
Comparison of microstructure and corrosion properties of Al-Zn-Mg-Cu alloys 7150 and 7010
MENG Qing-chang(孟庆昌), FAN Xi-gang(樊喜刚), REN Shi-yu(任时宇),
ZHANG Xin-mei(张新梅), ZHANG Bao-you(张宝友)
Analysis and Measurement Center, Harbin Institute of Technology, Harbin 150001, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
The influence of coarse Cu-bearing particles, matrix and subgrain boundary precipitates on the stress corrosion susceptibility of the Al-Zn-Mg-Cu alloys was investigated. The strength of 7150 alloy is about 15 MPa higher than that of 7010 alloy. The 7010 alloy exhibits higher resistance to stress corrosion cracking as compared with the 7150 alloy. The coarse Cu-bearing particles are detrimental to the resistance to stress corrosion cracking. The increase of size of matrix and subgrain boundary precipitates decreases the susceptibility of stress corrosion. The anodic dissolution and hydrogen embrittlement govern the cracking process. The severity of stress corrosion cracking is shown to be related to the coarse Cu-bearing particles, matrix and subgrain precipitates in Al-Zn-Mg-Cu alloys.
Key words:
Al-Zn-Mg-Cu alloy; stress corrosion resistance; heat treatment; Al2CuMg;
1 Introduction
The Al-Zn-Mg-Cu alloys are widely used in aircraft application for structural parts such as frames and stringers, which are often susceptible to stress corrosion cracking (SCC) in the practical working environments [1-2]. Lots of investigations indicated that there are three main mechanisms for SCC in aluminium alloys. They are anodic dissolution, hydrogen induced cracking and passive film rupture [3-4]. But there are still many controversies on the proposed mechanisms that operate at a microscopic level for many materials.
Studies of stress corrosion attack have been mainly focused on three microstructural features including the precipitate free zone (PFZ), matrix precipitate structure, and grain boundary precipitate structure[5]. It is generally reported that the precipitate structure at the grain boundary is of primary importance to stress corrosion susceptibility. The increase of the grain boundary precipitate spacing and size will improve resistance to SCC[6]. Matrix precipitate structure has also been suggested as the source of susceptibility[7]. The precipitate coherency is responsible for the susceptibility of SCC. Overaging can increase the resistance to SCC. This is attributed to the homogeneous deformation through alteration on η' and η precipitate-dislocation interactions, an increase of the electrochemical nobility of η' and η phases[8]. The relative effect of precipitate free zone is still controversial. More studies show that wider PFZ will reduce the susceptibility to SCC[9]. Copper as one of the major alloying elements can influence the precipitation sequence and improve the mechanical properties and SCC resistance. It can alter the electrochemical properties of a region or a phase. Coarse copper bearing particles are often present in Al-Zn-Mg-Cu alloys, such as Al2Cu, Al2CuMg and Al7Cu2Fe [10-12]. These particles will bring a detrimental effect on the properties directly[13]. Furthermore, coarse copper bearing particles reduce the copper content in the solid solution or precipitates, and then decrease the effective influence of copper on the properties. Therefore, a detailed investigation on the effect of copper content on the strength and SCC in the Al-Zn-Mg-Cu alloys is necessary.
The present work seeks to clarify the role of microstructural factors and alloyed copper level to both the mechanical properties and stress corrosion susceptibility of Al-Zn-Mg-Cu alloys. Especially, the effect of Cu-bearing particles on the properties is investigated.
2 Experimental
The two alloys with different copper contents were provided in the hot extruded condition, as 25 mm×100 mm extrusion. The chemical composition of each alloy is given in Table 1.
Table 1 Chemical compositions of two alloys (mass fraction, %)
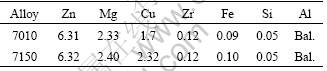
Samples were taken at the center position through the thickness direction of the extrusion. The samples were solution heat treated at 475 ℃ for 2 h, then quenched in water at approximately room temperature. Four aging heat treatments were used, as summarized in Table 2.
Table 2 Aging temper designations and heat treatments

Optical and scanning electron microscopy (SEM) were used to analyze the experimental results. For the transmission electron microscopy (TEM) analysis, the 3 mm discs about 70 μm in thickness were obtained, and electropolished in a double jet operated at 15 V and -20 ℃ with a 30% nitric acid and 70% methanol solution. The disks were observed in a Tecnai 12 microscope at 120 kV.
The stress corrosion test specimens were subjected to a constant load equivalent to 75% of the average yield stress. The specimens were tested in 3.5%NaCl+ 0.3%H2O2 solution. The tests were stopped when the SCC specimens did not rupture after testing for 720 h.
3 Results
3.1 Effect of temper and copper content on mechanical properties
The influences of the heat treatment conditions on the properties are summarized in Table 3. The yield strength of the two alloys evidently decreases from T6 to T73 tempered condition. The strength in the RRA condition is close to that in T6 condition. The yield strength of the 7150 alloy is about 15 MPa higher than that of the 7010 alloy in all tempers. The electrical conductivity of the 7010 and 7150 alloy at T6 tempered condition is as low as 31.5%IACS and 30.7%IACS, respectively. On the contrary, in RRA process, the conductivity is over 38%IACS while the yield strength is not inferior as compared to the case of T6 process.
Table 3 Properties of two alloys after different aging tempers
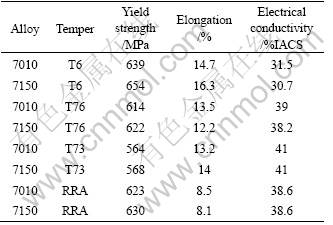
3.2 Effect of temper and copper content on SCC
The results of SCC tests are listed in Table 4. It can be seen that the two alloys fail within 30 d in the T6 condition. The 7150 alloy fails more rapidly than the 7010 alloy. It is obviously that the 7010 alloy exhibits better SCC resistance. In the T76 condition, only the 7150 alloy fails. The T73 and RRA tempered specimens do not fail within 30 d.
Table 4 SCC test results of two alloys for different aging tempers
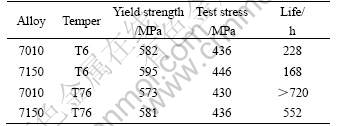
The exposed surfaces of the gauge length of the specimens are unevenly stained with white and dark gray corrosion product. This indicates that pitting corrosion has occurred. The wider defect can be clearly observed, as shown in Fig.1. The stress corrosion attack may initiate from some of these local defects.
Several cracks initiation sites are observed in the fracture surfaces. The crack surface of SCC is shown in Fig.2. Two specific regions can be distinguished: (a) near the external surface, a first region A with metal consumption that can be associated with crack initiation by anodic dissolution; (b) a deeper region B that can be associated with the crack propagation which is brittle in nature and occurs on crystallographic planes. Two types of cracks initiation sites can be observed: one is associated with the intense localized anodic dissolution of matrix, the other is associated with the constituent particles (Fig.2(b) and (d)). The cleavage-like fracture can be associated with crack propagation due to the hydrogen embrittlement[14].
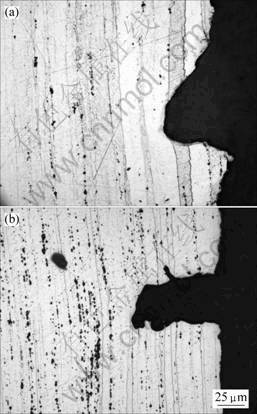
Fig.1 Cross sectional morphologies of SCC tested specimens for T6 condition: (a) 7010 alloy; (b) 7150 alloy
3.3 Microstructural characterization
The quantitatively measured results on the precipitate size and inter-particle spacing for the 7150 alloy samples aged at different tempers are summarized in Table 5. For the data of subgrain boundary precipitates, each datum point is the arithmetic mean of at least 100 measured data obtained at ten different subgrain boundaries. These results indicate that the precipitate size and inter-particle spacing increase with the increase of aging degree. Typical grain boundary precipitates for the 7150 alloy under various tempered conditions are shown in Fig.3. The RRA treatment shows the largest subgrain boundary precipitate size and spacing.
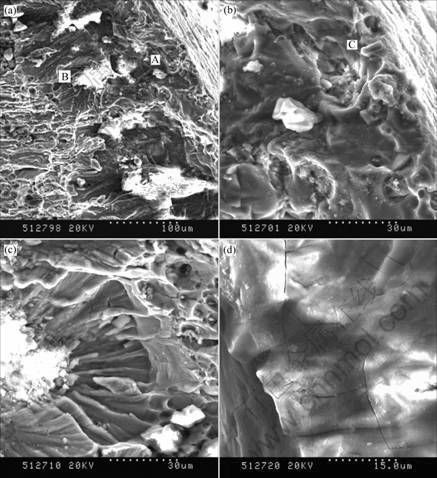
Fig.2 SEM images of crack surface for 7150-T76 alloy: (a) Typical fractograph of cracks; (b) Anodic dissolution associated with coarse particles; (c) Transgranular cleavage-like crack region; (d) Transgranular crack initiation (Region A: Crack initiation by localized anodic dissolution; Region B: Transgranular cleavage-like propagation induced by hydrogen embrittlement)
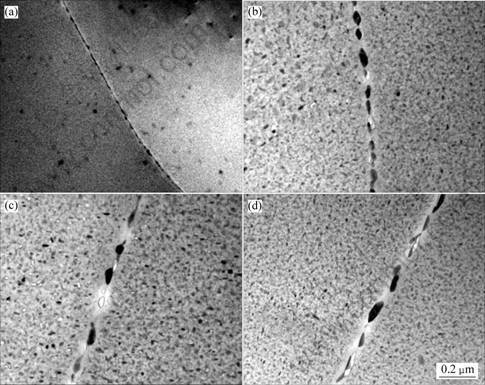
Fig.3 TEM photographs showing grain boundary precipitates under various heat treatment conditions
Table 5 Characteristics of microstructures for 7150 alloy under various heat treatment conditions

4 Discussion
4.1 Effect of microstructure
It can be observed that the elongated grains are perpendicular to the crack propagation direction(Fig.1), and the crack path is transgranular. So, the grain boundary structures exhibit a little contribution to the SCC. It should be noted that the intergranular crack is observed from the crack initiation regions(Fig.2(d)). The observation of fracture surface shows that the anodic dissolution and hydrogen embrittlement govern the cracking, as shown in Fig.2. The factors associated the anodic dissolution or hydrogen concentration of the extreme tip of critical defects will influence the stress corrosion behaviour[14].
The results show that the size and spacing of subgrain boundary precipitates for T76 temper increase greatly, as compared with those for T6 temper. At the same time, the size and amount of η' of the alloy for T76 temper are larger than those for T6 temper. So the alloy subjected to T76 temper exhibits a better resistance to stress corrosion, which may be attributed to the increase of the matrix and subgrain boundary precipitates size and the subgrain boundary precipitates spacing.
The susceptibility to SCC of the 7150 alloy for the T73 temper is reduced significantly as compared with that for the T76 temper. The matrix precipitate size increases greatly for the T73 tempered alloy. No significant difference of the size and spacing of grain boundary precipitates could be observed. In this case, it is indicated that the improvement of SCC resistance for T73 tempered alloy is mainly attributed to the coarsening of the matrix precipitates. The precipitation of η' or η phase within the grain reduces the potential difference between the grain boundary and the matrix, which decreases the anodic dissolution rate. Secondly, the increasing of size of η' or η precipitates can result in the reduction of planar slip and a more homogeneous slip mode. Hydrogen atoms are transported by dislocations until a critical concentration is reached locally to cause macroscopic embrittlement[8]. The homogeneous slip mode can effectively reduce hydrogen transporting rate and decrease the tendency to hydrogen embrittlement. Therefore, it appears that the increase of size and amounts of η' and η precipitates renders T73 more resistant to stress corrosion attack.
The lower electron conductivity implies that it may be difficult to achieve better stress corrosion resistance through T6 temper. On the contrary, the RRA treatment exhibits higher electrical conductivity, indicating that higher resistance to SCC may be achieved [2]. The results of SCC test show that the alloy in the RRA condition exhibits good resistance to SCC. It is noted that the matrix precipitates size of the RRA alloy is close to that of the T76 alloy. But the size and spacing of subgrain boundary precipitates increase significantly as compared with that of the T76 alloy. The larger η precipitates on the grain boundaries can act as trapping sites for atomic hydrogen, thereby reducing hydrogen concentration below a critical value to retard hydrogen embrittlement and improve the SCC resistance. It is indicated that the changing of subgrain boundary precipitates contributes to the improvement of stress corrosion resistance.
4.2 Effect of copper content on SCC
The strength of the 7010 alloy is about 15 MPa lower than that of the 7150 alloy in all tempers. This is attributed to the difference of copper content in the two alloys. The higher copper content in supersaturation solution increases the volume fraction of strengthening precipitates. Cu is added in the 7××× series alloys to improve the age hardenability as well as increase the resistance to SCC [10]. But in present experiment, the 7150 alloy with higher copper content exhibits higher susceptibility to SCC. It is found that the main difference between the two alloys is induced by the fraction of coarse Cu-bearing particles. The 7150 alloy contains higher fraction of coarse particles as compared with the 7010 alloy. This means that a great amount of copper is consumed by the coarse particles, leading to the decrease of copper content in the supersaturation solution. This will reduce the beneficial effect of copper on the resistance to SCC.
The coarse intermetallic particles play a crucial role in the corrosion behavior of aluminium alloys. It is reported that the anodic dissolution of matrix around Cu-bearing particles easily occurs during stress corrosion cracking process [15]. The 7150 alloy contains more Cu-bearing particles, which contributes to the pitting corrosion, as shown in Fig.2(b). The defects formed by localized anodic dissolution promote not only localization of plasticity but also a localized hydrogen discharge, entry and subsequent embrittlement. The hydrogen effects only take place after an initiation period related to the formation of critical defects by localized dissolution. So, fast anodic dissolution rate will promote the formations to critical defect, increasing the susceptibility to SCC. The 7010 alloy contains small amount of coarse particles, leading to the limitation of localized anodic dissolution. This delays the formation of critical defects for stress corrosion cracking, resulting in an improvement of the macroscopic SCC resistance.
5 Conclusions
1) The alloy with lower copper content exhibits better stress corrosion resistance. The coarse Cu-bearing particles decrease the aging hardenability and resistance to stress corrosion cracking.
2) The subgrain and matrix precipitates all play a significant role on the SCC resistance. For the alloy subjected to T76 temper, the increased resistance to SCC is attributed to the increase of the matrix and subgrain boundary precipitates size and the subgrain boundary precipitates spacing. For the alloy subjected to T73 temper, it is attributed to the increase of size and amounts of η' and η precipitates.
3) The RRA treatment can achieve a better combination of strength and resistance to stress corrosion cracking, resulting from a perfect microstructure with the combination of fine matrix precipitates and coarse subgrain boundary precipitates.
4) The SCC mechanism for the two types of Al-Zn-Mg-Cu alloys is associated with the anodic dissolution and hydrogen induced embrittlement. The anodic dissolution process plays a major role in the initiation and propagation of the crack.
References
[1] CHEN Kang-hua, LIU Yun-zhong, LIU Hong-wei. Microstructure and mechanical properties of enhanced solution treated 7075 and 2024 aluminium alloys [J]. The Chinese Journal of Nonferrous Metals, 2000, 10(6): 819-822. (in Chinese)
[2] YUE T M, YAN L J, CHAN C P, DONG C F, MAN H C, PANG G K H. Excimer laser surface treatment of aluminum alloy AA 7075 to improve corrosion resistance [J]. Surface and Coatings Technology, 2004, 179: 158-164.
[3] KURAMOTO S, OKAHANA J, KANNO M. Hydrogen assisted intergranular crack propagation during environmental embrittlement in an Al-Zn-Mg-Cu alloy [J]. Materials Transactions, 2001, 42(10): 2140-2143.
[4] JIN Y, LI C, RU J, YAN M. On the stress corrosion behavior of 7050 Al alloys [J]. Materials Letters, 1991, 12(5):376-380.
[5] LEE W, PYUM S. Role of prior cathodic polarization in the pitting corrosion of pure aluminium in acidic chloride solution [J]. Materials Science and Engineering A, 2000, 279: 130-137.
[6] ROBINSON J S. Influence of retrogression and reaging on the stress corrosion cracking resistance of 7010 [J]. Materials Science Forum, 2000, 331/337: 1653-1658.
[7] PUIGGALI M, ZIELINSKI A, OLIVE J M, RENAULD E, DESJARDINS D, CID M. Effect of microstructure on stress corrosion cracking of an Al-Zn-Mg-Cu alloy [J]. Corrosion Science, 1998, 40(4/5): 805-819.
[8] SONG R G, DIETZEL W, ZHANG B J, LIU W J, TSENG M K, ATRENS A. Stress corrosion cracking and hydrogen embrittlement of an Al-Zn-Mg-Cu alloy [J]. Acta Materialia, 2004, 52: 4727-4743.
[9] RAMGOPAL T. Role of Grain Boundary Precipitates and Solute Depleted Zone in the Intergranular Corrosion of Aluminum Alloy AA 7150[D]. The Ohio State University, 2001: 15.
[10] FAN X G, JIANG D M, MENG Q C, ZHANG B Y, WANG T. Evolution of eutectic structures in Al-Zn-Mg-Cu alloys during heat treatment [J]. Trans Nonferrous Met Soc China, 2006, 16: 577-581.
[11] LI X M, STARINK M J. Effect of compositional variations on characteristics of coarse intermetallic particles in overaged 7000 aluminium alloys [J]. Materials Science and Technology, 2001, 17: 1324-1328.
[12] CHEN K H, LIU H W, ZHANG Z, LI S, TODD R I. The improvement of constituent dissolution and mechanical properties of 7055 aluminum alloy by stepped heat treatments [J]. Journal of Materials Processing Technology, 2003, 142: 190-196.
[13] LI J F, ZHENG Z, JIANG N, TAN C. Localized corrosion mechanism of 2×××-series Al alloy containing S (Al2CuMg) and θ prime (Al2Cu) precipitates in 4.0% NaCl solution at pH 6.1 [J]. Materials Chemistry and Physics, 2005, 91(2-3): 325-329.
[14] NAJJAR D, MAGNIN T, WARNER T J. Influence of critical surface defects and localized competition between anodic dissolution and hydrogen effects during stress corrosion cracking of a 7050 aluminium alloy [J]. Materials Science and Engineering A, 1997, 238: 293-302.
[15] BUCHHEIT R G. Electrochemistry of θ (Al2Cu), S (Al2CuMg) and T1 (Al2CuLi) and localized corrosion and environment assisted cracking in high strength Al alloys [J]. Materials Science Forum, 2000, 331: 1641-1646.
(Edited by YUAN Sai-qian)
Foundation item: Project(2004AA5BG018) supported by the Science and Technology Development Fund of Harbin, China
Corresponding author: FAN Xi-gang; Tel: +86-451-86417617; E-mail: xigangfan@163.com


