by the redox potential and ferric/ferrous iron ratio at 22 °C [J]. International Journal of Mineral Processing, 2014, 132: 1-7. DOI: 10.1016/j.minpro.2014.08.008.
[43] ZHAO Hong-bo, WANG Jun, YANG Cong-ren, HU Ming-hao, GAN Xiao-wen, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Effect of redox potential on bioleaching of chalcopyrite by moderately thermophilic bacteria: An emphasis on solution compositions [J]. Hydrometallurgy, 2015, 151: 141-150. DOI: 10.1016/j.hydromet.2014.11.009.
[44] ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, HU Ming-hao, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Role of pyrite in sulfuric acid leaching of chalcopyrite: An elimination of polysulfide by controlling redox potential [J]. Hydrometallurgy, 2016, 164: 159-165. DOI: 10.1016/ j.hydromet.2016.04.013
[45] ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, ZHENG Xi-hua, TAO Lang, HU Ming-hao, LI Yi-ni, QIN Wen-qing, QIU Guan-zhou. Effects of pyrite and bornite on bioleaching of two different types of chalcopyrite in the presence of Leptospirillum ferriphilum [J]. Bioresource Technology, 2015, 194: 28-35. DOI: 10.1016/j.biortech.2015.07.003.
[46] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, GONZALEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part IV: The role of redox potential in the presence of mesophilic and thermophilic bacteria [J]. Hydrometallurgy, 2008, 93: 106-115. DOI: 10.1016/ j.hydromet.2007.11.005.
[47] VILCAEZ J, SUTO K, INOUE C. Bioleaching of chalcopyrite with thermophiles: Temperature–pH–ORP dependence [J]. International Journal of Mineral Processing, 2008, 88: 37-44. DOI: 10.1016/j.minpro.2008.06.002.
[48] DREISINGER D, ABED N. A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: Kinetic analysis [J]. Hydrometallurgy, 2002, 66: 37-57. DOI: 10.1016/S0304-386X(02)00079-8.
[49] GHAHREMANINEZHAD A, DIXON D G, ASSELIN E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution [J]. Electrochimica Acta, 2013, 87: 97-112. DOI: 10.1016/j.electacta.2012.07.119.
[50] WU Z H, DREISINGER D B, URCH H, FASSBENDER S. The kinetics of leaching galena concentrates with ferric methanesulfonate solution [J]. Hydrometallurgy, 2014, 142: 121-130. DOI: 10.1016/j.hydromet.2013.10.017.
(Edited by YANG Hua)
中文导读
微生物作用下含银固体废弃物催化黄铜矿浸出动力学研究
摘要:银能有效地催化黄铜矿浸出,本文使用含银固体废弃物作为催化剂,研究了不同黄铜矿/含银固体废弃物配比下的浸出动力学行为。体系的生物浸出行为表明,含银固体废弃物能有效催化浸出黄铜矿。固体废弃物的加入使得体系的氧化还原电位远高于其他学者所提出的最适电位区间(380~480 mV vs Ag/AgCl),体系浸出温度与铜浸出率呈正相关性。在此浸出条件下,黄铜矿的生物浸出主要由扩散控制,表面活化能Ea为28.24 kJ/mol。本工作对推广微生物作用下银催化黄铜矿浸出的工业应用有参考价值。
关键词:黄铜矿;含银固体废弃物;生物浸出;动力学
Foundation item: Project(2018JJ1041) supported by the Natural Science Foundation of Hunan, China; Projects(51774332, U1932129, 51804350 and 51934009) supported by the National Natural Science Foundation of China
Received date: 2019-06-28; Accepted date: 2020-04-28
Corresponding author: WANG Jun, PhD, Professor; Tel: +86-731-88876557; Email:wjwq2000@126.com; ORCID: 0000-0003-0931- 3946
Abstract: Silver ion can be useful in improving chalcopyrite bioleaching efficiency. In this work, leaching kinetics of this process was investigated using silver-bearing solid waste under different chalcopyrite/solid waste ratios. Bioleaching behavior indicates that silver-bearing solid waste can enhance the bioleaching process, and the redox potential is much higher than the proposed appropriate range (380-480 mV vs Ag/AgCl) with the solid waste added. There is a positive correlation between temperature and copper extraction rate. The kinetics data fit well with the shrinking-core model. Under these leaching conditions, the bioleaching of chalcopyrite is controlled by internal diffusion with calculated apparent activation energy (Ea) of 28.24 kJ/mol. This work is possible benificial to promote the industrial application of silver catalyst in leaching of chalcopyrite.


