
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 215-221
Carbothermal reduction-chlorination-disproportionation of alumina in vacuum
FENG Yue-bin1, 2, YANG Bin2, DAI Yong-nian2
1. Faculty of Science, Kunming University of Science and Technology, Kunming 650500, China;
2. National Engineering Laboratory of Vacuum Metallurgy, Faculty of Metallurgy and Energy Engineering,
Kunming University of Science and Technology, Kunming 650093, China
Received 4 January 2011; accepted 8 June 2011
Abstract:
The carbothermal reduction-chlorination-disproportionation of alumina in vacuum was investigated by XRD and thermodynamic analysis. The experiments on alumina and graphite at 1643-1843 K in vacuum were carried out. The results demonstrate that AlCl3(g) reacts with Al2O(g) or Al(g) generated from the carbothermal reduction of alumina to form AlCl(g), and the AlCl(g) disproportionates to aluminum and AlCl3(g) at a lower temperature and the reaction rate of AlCl(g) reaches 90% at 980 K and 100 Pa. The aluminum can absorb CO to catalyze its disproportionation to C and CO2, and react backward with CO to form Al4C3, Al2O3, C and CO2, resulting in the aluminum product containing C, Al4C3 and Al2O3. The impurities in the aluminum product decrease as the AlCl(g) disproportionation temperature decreases. AlCl3 condenses at a temperature approximated to the room temperature.
Key words:
carbothermal reduction; Al2O3; AlCl; vacuum; disproportionation;
1 Introduction
Despite the industrial supremacy of the Hall-Heroult electrolytic process, there have been sustained attempts to produce aluminum by the carbothermal reduction of alumina. The processes can be divided into two groups. In the first approach, alumina is directly reduced to aluminum using carbon as a reducing agent. The process has been studied extensively [1-3]. However, it remains to be a formidable technical challenge, due to the high temperature, and to the formation of aluminum carbide and oxycarbide [4]. In the second approach, aluminum is produced by carbothermal reduction with simultaneous chlorination of alumina. The present situation of the process was described in Ref. [5]. Recently, the researchers in Kunming University of Science and Technology, China have been carrying out research and developmental work on the latter approach to produce aluminum by carbothermal reduction-chlorination-disproportionation of alumina in vacuum, in which AlCl(g) is generated at high temperatures by the carbothermal reduction- chlorination of alumina and it will disproportionate into Al and AlCl3 at low temperatures [6]. It can be represented by the following overall reactions [6]:
Al2O3(s)+3C(s)+AlCl3(g)=3AlCl(g)+3CO(g) (1)
3AlCl(g)=2Al(l,s)+AlCl3(g) (2)
Compared with the direct carbothermal reduction, the temperatures required for the process are considerably decreased, and the aluminum products don’t need to be separated from residues because they are formed in the low temperature zone by the disproportionation of AlCl(g), apart from the carbothermal reduction-chlorination zone.
WANG et al [6] proved the practicality of the process through the experiments on the raw materials of bauxite and coal. YUAN et al [7-10] investigated the process by XRD, SEM and thermodynamic analysis, thereby proposing the following chlorination reactions:
Al4C3(s)+Al2O3(s)+3AlCl3(g)=9AlCl(g)+3CO(g),
Al4O4C(s)+3C(s)+2AlCl3(g)=6AlCl(g)+4CO(g),
Al4O4C(s)+Al4C3(s)+Al2O3(s)+3C(s)+5AlCl3(g)=
15AlCl(g)+7CO(g).
The purpose of this work is to investigate the mechanism of the formation of Al through the carbothermal reduction-chlorination-disproportionation of alumina at a temperature ranged from 1643 to 1843 K in vacuum.
2 Thermodynamic analysis
2.1 Carbothermal reduction-chlorination of alumina
The carbothermal reduction of alumina in Ar or vacuum was found to proceed through gas phase reactions, rather than direct solid-solid reactions [11-13]. Therefore, AlCl(g) should not be formed from the direct solid-solid-gas reactions of alumina, carbon and AlCl3, and be formed from the chlorination reactions of the carbothermal reduction products with AlCl3.
The carbothermal reduction of alumina in vacuum forms Al2O, Al and CO. The overall reactions are [13]:
Al2O3(s)+2C(s)=Al2O(g)+2CO(g) (3)
Al2O3(s)+3C(s)=2Al(g)+3CO(g) (4)
The initial reaction temperature for reaction (3) is lower than that for reaction (4) [14]. However, the Gibbs free energy change of reaction (3) decreases less sharply than that of reaction (4), so that the ΔG-T curves of reactions (3) and (4) intersect at temeprature where ΔG of reaction (3) equals that of reaction (4) [14]. The intersection temperature decreases with decreasing the system pressure, as shown in Fig. 1. The main Al-containing products of the carbothermal reduction of alumina are Al at the left-upper side of the curve and Al2O at the right-lower side of the curve [14].
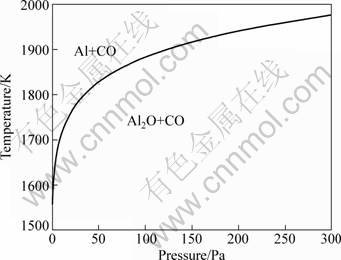
Fig. 1 Temperature at intersection of ΔG-T curves of reactions (3) and (4) vs system pressure [14]
The chlorination reaction of Al2O(g) to AlCl(g) is postulated:
2AlCl3(g)+3Al2O(g)=Al2O3(s)+6AlCl(g) (5)
Based on the data of chemical reaction and equilibrium of software (HSC), the Gibbs free energy change (ΔGΘ) of reaction (5) was calculated and shown in Fig. 2. As can be seen, ΔGΘ values are all negative and declined downwards in the range of 1200-1900 K. Furthermore, vacuum is benefical for reaction (5) because it is a volumn expansion reaction. Therefore, reaction (5) can occur under conditions studied.
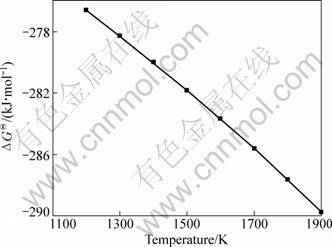
Fig. 2 ΔGΘ of reaction (5) vs temperature
The chlorination reaction of Al(g) to AlCl(g) is
2Al(g)+AlCl3(g)=3AlCl(g) (6)
Based on the data of HSC, the Gibbs free energy (ΔGΘ) of reaction (6) was calculated and shown in Fig. 3. As can be seen, ΔGΘ values are all negative and declined downwards in the range of 1200-1900 K. The system pressure has no influence on the Gibbs free energy change of reaction (6). Reaction (6) has ever been used to extract aluminum from Al alloy [15, 16].
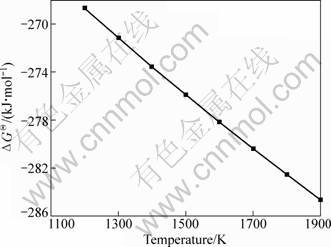
Fig. 3 ΔGΘ of reaction (6) vs temperature
2.2 Disproportionation of AlCl(g)
AlCl(g) disproportionates into Al and AlCl3(g) according to Eq. (2) at low temperature. When the mole ratio of AlCl(g) to AlCl3(g) was 0.5, the relationship of Gibbs free energy change (ΔG) of reaction (2) and temperature (T) was calculated based on the data of HSC using the method from Ref. [17], and shown in Fig. 4. As can be seen, ΔG value of reaction (2) decreases with decreasing the temperature and increasing the system pressure in the range of 300-1900 K. Therefore, the larger the system pressure is, and the higher the temperature is, the more easily the AlCl(g) disproportionation is carried out.
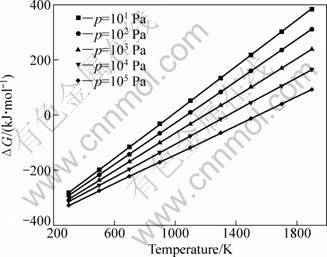
Fig. 4 ΔG of reaction (2) vs temperature at different pressures (the mole ratio of AlCl to AlCl3 is 0.5)
3 Experimental
3.1 Apparatus
The experiments were carried out in the furnace designed by ourselves, as shown in Fig. 5 [18].
3.2 Experimental procedure
Alumina (analytical grade) and graphite (fixed carbon content of 99.85%) with molar ratio of 1:3 were thoroughly mixed and pressed in a closed die of 20 mm in diameter under 2 MPa to produce cylindrical pellets
with mass of about 5 g.
1) Carbothermal reduction of alumina (Experments A)
The pellets were held in the graphite crucible placed in the vacuum furnace, and heated at a certain temperature for 30 min. The residues and condensates were then analyzed by X-ray diffraction technique using a D/max-3B diffractometer (Japan) with Cu Kα radiation.
The experiments were conducted at 1643, 1693, 1743, 1793 and 1843 K, respectively. The highest pressure in the system reached 150 Pa. The present conditions were mainly in the range for producing Al2O according to Fig. 1, and thereby alumina reacted with C to form Al2O chiefly according to reaction (3).
2) Carbothermal reduction-chlorination-dispropor- tionation of alumina (Experiment B)
The pellets were held in the graphite crucible placed in the vacuum furnace, and heated in the presence of AlCl3 at a certain temperature for 30 min. The residues and condensates were then analyzed by X-ray diffraction technique using a D/max-3B diffractometer (Japan) with Cu Kα radiation.
The experiments were conducted at 1643, 1693, 1743, 1793 and 1843 K, respectively. The highest pressure in the system reached 200 Pa.
4 Results and discussion
4.1 Phase composition of residues
The residues after heating the pellets in the absence of AlCl3(g) (Experiments A) consisted of Al2O3 and C as the same as the raw materials, which was in agreement with the earlier results that the products of the reactions of alumina and carbon in vacuum were all gases [13, 14].
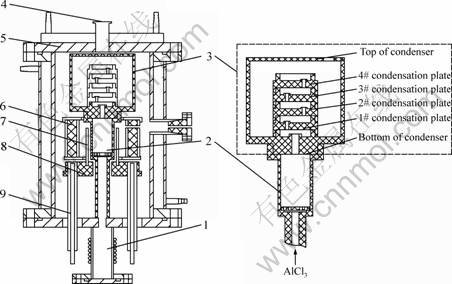
Fig. 5 Schematic diagram of vacuum furnace: 1—AlCl3 evaporator; 2—Reaction crucible; 3—Graphite condenser; 4–Vacuum pump; 5—Water-cooled cover of furnace; 6—Thermal insulating layer; 7—Graphite exothermic body; 8—Exothermic body base; 9—Water-cooled electrode
The residues after heating the pellets in the presence of AlCl3(g) (Experiments B) consisted of Al2O3 and C as well, which indicated that the reactions occurring were the same as those in the absence of AlCl3(g). Therefore, AlCl3(g) did not participate in the reaction of alumina and graphite, and AlCl should be formed by the reaction of AlCl3(g) and the Al-containing gaseous products generated from the carbothermal reduction of alumina. The main Al-containing gaseous products should be Al2O because the present conditions were mainly in the range for producing Al2O according to Fig. 1.
4.2 Phase composition of condensates
Table 1 presents the phase composition of the condensates formed in the experiments.
Table 1 Composition of condensates
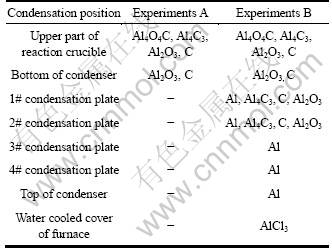
The XRD patterns of the condensates on the upper part of the reaction crucible formed by heating the pellets in the absence and the presence of AlCl3(g) at 1743 K for 30 min are shown in Fig. 6. As can be seen, the condensates formed in the carbothermal reduction and in the carbothermal reduction-chlorination-disproportiona- tion of alumina were identical in phase composition. The condensates could be the products of the back-reactions of Al2O and CO formed by carbothermal reduction of alumina [13, 14]. However, the diffraction peak assigned to C in the XRD patterns of the condensate formed in the carbothermal reduction- chlorination-disproportionation was considerably weaker than that in the carbothermal reduction. The result supported the above conclusion that AlCl3(g) did not participate in the reaction of alumina and graphite, and AlCl should be formed mainly by the reaction of AlCl3(g) and Al2O(g), and the reaction of Al2O(g) and AlCl3(g) occurred according to reaction (5) because Al2O3 formed by reaction (5) could react with carbon to result in a reduction in the amount of carbon.
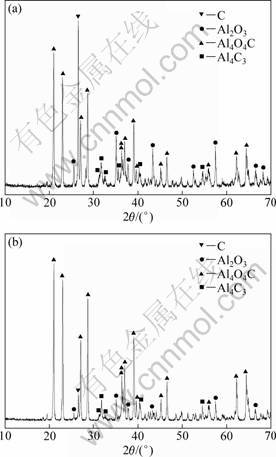
Fig. 6 XRD patterns of condensates on upper part of reaction crucible: (a) Carbothermal reduction; (b) Carbothermal reduction-chlorination-disproportionation
The XRD patterns of the condensates on the bottom of the condenser formed by heating the pellets in the absence and the presence of AlCl3(g) at 1743 K for 30 min are shown in Fig. 7. As can be seen, both the condensates consisted of Al2O3 and C. The condensates should be formed mainly by the back-reactions of Al2O and CO [13, 14]. However, the diffraction peaks assigned to Al2O3 in the patterns of the condensate formed in the carbothermal reduction–chlorination–disproportionation were considerably more intense than those in the carbothermal reduction, and the diffraction peaks assigned to δ-Al2O3 were also observed besides those assigned to α-Al2O3. It was further indicated that the chlorination reaction of Al2O(g) occurred according to reaction (5), because reaction (5) could generate Al2O3 to result in a growth in the amount of Al2O3, and the condenser bottom temperature was low enough to avoid reaction of Al2O3 with carbon.
For the carbothermal reduction, the gaseous products reacted backwards to condensate on the upper part of the reaction crucible and the bottom of the condenser. The gas from crucible into the condenser mainly consisted of CO, and consequently no condensate formed in the condenser. For carbothermal reduction-chlorination-disproportionation, besides back-reactions the gaseous products reacted with AlCl3(g) to form AlCl(g), and consequently the gas from crucible into the condenser consisted of CO, AlCl and AlCl3 unreacted. The gas condensed continuously with decreasing temperature as it went through the condensation plates and the top of the condenser to the cover of the furnace.
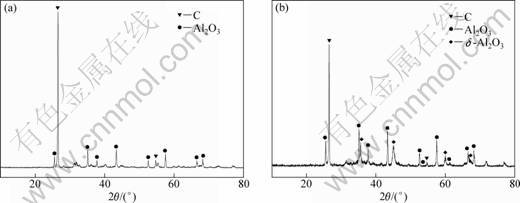
Fig. 7 XRD patterns of condensates on bottom of condenser: (a) Carbothermal reduction; (b) Carbothermal reduction- chlorination-disproportionation
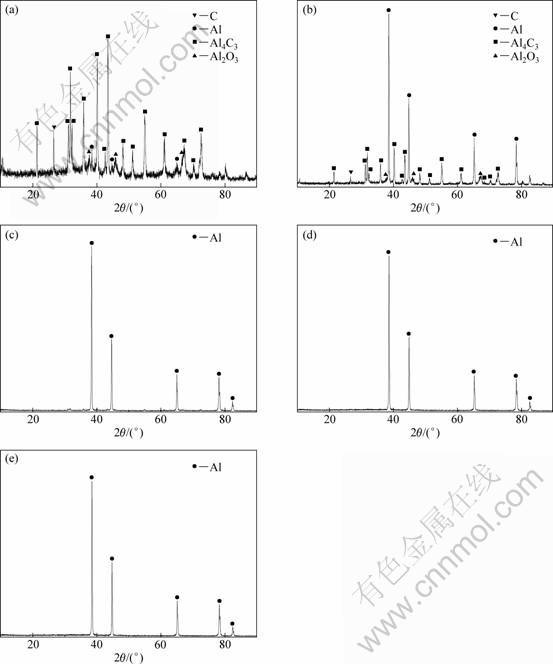
Fig. 8 XRD patterns of condensates on condensation plates and top of the condenser: (a) 1# condensation plate; (b) 2# condensation plate; (c) 3# condensation plate; (d) 4# condensation plate; (e) Top of the condenser
The XRD patterns of the condensates on the condensation plates and on the top of the condenser in the presence of AlCl3(g) at 1743 K for 30 min are shown in Fig. 8. AlCl(g) into condenser should disproportionate to form Al and AlCl3(g), and thereby the condensates should consist of Al. However, the condensates on 1# and 2# condensation plates contained C, Al4C3 and Al2O3 besides Al. The C should be formed by the disproportionation of CO according to reaction (7) because Al could absorb CO to catalyze its disproportionation to C and CO2.
2CO(g)=C(s)+CO2(g) (7)
The Al4C3 and Al2O3 should be formed by the back-reactions of Al and CO according to reactions (8) and (9).
4Al(s)+6CO(g)=Al4C3(s)+3CO2(g) (8)
2Al(s)+3CO(g)=Al2O3(s)+3C(s) (9)
From Fig. 8, the diffraction peaks assigned to C, Al4C3 and Al2O3 in the condensates grew weak with decreasing temperature. It was indicated that the extent of the disproportionation of CO and the backward reactions of Al and CO decreased as the temperature decreased.
AlCl3 condensed on the water-cooled cover of the furnace where the temperature approximated to the room temperature, due to the low sublimation temperature of AlCl3 in vacuum [19].
5 Conclusions
1) AlCl in the carbothermal reduction-chlorination- disproportionation of alumina in vacuum should be formed by two successive reaction steps. In the first step, alumina reacts with carbon to generate Al2O(g) or Al(g). In the second step, the Al2O(g) or Al(g) reacts with AlCl3(g) to form AlCl(g).
2) AlCl disproportionates to aluminum and AlCl3(g) at lower temperatures. The aluminum can absorb CO to catalyze its disproportionation to C and CO2, and react backward with CO to form Al4C3, Al2O3, C and CO2. The extent of the disproportionation of CO and the backward reactions of Al and CO decreases as the AlCl(g) disproportionation temperature decreases. AlCl3 condenses at a temperature approximated to the room temperature.
References
[1] Bruno M J. Aluminum carbothermic technology [R]. DOE/ID/13900. Pennsylvania: Alcoa, 2004.
[2] Grjotheim K, Motzfeldt K, Kvande H, Schei A. Carbothermal production of aluminium: Chemistry and technology [M]. Dusseldorf: Aluminium-Verlag, 1989.
[3] Motzfeldt K, Sanberg B. Chemical investigations concerning carbothermic reduction of alumina [C]//PETERSON W S. Light Metals. Warrendale: TMS, 1979: 411-428.
[4] Choate W, Green J. Technoeconomic assessment of the carbothermic reduction process for aluminum production [C]// GALLOWAY T J. Light Metals 2006, volume 2, Aluminum Reduction Technology. Warrendale: TMS, 2006: 445-450.
[5] FENG Yue-bin, DAI Yong-nian, WANG Ping-yan. Research of the production and refining of aluminum by disproportionation [J]. Light Metals, 2009(3): 12-15. (in Chinese)
[6] WANG Ping-yan, LIU Mou-sheng, DAI Yong-nian. Vacuum metallurgy of Al from bauxite by carbothermic reaction-chloridation [J]. Chinese Journal of Vacuum Science and Technology, 2006, 26(5): 377-380. (in Chinese)
[7] Yuan Hai-bin, YANG Bin, XU Bao-qiang, YU Qing-chun, FENG Yue-bin, DAI Yong-nian. Aluminum production by carothermo- chlorination reduction of alumina in vacuum [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1505-1510.
[8] Yuan Hai-bin, Yang Bin, Yu Qing-chun, Xu Bao-qiang, Zhu Yu-yan, Feng Yue-bin, Dai Yong-nian. Reaction mechanism of AlCl generated by carbothermic chloride to produce aluminum in vacuum [C]//BA De-chun. Proceeding of 9th Vacuum Metallurgy and Surface Engineering Conference. Beijing: Electronics Industry Press, 2009: 39-45.
[9] Yuan Hai-bin, FENG Yue-bin, YANG Bin, YU Qing-chun, XU Bao-qiang, WANG Peng-cheng, DAI Yong-nian. Thermal behavior of alumina in process of carbothermic reduction and chloride to produce aluminum [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(4): 777-783. (in Chinese)
[10] Yuan Hai-bin, FENG Yue-bin, XU Bao-qiang, YANG Bin, YU Qing-chun, DAI Yong-nian. Direct extraction of aluminum alumina by carbothermic reaction-chloridation and possible mechanisms [J]. Chinese Journal of Vacuum Science and Technology, 2010, 30(3): 259-264. (in Chinese)
[11] Yu J K, Ueno S, Li H X, Hiragushi K. Improvement of graphitization of isotropic carbon by Al2O3 formed from aluminum chelate compound [J]. Journal of the European Ceramic Society, 1999, 19(16): 2843-2848.
[12] Lefort B. Mechanism of AlN formation through the carbothermal reduction of Al2O3 in a flowing N2 atmosphere [J]. Journal
of the American Ceramic Society, 1993, 76(9): 2295-2299.
[13] FENG Yue-bin, YANG Bin, DAI Yong-nian. Carbothermal reduction of alumina in a vacuum [C]//Advanced Material Research. Switzland: Trans Tech Publications, 2011, 156-157: 1688-1691.
[14] FENG Yue-bin, YANG Bin, DAI Yong-nian. Thermodynamics of the carbothermal reduction of alumina in vacuum [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(7): 1748-1755. (in Chinese)
[15] Paul W. Process for the production and recovery of pure aluminum with the aid of halides: British, 734480 [P]. 1955-08-03.
[16] FRUCHTER, MOSHE, MOSCOVICI, ANUTZA. Process for the manufacture of pure metallic aluminum from aluminum ores and other aluminum-bearing materials: USA, 4430120 [P]. 1984-02-07.
[17] Dai Yong-nian, Yang Bin. Vacuum metallurgy for nonferrous metal material [M]. Beijing: Metallurgical Industry Press, 2000: 278-345. (in Chinese)
[18] DAI Yong-nian, FENG Yue-bin, YANG Bin, YANG Bu-zheng, LIU Yong-cheng, XU Bao-qiang, LIU Da-chun, YU Qing-chun, MA Wen-hui, QIN Bo. A kind of vacuum reaction furnace: China, 00920111773.1 [P]. 2009-08-06. (in Chinese)
[19] FENG Yue-bin, DAI Yong-nian, LIU Yong-cheng, YANG Bin. Vacuum sublimation of anhydrous aluminum chloride [J]. Chinese Journal of Vacuum Science and Technology, 2009, 29(3): 336-339. (in Chinese)
氧化铝的真空碳热还原-氯化-歧化反应
冯月斌1, 2,杨 斌2,戴永年2
1. 昆明理工大学 理学院,昆明 650500;
2. 昆明理工大学 冶金与能源工程学院,真空冶金国家工程实验室,昆明 650093
摘 要:通过XRD物相分析和热力学分析研究氧化铝的真空碳热还原-氯化-歧化反应。以氧化铝和石墨为原料,在真空下、1643-1843 K的温度范围内进行实验。结果表明,AlCl3(g)与氧化铝碳热还原产生的Al2O(g)或Al(g)反应生成AlCl(g),该AlCl(g)在较低温度下歧化分解为金属铝和AlCl3(g);当压力为100 Pa、温度为980 K时,AlCl(g)的歧化反应率达到90%。生成的金属铝可以吸附催化CO歧化为C和CO2,并可以与CO二次反应形成Al4C3、Al2O3、C和CO2,导致铝产物中含有C、Al4C3和Al2O3。产物铝中所含的这些杂质随着AlCl(g)歧化反应温度的降低而减少。AlCl3(g)在接近室温的温度下冷凝下来。
关键词:碳热还原;Al2O3;AlCl;真空;歧化
(Edited by YUAN Sai-qian)
Foundation item: Project (u0837604) supported by the Joint Funds of the National Natural Science Foundation of China and Yunnan Province
Corresponding author: YANG Bin; Tel/Fax: +86-871-5161583; E-mail: kgyb2005@126.com
DOI: 10.1016/S1003-6326(11)61163-1
Abstract: The carbothermal reduction-chlorination-disproportionation of alumina in vacuum was investigated by XRD and thermodynamic analysis. The experiments on alumina and graphite at 1643-1843 K in vacuum were carried out. The results demonstrate that AlCl3(g) reacts with Al2O(g) or Al(g) generated from the carbothermal reduction of alumina to form AlCl(g), and the AlCl(g) disproportionates to aluminum and AlCl3(g) at a lower temperature and the reaction rate of AlCl(g) reaches 90% at 980 K and 100 Pa. The aluminum can absorb CO to catalyze its disproportionation to C and CO2, and react backward with CO to form Al4C3, Al2O3, C and CO2, resulting in the aluminum product containing C, Al4C3 and Al2O3. The impurities in the aluminum product decrease as the AlCl(g) disproportionation temperature decreases. AlCl3 condenses at a temperature approximated to the room temperature.


