
Environmentally friendly hybrid coating prepared by electro-deposition method at various seawater conditions
Myeong-Hoon LEE1, Kyung-Man MOON1, Jong-Do KIM1, Kang JUN1, Kwang-Ho KIM2
1. Division of Marine System Engineering, Korea Maritime University, Dongsam-Dong, Youngdo,
Busan, 606-791, Korea;
2. National Core Research Center for Hybrid Materials Solution, Pusan National University, Busan, 609-735, Korea
Received 2 March 2009; accepted 30 May 2009
Abstract:
The formation mechanism of calcareous deposit films in seawater involves an increase in pH at the metal/solution interface due to cathodic reactions, a raised carbonate ion concentration at the interface and precipitation of inorganic deposits such as CaCO3, Mg(OH)2. Environmentally friendly hybrid calcareous deposit films were formed by an electrochemical technique on steel substrates in various solution environments. And the influence of dissolved gas on formation of calcareous deposit films was investigated by scanning electron microscopy(SEM), energy dispersive spectroscopy(EDS) and X-ray diffractometry(XRD). Consequently, these results showed that formation of good overall calcareous deposited films by dissolved gas in seawater environments can be achieved by controlling the material composition and structure through effective use of electrochemical method.
Key words:
seawater; dissolved gas; electro-deposition; calcareous deposit coatings;
1 Introduction
Sea occupies three quarters of the earth and has various kinds of chemical ions. Meanwhile, some of these chemicals have corrosiveness for metal and others can be very useful in protecting metal from corrosion by means of electrochemical technique. Cathodic protection is one of such methods. The peculiar feature of cathodic protection in seawater is its capability to form mineral calcareous deposits such as Mg and Ca on metal surfaces. Here, mineral calcareous deposits can be ultimately obtained by lowering the potential of the metal to a higher negative value. The principles of calcareous deposit formation in seawater had been known for a long time. Cathodic current is assumed to increase OH- of the solution neighboring to the metal surface. These reactions increase the pH of the metal/seawater interface in accordance with the following formulae:
O2+2H2O+4e→4OH- (1)
or
2H2O+2e→H2+2OH- (2)
High pH causes precipitation of Mg(OH)2 and CaCO3 in accordance with the following formulae[1-2]:
Mg2++2OH-→Mg(OH)2 (3)
and
Ca2++ HCO3-+OH-→H2O+CaCO3 (4)
These deposit layers are very useful in controlling the corrosion of ferrous structures. And they obviously have several advantages compared with conventional coatings, since the environmentally friendly calcareous deposits are formed from elements (Ca2+, Mg2+, etc.) naturally present in seawater. However, there are some difficulties in having both fast deposition rate and strong adhesion between deposit films and metal substrate.
From this point of view, calcareous deposit films were formed using dissolved CO2 gas and the effect of dissolved gas on the formation of calcareous deposit films was investigated in this study.
2 Experimental
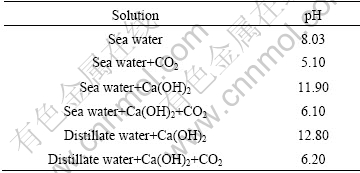
SPCC steel specimens were used for cathodic protection experiments in seawater. They were cut into 30 mm×40 mm×2 mm samples and a copper wire was soldered at the upper end of specimen. The surfaces of the samples were ground using sand paper starting with grit No.100 and finishing with grit No.600. The upper 10 mm part and backside of the specimen were coated with coated with epoxy resin. Carbon rods were used for the anodes with 100 mm in length and 7 mm in diameter. A steel specimen and a carbon rod anode were vertically fixed in it and the distance between the two was set as 1 cm. Used solutions were natural seawater and distillate water and Ca(OH)2 was added into solutions to increase Ca+. Calcareous deposit formation was conducted with supply of CO2 gas used to adjust pH of solution. The pH of solutions is presented in Table 1. All experiments were carried out in a closed system with agitation at 25 ℃ and the volume of solutions was 3 000 mL.
Table 1 Experimental conditions used in this study
The cathodic current density was kept at 10 mA/cm2 by a rectifier and deposit times were 1, 3 and 6 h. Fig.1 shows the set-up of experiments for calcareous deposits.

Fig.1 Schematic of experimental set-up
3 Results and discussion
Fig.2 shows the dependence of the total calcareous amount deposited on specimens on time, dissolved CO2 gas and Ca(OH)2. With the supply of dissolved CO2 gas, the mass of calcareous deposit films sharply increases. Also, specimens with Ca(OH)2 formed in seawater obtained more calcareous deposits than specimens without Ca(OH)2.

Fig.2 Variation of mass gain with time for electrodeposit films formed in various solutions
Figs.3 and 4 show SEM photographs of calcareous deposited on specimens. Calcium carbonate can deposit as calcite or aragonite. When calcareous deposits are formed in natural seawater, SEM photos show well defined aragonite structure of calcium deposit. XRD results also confirmed that the precipitates are aragonite. This is due to the inhibiting effect of magnesium on nucleation and growth of calcite. And brucite structure of magnesium deposit was not observed, because Mg(OH)2-based layer is formed faster than aragonite crystals on metal surface. Therefore, only the aragonite crystal was observed in calcareous deposits even though Mg2+ ions were included in these films. This phase is easily recognizable by appearance of flower shape for its crystal[3-4]. When calcareous deposits are formed with CO2 gas, SEM photo shows the calcite crystals associated with aragonite and its shape is rhombohedral. This is because Mg2+ ion is dissolved in calcite lattices and forms CaCO3 and MgCO3 solid solution. Mg2+ ion is not dissolved in aragonite lattices because MgCO3 has a calcite-type structure. Mg2+ ion can be easily contained in calcite lattices and form magnesium calcite. X-ray diffraction (XRD) was used to confirm that these precipitates are indeed calcites (Fig.5). High peaks of calcite crystal formed on specimens are shown clearly and aragonite is scarcely found.
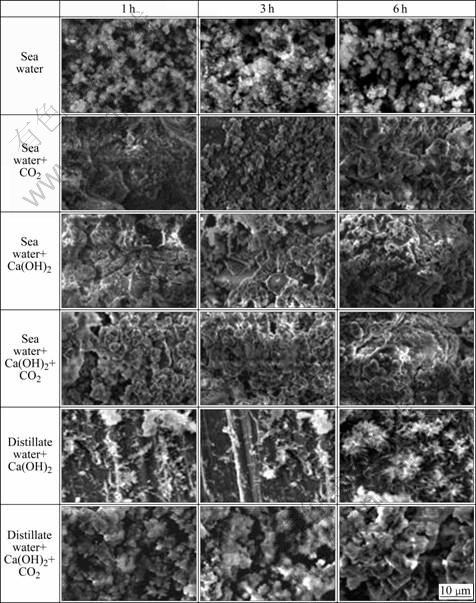
Fig.3 SEM photographs of electrodeposit films in various solutions
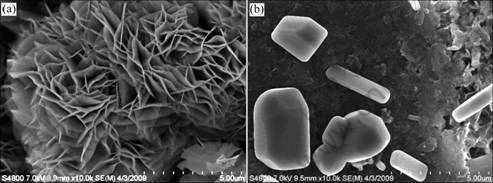
Fig.4 Detailed SEM images of aragonite (a) and calcite crystal (b) in calcareous deposits
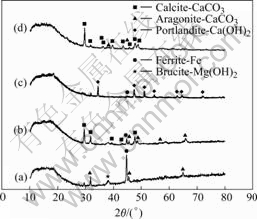
Fig.5 XRD patterns of electrodeposit films formed in various solutions: (a) Sea water, 6 h; (b) Sea water+CO2, 6 h; (c) Sea water+Ca(OH)2, 6 h; (d) Sea water+Ca(OH)2+CO2, 6 h
On the other hand, specimens formed in distillate water with no supply of CO2 gas did not form any precipitation. This is due to insufficiency of ions. Specimens under the same conditions with supply of CO2 gas formed calcareous deposit, but the adhesion of films is very poor because there are ions smaller than seawater.
Another peculiar feature is the formation of portlandite. It is one of Ca(OH)2 crystals and is only formed from seawater with addition of Ca(OH)2 without CO2 gas.
Fig.6 shows the anodic polarization curve of specimens formed in various solutions for 3 h. All coated specimens show better corrosion resistance than specimens without coating. Among them, specimens formed from seawater with CO2 gas and Ca(OH)2 are extremely good. It is therefore thought that calcite crystals are densely formed on specimens by supplying CO2 gas and calcite crystals are more effective in covering the surface than aragonite crystals. They are more useful in preventing corrosion of specimens surface.
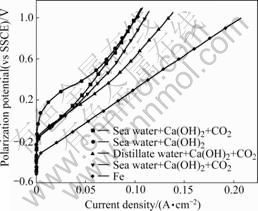
Fig.6 Anodic polarization curves of all specimens
4 Conclusions
1) The influence of dissolved CO2 gas on formation of non-ferrous calcareous deposit films was studied. This study was aimed to provide a better understanding on the growth of CaCO3 (aragonite or calcite) compared with that of Mg(OH)2 (brucite) during formation of film on steel substrate under cathodic electro-deposition in seawater environments.
2) Good overall properties of electrodeposited films can be achieved by effectively controlling dissolved CO2 gas and Ca(OH)2 in seawater.
Acknowledgements
This paper was done as a joint research with BNT Engineering Co. and supported by the Ministry of Construction & Transportation, Korea.
References
[1] NEVILLE A, MORIZOT A P J. Calcareous scales formed by cathodic protection—An assessment of characteristics and kinetics [J]. Crystal Growth, 2002, 243: 490-502.
[2] LEE M H, LEE C S, MOON K M, OH J S, LEE K H. Calcareous deposit films formed at various current densities and electrodeposition times in natural seawater [C]// Fifth International Symposium on Biomimetic Materials Processing. Nagoya, 2005: 54.
[3] MORSE J W, MUCCI A, MILLERO F J. The solubility of calcite and aragonite in seawater of 35% salinity at 25 ℃ and atmospheric pressure [J]. Geochimica et Cosmoxhimica Acta, 1980, 44: 85-94.
[4] BARCHICHE C, DESLOUIS C, FESTY D, GIL O, MAILLOT V, TOUZAIN S, TRIBOLLET B. Characterization of calcareous deposits in artificial sea water by impedances techniques: 3-deposit of CaCO3 in the presence of Mg2+ [J]. Electrochimica Acta, 2003, 48: 1645-1654.
Corresponding author: Myeong-Hoon LEE; Tel: +82-51-4104264; E-mail: leemh@hhu.ac.kr
(Edited by YANG Hua)


