Trans. Nonferrous Met. Soc. China 29(2019) 242-252
Thermal stability of nanocrystalline Al-10Fe-5Cr bulk alloy
Muneer BAIG1, Hany R. AMMAR2,3, Asiful H. SEIKH4, Jabair A. MOHAMMED4, Fahad AL-MUFADI2, Abdulaziz ALABOODI2
1. Engineering Management Department, College of Engineering, Prince Sultan University, Riyadh, KSA;
2. Mechanical Engineering Department, College of Engineering, Qassim University, Buraidah, KSA;
3. Metallurgical and Materials Engineering Department, Faculty of Petroleum and Mining Engineering, Suez University, Suez, Egypt;
4. Center of Excellence for Research in Engineering Material, King Saud University, Riyadh, KSA
Received 5 April 2018; accepted 12 October 2018
Abstract:
Thermal stability of nanocrystalline Al-10wt.%Fe-5wt.%Cr bulk alloy was investigated. The initial micro-grained mixture of powders was processed for 100 h using mechanical alloying (MA) to produce nano-grained alloy. The processed powders were sintered using high frequency induction heat sintering (HFIHS). The microstructures of the processed alloy in the form of powders and bulk samples were investigated using XRD, FESEM and HRTEM. Microhardness and compression tests were conducted on the bulk samples for evaluating their mechanical properties. To evaluate the thermal stability of the bulk samples, they were experimented at 573, 623, 673 and 723 K under compression load at strain rates of 1×10-1 and 1×10-2 s-1. The annealed samples exhibited a significant increase in their microhardness value of 2.65 GPa when being annealed at 723 K, as compared to 2.25 GPa of the as-sintered alloy. The bulk alloy revealed compressive strengths of 520 MPa and 450 MPa at 300 K and 723 K, respectively, when applying a strain rate of 1×10-1 s-1. The microstructural stability of the bulk alloy was ascribed to the formation of iron and chromium containing phases with Al such as Al6Fe, Al13Fe4 and Al13Cr2, in addition to the supersaturated solid solution (SSSS) of Cr and Fe in Al matrix.
Key words:
nanocrystalline; Al-Fe-Cr alloy; mechanical alloying; consolidation; thermal stability;
1 Introduction
Aluminum (Al) and its alloys are important materials in increasing demand due to their appealing mechanical and corrosion-resistant properties and high specific strength. However, there is an increasing demand to produce new Al alloys that exhibit higher specific strength. One of the prominent ways to improve the strength of Al alloys is the selective addition of transition metals (TM). The increase in the strength of Al-TM alloy is attributed to the formation of super- saturated solid solution (SSSS). Several investigations have been performed earlier to study the properties of the alloys with incorporation of TM [1-8]. The results obtained in these investigations have shown an increase in the mechanical properties of the alloys. In another investigation [9], it is shown that the strength of the Al alloy increased with the volume fraction of the alloying elements. Thus, to produce new Al alloys with increased strength, it is desirable to produce alloys with higher content of TM. However, it is known that the solubility of TM in pure metals even at elevated temperature is limited to 0.03% if the traditional manufacturing processes are employed [10]. The effect of Mg addition on the mechanical and thermo-electrical properties of Al-Al2O3 nanocomposite was studied and it was noticed that the Mg addition resulted in enhancing the mechanical and thermal properties of the Al-based nanocomposite [11].
Another way to produce high strength Al alloys is to improve the traditional or conventional manufacturing processes. In the last decade, significant progress has been made in investigating alternate manufacturing techniques that help to extend the limit of solubility of the TM in pure material. These techniques include chemical vapor deposition [12-14], rapid solidification [15,16] and mechanical alloying [17-21]. In addition toextending the limit of solubility, the mechanical alloying (MA) process further leads to the grain refinement.
In general, the yield strength of engineering material is related to its average grain size through the Hall-Petch relationship [22,23]. Based on this relationship, several studies and theories [24-26] have been proposed that relate the strength of the material to microstructure-based models. In addition to these theories, several investigations were performed based on the direct application of Hall-Petch relationship. It is deemed imperative to produce nanocrystalline (NC) materials from the coarse-grained polycrystalline materials as a means to improve the mechanical properties. The NC materials are known to exhibit excellent mechanical properties at room temperature. However, the properties of such materials tend to deteriorate upon exposure to elevated temperatures. It is observed that loss in mechanical properties of NC materials at elevated temperatures is largely associated to the grain growth mechanism. The NC materials, in general, tend to be unstable due to the possibility of grain growth when being subjected to mechanical or thermal loads [27]. In some investigations, a significant grain growth was observed in NC materials even at lower temperatures [28], but others suggested that the grain growth in NC materials due to thermal loads is limited at intermediate temperatures [29,30]. The grain growth in NC materials is usually attributed to the grain boundary diffusion, which tends to be more active at higher temperatures [31]. Also, substantial decomposition of microstructure takes place at higher temperatures, leading to the decrease in thermal stability. The grain growth mechanism in combination with the microstructural instability at elevated temperatures is responsible for altering the mechanical properties of the NC materials. Thus, the studies of the thermal stability of these alloys have been an area of significant interest lately [20,32-34]. An investigation into the thermal stability of NC AA5083 prepared by cryomilling revealed the stability of the alloy up to 0.61Tm. The achieved stability was associated to Zener pinning and solute drag mechanisms [35]. Another investigation on the thermal stability of ultrafine structured Al 2014 alloy, revealed the stability of the microstructure to 0.55Tm. The thermal stability of the alloy in this case was associated to pinning action of secondary precipitates along the grain boundaries [36].
The present study was accomplished to study two aspects: production of bulk NC Al-10wt.%Fe-5wt.%Cr alloys and thermal stability of the alloys at elevated-temperatures. The thermal stability of the fabricated alloy was determined using high temperature compression experiments. In addition, the current study further explores the synergistic effect of annealing temperature on the elevated-temperature mechanical properties of the bulk alloy.
2 Experimental
The starting mixture of high purity (>99.9%) elemental powders used in the present study includes 85 wt.% Al, 10 wt.% Fe and 5 wt.% Cr. Prior to mechanical alloying (MA), the starting mixture was degassed for 24 h at 373 K in vacuum. The degassed mixture was cooled to room temperature in the oven. The mixture was later poured into the mill containers (vials) with 10 mm-in-diameter stainless steel balls under controlled atmosphere. The ball-to-powder mass ratio (BPR) was selected to be 10:1. The MA of the mixture was accomplished in a planetary ball mill set to 120 r/min. To avoid excessive heating, milling for 15 min was alternated with hold time of 15 min. It is well known that during MA, the metal powders are subjected to recurrent cycles of welding, splintering and re-welding [37]. This process leads to the formation of clusters of powder particles. Process control agents (PCA) are usually used to avoid the formation of clusters during milling process [38-40]. In this study, 1 wt.% stearic acid (PCA) was used to avoid the formation of powder clusters. The milled powder was charged into a graphite mold retained under controlled atmosphere in a glove box. The graphite mold used was 40 mm in diameter and length with a through hole of 10 mm in diameter. A thin graphite wafer was used to cover the upper and lower surface of the charged powders in the graphite mold to protect these powders from contaminating during the transportation to the sintering machine. The powder samples were consolidated in a high-frequency induction heat sintering (HFIHS) machine. The rate of heating and the temperature of sintering were 823 K/min and 823 K, respectively. Upon reaching 823 K, the sintering was accomplished for 6 min, while keeping a pressure of 50 MPa. The experimental density of the bulk samples was evaluated based on Archimedes principle. The density of the bulk sample was measured to be 2.94 g/cm3.
The measurements of Vickers microhardness were accomplished on the bulk samples using Buehler microhardness tester. The indentation load was 1 N. Before taking the measurements, the samples were ground and polished using sand papers. To obtain a good quality surface, the samples were finally polished in a 0.1 μm colloidal silica solution. A number of hardness measurements were performed on the sample surface, each separated by a distance of 0.25 mm along the diameter of the sample.
To study the thermal stability, the bulk samples were experimented under compression load at strain rates of 1×10-1 and 1×10-2 s-1, and the tests were conducted at 573, 623, 673 and 723 K. Since it is difficult to measure the strain using strain gage during high temperature experiment, the machine displacement was corrected for compliance. The strain was calculated using the corrected displacement data. A temperature-resistant grease lubricant, produced by Dow Corning, was used to reduce the effect of friction during the experiment. The specimen was heated to the desired temperature, before carrying out the test, using a high temperature furnace and maintained for 30 min at that temperature to ensure the homogeneity of the temperature through the sample. A thermocouple (J-type) was attached onto the specimen surface to further ensure the accuracy of the sample temperature.
The sample surfaces were finely polished with different grit sand papers starting with a coarse and gradually to fine polishing using the colloidal silica solution. The XRD was performed on the polished sample using a Discover D8 diffractometer using the standard Cu Kα (λ=0.154 nm) radiation. The correction for instrument broadening was performed using a standard silicon sample with an assumption of Gaussian function for the instrument and sample. The peak width βcorrsample(2θ) after the instrument correction was calculated using the equation provided by earlier investigation [41]:
β2corrsample(2θ)=β2sample(2θ)-β2stdsample(2θ) (1)
where βsample(2θ) and βstdsample(2θ) represent the measured peak width of the sample and the peak width of the standard sample, respectively.
The scan rate of the sample surface was 2 (°)/min for a 2θ range from 30° to 90°. It should be noted that the broadening of XRD profiles exhibited by low angle reflection is governed by small grain size while the higher angle reflection corresponds to the internal strain [42]. Since the MA process involves the imposition of lattice strain in the milled powders, it is imperative to incorporate the contribution of the lattice strain in the calculation of the average crystallite size. Thus, in this study, the average crystallite size and lattice strain were calculated using the Williamson-Hall analysis. The lattice strain ε present in the milled powder can be calculated using the relation:
 (2)
(2)
where βhkl represents the measured full width half maximum (FWHM) value of the peak (rad). The details of the analysis can be found elsewhere [43].
The microstructures of the as-mixed and milled powders in addition to the bulk samples were characterized using JEOL model JSM-6610LV FESEM containing an energy dispersive X-ray analyzer and JEM-2100F HRTEM. The elemental composition of the sintered alloy was determined using elemental mapping by means of energy dispersive X-ray. The sample was prepared by sintering the MA powder at 823 K for 6 min without the application of pressure (pressureless sintering). The pressureless sintering followed in this investigation did not result in the formation of the bulk sample. The pressureless sintering resulted in the formation of sintered powder that was later used to perform elemental mapping. The samples for TEM investigation were prepared by sonicating a small quantity of the pressureless sintered powders in ethanol. The sonication was performed for 15 min using a high frequency probe sonicator. Thereafter, a drop of the sonicated solution was dispensed on a carbon wafer, which was later dried to evaporate the solution. To further validate the thermal stability of the developed alloy, the sintered samples were annealed at 673, 723 and 773 K in vacuum for 30 min.
3 Results
3.1 Microstructure characteristics of processed alloys
Figure 1 shows the morphologies of the starting powders including Al, Fe and Cr along with the milled mixture for 100 h. A spherical Al powder was used with an average diameter of 3 μm, as shown in Fig. 1(a). The as-received Fe and Cr powders were spherically shaped with an average particle size of 45 μm. These powders were independently milled for 6 h in an attritor under the inert atmosphere for reducing the difference in the particle size between Al and the alloying elements (Fe and Cr). After milling for 6 h, the particle size of Fe and Cr powders reduced to 8 and 10 μm, respectively, while the morphology of these powders changed to flake-like shape, as observed in Figs. 1(b, c). It is well known that milling process induces significant strain on the powder particles as a result of repetitive welding, fracturing and re-welding process. As observed from Fig. 1(d), the morphology of the alloyed mixture (after 100 h) was also in the form of flakes. The modification in the morphology of the milled powders (spherical to flakes) could be ascribed to the significant straining of the powders during the milling process.
The XRD patterns of the as-mixed and mechanically alloyed powders (after 100 h of milling) are shown in Fig. 2. The major peaks detected were observed with be associated to the diffraction peaks of Al. However, the major diffraction peaks of Fe (110) and Cr (110) were not observed in the XRD profiles probably due to their overlapping with Al (200). Al peaks was observed to shift to a higher 2θ angles in the milled powder when being compared to the as-mixed powder. The observed 2θ peak shift shown in Table 1 can be associated to the dissolution of the alloying elements Fe and Cr to form Al based supersaturated solid solution (SSSS). In addition to the peak shift, a considerable decrease in the peak intensities along with broadening of the peaks was also observed in the XRD peak profile of the alloyed powder. These observations imply that the MA of the powders resulted in refining the structure and increasing the lattice strain.

Fig. 1 SEM images of powders

Fig. 2 XRD patterns of as-mixed and milled (for 100 h) powders of Al-10wt.%Fe-5wt.%Cr
Table 2 displays the variation in the values, cal- culated using the Williamson-Hall analysis, of crystallite size and lattice strain of the milled powder and the sintered bulk alloy. In this analysis, Al peaks (111), (200) and (220) were considered in calculating the crystallite size and the accumulated lattice strain. For the 100 h-milled powder, the estimated average crystallite size was (53±2) nm while the crystallite size of (56±2) nm was found in the bulk sintered alloy. The average lattice strain for the milled powder and the sintered bulk alloy were found to be 0.001479 and 0.0013495, respectively. The decrease in the lattice strain of the sintered bulk samples could be due to the annealing that occurred during the sintering process.
Table 1 XRD peak position before and after milling

Table 2 Variation in crystallite size and lattice strain of as-milled and sintered alloy obtained using Williamson-Hall analysis
FESEM-energy dispersive X-ray (EDX) analysis was performed on the milled powder to confirm the existence of Fe and Cr in the Al matrix. The result obtained from the EDX is shown in Fig. 3, which refers to the existence of Fe and Cr in the milled powder. The mass fractions of Al, Fe and Cr in the selected spectrum were found to be 84.5%, 10.64% and 4.86%, respectively. The EDX results alone are not enough to confirm the formation of SSSS. However, the results of EDX analysis were in good agreement with the XRD results of the milled powder. Thus, it can be assumed that alloying of 10 wt. %Fe and 5 wt.% Cr with Al led to the dissolution of Fe and Cr in Al base matrix, resulting in the formation of aluminum super-saturated solid solution (SSSS).

Fig. 3 SEM micrograph and its corresponding elemental mapping using EDX for 100 h-milled powders
Figure 4 displays the XRD patterns of the milled powder and the sintered Al-10wt.%Fe-5wt.%Cr (bulk) alloy produced through the sintering of the milled powder in HFIHS system. On comparing the XRD results of the milled powder, the evolution of new peaks was observed in the XRD profile of the bulk alloy (in Fig. 4). A similar observation was made in earlier investigations [9,44]. Based on the Al-Fe phase equilibrium diagram, it is expected to find α(Al) and stable Al3Fe phase in the bulk alloy. However, the existence of Al3Fe phase was not established in the current investigation. This could be due to the MA process itself where the material is subjected to severe straining and also due to the high temperatures that are encountered during the sintering process [9]. It should be noted that the sintering time and temperature selected in this study were optimized so as to increase the precipitation of the intermetallics without significant grain growth in the sintered alloy. On comparing the XRD peak profile of the sintered alloy with the standard data on the ICDD database, the new peaks were correlated to the standard peak of Al6Fe intermetallics, confirming the precipitation of the secondary phases during the sintering process.
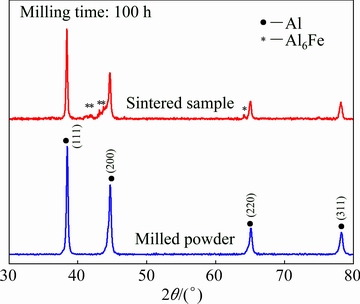
Fig. 4 XRD patterns of sintered Al-10wt.%Fe-5wt.%Cr alloy in comparison with 100 h-milled powder
Figure 5 illustrates the backscattered SEM image of Al-10wt.%Fe-5wt.%Cr sintered powder (pressureless sintered) along with its corresponding elemental mapping. Figure 5(a) illustrates the SEM image of the sintered powder sample. Figure 5(b) displays the contrast SEM image illustrating the Al distribution in the sintered sample. It is observed that Al is dispersed homogeneously throughout the scanned area. Similarly, Figs. 5(c) and (d) indicate the distributions of Fe and Cr in the matrix of Al, respectively. Based on the results from the elemental mapping of the sintered sample, it could be interpreted that the MA performed in this study resulted in the homogeneous dissolution of the alloying elements into the matrix.
Figure 6 shows the high resolution micrograph of the sintered powder (pressureless sintering) obtained from the HRTEM. The sintered powder for TEM imaging was prepared by performing pressureless sintering wherein; the processed powder was first charged in the graphite die, followed by sintering at 823 K for 6 min at a heating rate of 823 K/min. This is the similar sintering condition that was used to produce the bulk sample. It is observed that the sintered powder consists of NC aluminum. From the micrograph shown in Fig. 6, the crystallite size of the pressureless sintered powder was found to be in the range of 15-30 nm. The average crystallite size of the sintered bulk alloy calculated using the XRD data was found to be (34±3) nm. In addition, the existence of sharp and bright rings in the SAD pattern confirms the crystallinity of the sintered alloy and specifies the existence of NC particles.

Fig. 5 Elemental mapping of sintered powder (pressureless sintering) obtained from 100 h ball milling
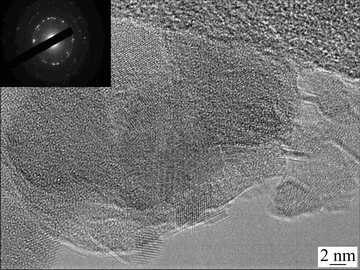
Fig. 6 HRTEM micrograph of sintered powder after 100 h milling along with SAD pattern (insert)
3.2 Hardness and compressive stress-strain behavior of processed bulk alloy

Fig. 7 Variation in hardness of sintered alloy
Figure 7 shows the hardness distribution profiles obtained on pure Al, Al-10wt.%Fe and Al-10wt.%Fe- 5wt.%Cr bulk alloys. As expected, the hardness of the alloy increased with the addition of TM as alloying elements. It is also noticed that the sintered alloys displayed similar behavior in the hardness values when being compared with the pure Al. This is due to the fact that the 100 h MA resulted in homogeneous dispersion of Fe and Cr atoms in the Al matrix. This implies that the processed alloys by mechanical milling for long duration could reveal similar properties through the entire sintered samples. The maximum hardness of 2.25 GPa was exhibited by Al-10wt.%Fe-5wt.%Cr alloy. The hardness increased by over 6 times when being compared to the hardness of pure Al sample. The significant increase in the hardness values from 0.34 to 1.7 and to 2.25 GPa for Al, Al-10wt.%Fe and Al-10wt.%Fe-5wt.%Cr bulk alloys, respectively, is attributed to the influence of adding alloying elements such as Fe and Cr to pure Al. The addition of Fe and Cr to pure Al resulted in several hardening mechanisms in the alloys. In the case of Al-10wt.%Fe bulk alloy, the hardness was observed to increase to 1.7 GPa, as compared to 0.34 GPa in the case of pure Al. This increase in hardness is related to the formation of a super-saturated solid solution of Fe in Al and the precipitation of intermetallic phases such as Al6Fe and Al13Fe4. The addition of Cr to produce Al-10wt.%Fe-5wt.%Cr bulk alloy resulted in a further increase in the hardness to 2.25 GPa, which is attributed to the additional effect of solid solution hardening due to the dissolution of Fe and Cr in the Al matrix and also an effective hardening mechanisms due to the presence of several intermetallic phases in the Al matrix such as Al6Fe, Al13Fe4 and Al13Cr2. These hardening mechanisms resist the dislocation motion and increase the hardness of the bulk alloys under study. It should be empathized that additional hardening mechanisms are active in the alloys under investigation due to the application of MA process which leads to the formation of nanocrystalline bulk alloys (grain size hardening) with a considerable lattice strain (strain hardening).
To further demonstrate the thermal stability of the developed alloy, compressive experiments were performed on the alloy at a constant engineering strain rate of 1×10-1 and 1×10-2 s-1. The temperatures experimented include 573, 623, 673 and 723 K. Three samples were experimented for each temperature and a good repeatability was observed in each experiment. Figure 8 shows the high temperature compressive true stress-strain of Al-10wt.%Fe-5wt.%Cr sintered alloy at a constant engineering strain rate of 1×10-1 s-1. From the experimental results, it is noticed that the sintered alloy exhibited enhanced thermal stability even at elevated temperatures up to 723 K. Similar behavior was observed from the high temperature experiments performed at a strain rate of 1×10-2 s-1, as shown in Fig. 9. The strain to failure was found to increase with an increase in temperature up to 723 K.

Fig. 8 True compressive stress-strain responses of sintered alloy at different temperatures and strain rate of 1×10-1 s-1

Fig. 9 True compressive stress-strain responses of sintered alloy at different temperatures and strain rate of 1×10-2 s-1

Fig. 10 Variation in yield strength of Al-10wt.%Fe-5wt.%Cr alloy with experimental temperature at different strain rates
Figure 10 shows the effect of test temperature on the yield strength of the alloy for the two strain rates. From the figure, it is noticed that the yield strength of the alloy decreased with an increase in the experimental temperature. The yield strength of the developed alloy at 723 K and at a strain rate of 1×10-2 s-1 was found to be ~430 MPa which is 10 times higher than the strength of an aviation alloy Al7085 (~40 MPa) at the same strain rate and temperature [45]. From this comparison, it is clear that the sintered alloy in this investigation exhibited more significant thermal stability than the high strength aviation alloy Al7085. The higher thermal stability is related to the addition of transition metals, producing nano-structured alloy and the precipitation of intermetallics during sintering process.
A further investigation of the formation of different intermetallic phases was accomplished. For this purpose, the sintered alloy was annealed at 673, 723 and 773 K for 30 min. This annealing time was selected based on the hold time (before performing the experiment) during the compression experiment. The XRD patterns of the annealed samples are shown in Fig. 11. From the figure, the presence of an orthorhombic metastable phase Al6Fe intermetallic phase was identified in the alloy after the sintering process. The intermetallic Al6Fe is reported to be a metastable phase. The annealing of sintered sample below 673 K for 30 min did not result in the formation of new intermetallic phases. However, at an annealing temperature of 673 K, the formation of a new stable Al13Fe4 intermetallic was observed along with the metastable Al6Fe intermetallic. For the annealing temperatures at 723 K and above this temperature, the precipitation of an additional intermetallic phase Al13Cr2 was also observed in the annealed alloy. The thermal stability of the alloy at elevated temperatures (shown in Figs. 8 and 9) could be attributed to the formation of thermally stable intermetallic phases Al6Fe, Al13Fe4, and Al13Cr2 in the bulk alloy before performing the compression experiment. The formation of these new phases is believed to be responsible for improving the stability of the microstructure of the alloy, thereby imparting significant strength to the alloy at elevated temperature up to 723 K.
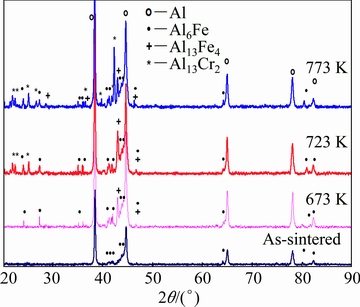
Fig. 11 XRD patterns of sintered Al-10wt.%Fe-5wt.%Cr alloy annealed at different temperatures for 30 min
To further investigate the variation in the crystallite size of the alloy after 30 min of annealing, the XRD patterns of the annealed powder samples were used to determine the crystallite size. Figure 12 illustrates the effect of annealing temperature on the crystallite size of the annealed alloy calculated using the Williamson-Hall analysis. From the figure, it is found that the crystallite size of the bulk alloy increased with an increase in annealing temperature with the maximum being 78 nm at 773 K. The limited grain growth at or below 573 K can be attributed to the solute drag. It is known that the formation of new particles has an influence on the grain growth mechanisms through the grain boundary pinning and Zener drag mechanisms [46]. The grain size stabilization in this investigation could be ascribed to the pinning of the grain boundary by Cr- and Fe-containing phases.

Fig. 12 Variation in crystallite size of sintered alloy with annealing temperature

Fig. 13 Variation in lattice strain of sintered alloy with annealing temperature
Figure 13 depicts the effects of annealing temperature on the lattice strain accumulated due to the milling process. The milling process introduces the lattice strain due to the repeated deformation/fracturing of powder particles. Thus, the lattice strain is expected to be higher in the processed powders. However, annealing tends to relieve the accumulated strain. As expected, the lattice strain was observed to decrease with an increase in annealing temperature. It was observed that the change in the lattice strain at high temperatures was not as significant as that noticed at lower temperature due to the fact that the retained lattice strain decreases as the annealing temperature increases.
Figure 14 shows the hardness plots of the annealed samples. It is observed that there exists a difference of about 100 MPa in the average hardness values of the samples annealed at 673 and 723 K. The improvement in the hardness of the annealed alloy when being compared with the pure Al may be related to the formation of SSSS of Fe and Cr in Al matrix, in addition to the evolution of the Fe- and Cr-containing phases during the sintering and followed by the annealing process. With regard to Fig. 11, it may be noticed that annealing the bulk samples resulted in forming intermetallic phases such as Al6Fe at 673 K, Al13Fe4 at 673 K and Al13Cr2 at 773 K. Increasing the temperature of annealing was observed to give more driving force for evolution of new intermetallic phases where all phases including Al6Fe, Al13Fe4 and Al13Cr2 were detected together only when the alloy was annealed at 723 K, as observed from the XRD pattern in Fig. 11. Accordingly, after annealing, the hardness was observed to increase due to the formation of the intermetallic phases which obstruct the dislocation motion and harden the material. A further increase in the annealing temperature from 673 to 773 K was observed to display an additional increase in the hardness value due to the formation of new intermetallic phases which play the main role in hardening the alloy under study through restricting the dislocation motion. The compression test results presented in Figs. 8 and 9 are in a full agreement with the microhardness measurements shown in Fig. 14.

Fig. 14 Variation in Vickers microhardness of sintered alloy annealed at 673 K (a) and 723 K (b)
The majority of the earlier studies in developing nanocrystalline alloys were based on the analysis and characterization of the milled powder. However, the metallic bulk sample was prepared from the milled powders in the present investigation. These samples were used to characterize the thermal stability of the developed alloy through high temperature compression experiments. Based on the experimental results obtained in this investigation from XRD, uniaxial compression experiments at high temperatures and Vickers microhardness, it can be concluded that the developed nanocrystalline alloy showed enhanced strength at extremely high temperatures (above 0.5Tm). The attainment of higher thermal stability of the developed alloy is related to the precipitation of new phases during sintering and through a process of controlled annealing. Furthermore, the formation of SSSS of low diffusivity elements, Fe and Cr, in Al matrix is another contributing mechanism in improving the thermal stability of the developed alloy.
4 Discussion
The transition metals (TMs), Fe and Cr, have been selected as alloying elements in the current investigation. These alloys are known to have limited diffusivity and solubility in Al under equilibrium conditions, which is suitable to prevent the Ostwald ripening [47] during the sintering process and could lead to the production of materials that exhibit excellent thermo-mechanical properties. In addition, due to their limited solubility, the bulk alloy produced using the alloyed mixture is expected to display higher thermal stability levels than the alloy produced through the traditional methods.
In the current investigation, it is observed that the MA process leads to the formation of nanocrystalline Al-10wt.%Fe-5wt.%Cr alloy including the super- saturated solid solution of Fe and Cr. However, the super-saturated solutions are usually found to be metastable and result in the precipitation of the secondary phases or intermetallics, when being subjected to sintering followed by annealing at higher temperatures. With an increase in the annealing temperatures, the formation of several intermetallic phases was observed. These phases include the metastable Al6Fe at low annealing temperatures, and Al6Fe, Al13Fe4 and Al13Cr2. The existence of these phases in the microstructure resulted in the superior thermal stability of the alloy even at elevated temperatures.
The observed retardation in the grain growth at/or below 573 K (in Fig. 12) can be related to the solute drag mechanism, wherein the segregation of solute atoms at the grain boundaries tend to decrease the energy of grain boundary which is responsible for restricting the grain growth. However, at annealing temperatures above 573 K, the grain growth is controlled by the second phase or Zener drag mechanism.
Furthermore, the initial reduction in the lattice parameter of the alloy is an indication of the alloying elements dissolution into the Al matrix. A rather sharp increase in the lattice parameter of the annealed alloy from 573 K indicates that the alloying element Fe was mainly responsible for the decrease in the lattice parameter of the MA powders. The enhancement in the microhardness of the annealed alloy when being compared to the sintered alloy is also an indication for the precipitation of the hard intermetallics that are responsible for holding the microstructure of the alloy.
5 Conclusions
(1) The MA of initial mixture resulted in producing nanocrystalline Al-10wt.%Fe-5wt.%Cr alloy powders in the form of SSSS of Fe and Cr in α(Al).
(2) The precipitated metastable Al6Fe and the stable Al13Fe4, Al13Cr2 intermetallics provided the thermal stability to the alloy at elevated temperature up to 723 K. The alloy under study revealed a compressive strength of 520 MPa and 450 MPa at 300 K and 723 K, respectively, when applying a strain rate of 1×10-1 s-1.
(3) The yield strength of the sintered alloy was found to be 10 times higher than that of high strength Al7085 alloy at high temperature.
(4) Annealing of the bulk nanocrystalline alloy at 673 and 723 K for 30 min resulted in the precipitation of new phases, namely Al13Fe4 and Al13Cr2. The presence of these precipitates enhanced thermal stability of the alloy under study.
(5) The annealed samples showed a significant increase in their microhardness value of 2.65 GPa when being annealed at 723 K, as compared to 2.25 GPa of the as-sintered alloy.
Acknowledgments
This work was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12-NAN2635-02).
References
[1] MILEA A, GINGU O, PREDA S, SIMA G, NICOLICESCU C, TANASESCU S. Thermodynamic measurements on Ag-28%Cu nanopowders processed by mechanical alloying route [J]. Journal of Alloys and Compounds, 2015, 629: 214-220.
[2] NEAMTU B, CHICINAS H, MARINCA T, ISNARD O, CHICINAS I, POPA F. Synthesis of amorphous Fe75Si20-xMxB5 (M=Ti, Ta, Zr) via wet mechanical alloying and its structural, thermal and magnetic characterization [J]. Advanced Powder Technology, 2016, 27: 461-470.
[3] SHYNI P C, PERUMAL A. Structural and magnetic properties of nanocrystalline Fe-Co-Si alloy powders produced by mechanical alloying [J]. Journal of Alloys and Compounds, 2015, 648: 658-666.
[4] MAURYA R S, LAHA T. Effect of rare earth and transition metal elements on the glass forming ability of mechanical alloyed Al-TM-RE based amorphous alloys [J]. Journal of Materials Science and Technology, 2015, 31: 1118-1124.
[5] MUSU E, MURA G, LIGIOS G, DELOGU F. Formation of metastable solid solutions by mechanical alloying of immiscible Ag and Bi [J]. Journal of Alloys and Compounds, 2013, 576: 80-85.
[6] NUTHALAPATI M, KARAK S, BASU A. Synthesis and characterization of nano-Y2O3 dispersed Zr-based alloys by mechanical alloying and conventional sintering [J]. Materials Today: Proceedings, 2015, 2(4-5): 1109-1117.
[7] SLIMI M, AZABOU M, ESCODA L, SUNOL J, KHITOUNI M. Structural and microstructural properties of nanocrystalline Cu-Fe-Ni powders produced by mechanical alloying [J]. Powder Technology, 2014, 266: 262-267.
[8] YUAN Y, WANG Z, ZHENG R, HAO X, AMEYAMA K. Effect of mechanical alloying and sintering process on microstructure and mechanical properties of Al-Ni-Y-Co-La alloy [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 2251-2257.
[9] NAYAK S S, WOLLGARTEN M, BANHART J, PABI S, MURTY B. Nanocomposites and an extremely hard nanocrystalline intermetallic of Al-Fe alloys prepared by mechanical alloying [J]. Materials Science and Engineering A, 2010, 527: 2370-2378.
[10] KATTNER U, MASSALSKI T. Binary alloy phase diagrams [M]. OH: ASM International Material Park, 1990.
[11] WAGIH A. Effect of Mg addition on mechanical and thermo electrical properties of Al-Al2O3 nanocomposite [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 2810-2817.
[12] BRIEN V, KENZARI S, WEISBECKER P, WEBER S, MACHIZAUD F, DUBOIS J M. Synthesis, characterization and optical behaviour of vapour grown nano-crystalline Al-Cr-Fe-Mo thin films [J]. Journal of Alloys and Compounds, 2005, 391: 206-211.
[13] HU J, ZHANG F, WANG J, XIAO J Q. Synthesis of single- crystalline Fe nanowires using catalyst-assisted chemical vapor deposition [J]. Materials Letters, 2015, 160: 529-532.
[14] WANG X H, HUANG L Q, NIU L J, LI R B, FAN D H, ZHANG F B, CHEN Z W, WANG X, GUO Q X. The impacts of growth temperature on morphologies, compositions and optical properties of Mg-doped ZnO nanomaterials by chemical vapor deposition [J]. Journal of Alloys and Compounds, 2015, 622: 440-445.
[15] NAYAK S S, PABI S K, MURTY B S. Structure of nanocomposites of Al-Fe alloys prepared by mechanical alloying and rapid solidification processing [J]. Bulletin of Materials Science, 2008, 31: 449-454.
[16] HUANG Y, LI X, XU L, MENG C. One new method for preparing nanocrystalline metal [J]. Materials Letters, 2016, 166: 78-80.
[17] AL-JOUBORI A A, SURYANARAYANA C. Synthesis of metastable NiGe2 by mechanical alloying [J]. Materials & Design, 2015, 87: 520-526.
[18] MAULIK O, KUMAR V. Synthesis of AlFeCuCrMgx (x=0, 0.5, 1, 1.7) alloy powders by mechanical alloying [J]. Materials Characterization, 2015, 110: 116-125.
[19] MASMOUDI M, MHADHBI M, ESCODA L, SUNOL J, KHITOUNI M. Microstructural evolution and corrosion behavior of nanocrystalline FeAl synthesized by mechanical alloying [J]. Journal of Alloys and Compounds, 2016, 657: 330-335.
[20] BAIG M, AMMAR H R, SEIKH A H. Thermo-mechanical responses of nanocrystalline Al-Fe alloy processed using mechanical alloying and high frequency heat induction sintering [J]. Materials Science and Engineering A, 2016, 655: 132-141.
[21] YUAN Y, WANG Z, ZHENG R, HAO X, AMEYAMA K, MA C. Effect of mechanical alloying and sintering process on microstructure and mechanical properties of Al-Ni-Y-Co-La alloy [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 2251-2257.
[22] HALL E. The deformation and ageing of mild steel: III discussion of results [J]. Proceedings of the Physical Society: Section B, 1951: 64(9): 747-752.
[23] PETCH N. The cleavage strength of polycrystals [J]. Journal of the Iron and Steel Institute, 1953, 174: 25-28.
[24] COTTRELL A H. Theory of brittle fracture in steel and similar metals [J]. Transactions of Metallurgical Society AIME, 1958, 212: 192-203.
[25] CONRAD H, FEUERSTEIN S, RICE L. Effects of grain size on the dislocation density and flow stress of niobium [J]. Materials Science and Engineering, 1967, 2: 157-168.
[26] LI J C, CHOU Y T. The role of dislocations in the flow stress grain size relationships [J]. Metallurgical and Materials Transactions B, 1970, 1: 1145-1159.
[27] JEONG G, PARK J, KANG S, CHOI H. Strategies to suppress grain growth of nanocrystalline aluminum [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(S): s112-s118.
[28] KLEMENT U, ERB U, EL-SHERIK A, AUST K. Thermal stability of nanocrystalline Ni [J]. Materials Science and Engineering A, 1995, 203: 177-186.
[29] BAI X M, VOTER A F, HOAGLAND R G, NASTASI M, UBERUAGA B P. Efficient annealing of radiation damage near grain boundaries via interstitial emission [J]. Science, 2010, 327: 1631-1634.
[30] CHOI P, da SILVA M, KLEMENT U, AL-KASSAB T, KIRCHHEIM R. Thermal stability of electrodeposited nanocrystalline Co-1.1at.%P [J]. Acta Materialia, 2005, 53: 4473-4481.
[31] GLEITER H. Nanostructured materials: Basic concepts and microstructure [J]. Acta Materialia, 2000, 48: 1-29.
[32] ZHU J, WANG Y. Effect of Al addition on the glass forming ability, thermal stability and soft magnetic properties of (Fe0.83P0.16Cu0.01)100-xAlx nanocrystalline alloys [J]. Journal of Alloys and Compounds, 2015, 652: 220-224.
[33] SHAW L, LUO H, VILLEGAS J, MIRACLE D. Thermal stability of nanostructured Al93Fe3Cr2Ti2 alloys prepared via mechanical alloying [J]. Acta Materialia, 2003, 51: 2647-2663.
[34] RAJABI M, SEDIGHI R M, RABIEE S M. Thermal stability of nanocrystalline Mg-based alloys prepared via mechanical alloying [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 398-405.
[35] TELLKAMP V, LAVERNIA E, MELMED A. Mechanical behavior and microstructure of a thermally stable bulk nanostructured Al alloy [J]. Metallurgical and Materials Transactions A, 2001, 32: 2335-2343.
[36] DHAL A, PANIGRAHI S, SHUNMUGAM M. Precipitation phenomena, thermal stability and grain growth kinetics in an ultra-fine grained Al 2014 alloy after annealing treatment [J]. Journal of Alloys and Compounds, 2015, 649: 229-238.
[37] SURYANARAYANA C. Mechanical alloying and milling [J]. Progress in Materials Science, 2001, 46: 1-184.
[38] SURYANARAYANA C, FROES F. Nanocrystalline titanium- magnesium alloys through mechanical alloying [J]. Journal of Materials Research, 1990, 5: 1880-1886.
[39] WANG J S, DONNELLY S G, GODAVARTI P, KOCH C C. Microstructures and mechanical behaviour of mechanically alloyed nickel aluminide [J]. International Journal of Powder Metallurgy, 1988, 24: 315-325.
[40] KHAN A S, FARROKH B, TAKACS L. Effect of grain refinement on mechanical properties of ball-milled bulk aluminum [J]. Materials Science and Engineering A, 2008, 489: 77-84.
[41] WARREN B E. X-ray Diffraction [M]. Courier Corporation, 1969.
[42] WILLIAMSON G, HALL W. X-ray line broadening from filed aluminum and wolfram [J]. Acta Metallurgica, 1953, 1: 22-31.
[43] SIVASANKARAN S, SIVAPRASAD K, NARAYANASAMY R, SATYANARAYANA P V. X-ray peak broadening analysis of AA 6061100-x–x wt.% Al2O3 nanocomposite prepared by mechanical alloying [J]. Materials Characterization, 2011, 62: 661-672.
[44] JONES H. The status of rapid solidification of alloys in research and application [J]. Journal of Materials Science, 1984, 19: 1043-1076.
[45] PRABHU T. An overview of high-performance aircraft structural Al alloy-AA7085 [J]. Acta Metallurgica Sinica (English Letters), 2015, 28: 909-921.
[46] MORRIS M, MORRIS D. Microstructural refinement and associated strength of copper alloys obtained by mechanical alloying [J]. Materials Science and Engineering A, 1989, 111: 115-127.
[47] ZAWRAH M, SHAW L. Microstructure and hardness of anostructured Al-Fe-Cr-Ti alloys through mechanical alloying [J]. Materials Science and Engineering A, 2003, 355: 37-49.
纳米晶Al-10Fe-5Cr块体合金的热稳定性
Muneer BAIG1, Hany R. AMMAR2,3, Asiful H. SEIKH4, Jabair A. MOHAMMED4, Fahad AL-MUFADI2, Abdulaziz ALABOODI2
1. Engineering Management Department, College of Engineering, Prince Sultan University, Riyadh, KSA;
2. Mechanical Engineering Department, College of Engineering, Qassim University, Buraidah, KSA;
3. Metallurgical and Materials Engineering Department, Faculty of Petroleum and Mining Engineering, Suez University, Suez, Egypt;
4. Center of Excellence for Research in Engineering Material, King Saud University, Riyadh, KSA
摘 要:研究具有纳米晶的Al-10wt.%Fe-5wt.%Cr 块体合金的热稳定性。采用机械合金化(MA)方法对初始微晶粒混合粉末进行100 h的球磨,得到纳米晶粒合金粉末,然后用高频感应加热烧结法(HFIHS)烧结成块体材料。采用X射线衍射分析(XRD)、场发射扫描电镜(EFSEM)和高分辨透射电镜(HRTEM)对合金粉末样品和块体样品的显微结构进行表征。对块体样品进行显微硬度和压缩试验,以评价其力学性能。为评价块体试样的热稳定性,分别在573、623、673和723 K下进行压缩试验,应变速率为1×10-1和1×10-2 s-1。与烧结态合金相比,退火后的试样其显微硬度值显著提高,在723 K退火后显微硬度值为2.65 GPa,而烧结态的为2.25 GPa。当应变速率为 1×10-1 s-1时,块体合金在300和723 K下的抗压强度分别为520和450 MPa。块体合金的显微结构稳定性归因于与铝形成的如Al6Fe、Al13Fe4和Al13Cr2等含Fe和Cr相以及铝基体中含Cr和Fe的过饱和固溶体。
关键词:纳米晶;Al-Fe-Cr合金;机械合金化;固结;热稳定性
(Edited by Bing YANG)
Corresponding author: Hany R. AMMAR; E-mail: hanyammar@qec.edu.sa
DOI: 10.1016/S1003-6326(19)64933-2
Abstract: Thermal stability of nanocrystalline Al-10wt.%Fe-5wt.%Cr bulk alloy was investigated. The initial micro-grained mixture of powders was processed for 100 h using mechanical alloying (MA) to produce nano-grained alloy. The processed powders were sintered using high frequency induction heat sintering (HFIHS). The microstructures of the processed alloy in the form of powders and bulk samples were investigated using XRD, FESEM and HRTEM. Microhardness and compression tests were conducted on the bulk samples for evaluating their mechanical properties. To evaluate the thermal stability of the bulk samples, they were experimented at 573, 623, 673 and 723 K under compression load at strain rates of 1×10-1 and 1×10-2 s-1. The annealed samples exhibited a significant increase in their microhardness value of 2.65 GPa when being annealed at 723 K, as compared to 2.25 GPa of the as-sintered alloy. The bulk alloy revealed compressive strengths of 520 MPa and 450 MPa at 300 K and 723 K, respectively, when applying a strain rate of 1×10-1 s-1. The microstructural stability of the bulk alloy was ascribed to the formation of iron and chromium containing phases with Al such as Al6Fe, Al13Fe4 and Al13Cr2, in addition to the supersaturated solid solution (SSSS) of Cr and Fe in Al matrix.


