
Treatment of nickel-ammonia complex ion-containing ammonia nitrogen wastewater
MIN Xiao-bo(闵小波), ZHOU Min(周 敏), CHAI Li-yuan(柴立元),
WANG Yun-yan(王云燕), SHU Yu-de(舒余德)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 26 September 2008; accepted 2 March 2009
Abstract:
Air stripping was adopted to treat nickel ammonia complex ion-containing wastewater in order to remove nickel and ammonia simultaneously in one technological process. The relationship among pH, the concentration of nickel ammonia complex ion and total ammonia concentration was analyzed theoretically. Influence of pH value, water temperature, airflow rate and time on air stripping was studied in detail by static experiment in laboratory. The results show that at pH 11, temperature of 60 ℃ and airflow rate of 0.12 m3/h, NH3 and Ni2+ concentrations remained in wastewater are less than 2 and 0.2 mg/L, respectively, after blowing for 75 min, which reaches the standard of the state discharge. When the tail gas is absorbed by 0.5 mol/L H2SO4 in order to avoid the secondary pollution, the absorption rate can achieve 70%.
Key words:
air stripping; nickel ammonia complex ion; nickel ammonia wastewater;
1 Introduction
The wastewater containing nickel-ammonia complex ion is one of the most important industrial pollution sources. This kind of wastewater mainly comes from the industries such as hard alloy, high efficiency catalysts, coated materials, and multilayer ceramic capacitor. Nickel and its compounds can greatly harm humans and aquatic organisms. They are accumulated in plants and soil and cannot be degraded in natural environment[1]. With development of industry, especially electroplating, this kind of wastewater brings more and more threats to environment. So, it is very imperative to research on such wastewater.
When the ammonia exists in nickel wastewater, nickel ion and free ammonia can form metal complex ion, which makes it difficult to treat. At present, some treatments to this kind of water merely concern with the heavy metal pollution, neglecting the ammonia pollution [2-5]; some aim at removing ammonia after nickel removal from wastewater[6]; and some only pay attention to ammonia removal which makes the effluent not meet the discharge standard[7]. The purpose of this work is to seek a technology treating the wastewater containing nickel ammonia complex ion and the influencing factors. It is expected that the ammonia and nickel can be removed simultaneously.
2 Experimental
2.1 Wastewater composition
The wastewater was of cyan with pH 9.42. It contained Ni2+ of 2.2×10-3 mol/L and NH3 of 0.118 mol/L.
2.2 Methods
200 mL wastewater was put into a 500 mL beaker and placed in a water bath to preheat to designated temperature. The pH was also adjusted using saturated NaOH solution. After that, an aerator was put in the beaker to start aerating and the flow velocity was controlled by an air rotameter; meanwhile, the starting time was recorded. The beaker was taken out of the water bath when the appointed time was arrived, and nickel and ammonia in the treated water were determined after being filtered. In order to reduce secondary pollution, the tail gas was absorbed with H2SO4.
2.3 Analytical methods
Ammonia at low and high concentrations were determined by salicylic acid spectrophotometry (GB7481—87) and by formaldehyde titration method, respectively. Nickel was tested with flame atomic absorption spectrometer (GB11912—89). pH was measured with PHS-3C precise acidity meter.
3 Experimental principles
The ammonia in water exists in two forms: ammonium ions and free ammonia. The equilibrium equation is NH4++OH-=NH3+H2O, which is strongly influenced by pH. The proportion of free ammonia increases with pH rising[8], and it can be calculated by following formula[9]:
![]() ×100%
×100%
=![]() ×100% (1)
×100% (1)
where A is the proportion of free ammonia; c(NH3) is the concentration of free ammonia; c(NH3)+![]() is the total concentration of ammonia; K0 is the acid ionization constant for ammonia, being 5.65×10-10; and c(H+) is the hydrogen ion concentration.
is the total concentration of ammonia; K0 is the acid ionization constant for ammonia, being 5.65×10-10; and c(H+) is the hydrogen ion concentration.
Using Eq.(1), we can get Fig.1. It can be seen that the proportion of free ammonia reaches over 98% when pH=11. Based on this principle, an air stripping method is offered, i.e. a large number of air is blown into the wastewater, and the dissolved free ammonia erupts from the water and is removed.

Fig.1 Relationship between pH and proportion of free ammonia
In order to know the distribution of nickel and ammonia complex irons, the generating function ![]() is introduced.
is introduced. ![]() is the average ligand number of the central ion in solution. For the Ni2+-NH3-H2O system,
is the average ligand number of the central ion in solution. For the Ni2+-NH3-H2O system, ![]() is expressed as [10]
is expressed as [10]
 (N≤6) (2)
(N≤6) (2)
where n is the coordination number.
At a certain temperature and constant iron intensity, there is a cumulative stability constant βn corresponding to the complex of nickel ![]() with n≥6:
with n≥6:
![]() (3)
(3)
Substituting Eq.(3) into Eq.(2), we get
 (4)
(4)
where β1=102.72, β2=104.89, β3=106.55, β4=107.67, β5=108.34 and β6=108.31[11-14].
When pH values are 7, 8, 9, 10,11,12,13 and 14, the generating function n are calculated by Eq.(4) to be 0.30, 1.50, 3.05, 3.77, 3.89, 3.90, 3.90 and 3.90, respectively. When pH is 7, there is nearly no nickel-ammonia coordinate ion in the solution; and pH is over 8, the main complex ion forms are ![]()
![]() and
and ![]()
When nickel ammonia complex ion exists in the solution, the concentration of free ammonia is influenced not only by pH value, but also by concentration of nickel ammonia complex ion:
![]() ×100%=
×100%=
 ×100%
×100%
(5)
where K2=107.67 , the stability constant of [Ni(NH3)4]2+; Ksp=2.0×10-15, solubility products of Ni(OH)2[11].
As shown in Fig.2 plotted according to Eq.(5), when the concentration of [Ni(NH3)4]2+ is 2.2×10-3 mol/L, the proportion of free ammonia increases with pH arising. When pH is over 11, free ammonia is over 98%, meaning that the effect of such low concentration of nickel ammonia complex ions on removal of ammonia can be ignored. So, as long as pH is controlled≥11, ammonia can be removed by air stripping.

Fig.2 Relationship between pH and proportion of free ammonia in nickel ammonia wastewater
Nickel can be precipitated as Ni(OH)2 when pH is between 9 and 10[15]. For the nickel-ammonia- containing wastewater, the situation of pH over 11 satisfies not only the ammonia removal, but also the precipitation of Ni(OH)2. In this research, appropriate conditions are chosen to remove Ni2+ and NH3 by simultaneous use of hydrolysis and air stripping method.
4 Results and discussion
4.1 Effect of pH on removal of NH3 and Ni2+
The effects of pH value on air striping at 60 ℃ and airflow rate of 0.12 m3/h for 1.5 h are shown in Fig.3 and Fig.4.

Fig.3 Effect of pH value on removal of ammonia

Fig.4 Effect of pH value on removal of nickel
As indicated in Fig.3, the concentration of residual ammonia decreases with pH arising. When pH is below 10, the removal rate for ammonia increases gradually; while pH=11, the residual ammonia concentration is much less than 15 mg/L which is the first grade of discharge standard of GB8978—1996; and then the removal efficiency of ammonia tends to stable. When pH is over 11, the change is similar to the ammonia stripping without nickel ammonia complex ion[16-18]. It can be seen from Fig.3 and Fig.4 that the residual ammonia concentration is highly correlated with residue of nickel. The lower the residual ammonia concentration is, the higher the nickel is removed. When pH is 11, the residual ammonia is 2.12 mg/L and the residual nickel is down to 0.26 mg/L. These results show that the effect of denickelefication increases apparently with pH arising. This is because nickel ion is free from nickel ammonia complex ion and then is hydrolyzed under high pH. So, pH=11 is chosen to be the optimal condition.
4.2 Effect of airflow rate on NH3 and Ni2+ removal
In intermittent operation, the treatment capacity of water quantity each time is fixed. The change in airflow rate equals changing the gas-to-liquid ratio for studying the effect of airflow rate on air stripping efficiency and the rule and the change of residual ammonia concentration at different airflow rates. In order to study the effect of airflow rate on removal of ammonia and nickel, an experiment was made at pH = 11 and 60 ℃ for 1.5 h. The results are shown in Fig.5 and Fig.6.

Fig.5 Effect of airflow rate on removal of ammonia

Fig.6 Effect of airflow rate on removal of nickel
It can be seen from Fig.5 that the removal of ammonia basically increases with the increase of airflow rate. When the airflow rate is over 0.10 m3/h (which equals the gas-to-liquid ratio of more than 750), the increase of air stripping efficiency of ammonia is slow. Finally, ammonia concentration is lower than 2.21 mg/L and the removal rate reaches 99%. As shown in Fig.6, residual nickel concentration decreases with the gas-to-liquid ratio rising. A reason may be that a small amount of nickel ammonia complex ion is destructed with the decrease of ammonia, which makes nickel hydrolyzed. When the airflow rate reaches 0.12 m3/h, residual nickel concentration is almost unchanged (about 0.20 mg/L). So, the best airflow rate of 0.12 m3/h is chosen.
4.3 Effect of temperature on NH3 and Ni2+ removal
In order to study the effect of temperature on nickel and ammonia, a experiment was made at airflow rate of about 0.12 m3/h and pH of 11 for 1.5 h. The results are presented in Fig.7 and Fig.8.

Fig.7 Effect of temperature on removal of ammonia
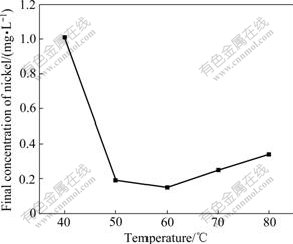
Fig.8 Effect of temperature on removal of nickel
From Fig.7, we know that as temperature increases from 40 ℃ to 50 ℃, the effect of temperature on ammonia removal efficiency is significant. When temperature is 50 ℃, the ammonia removal rate reaches 95% (the evaporization effect has been taken into consideration in calculating the residual concentration of ammonia at this temperature,) and the ammonia concentration is only 2.68 mg/L at 60 ℃. However, there is a reverse effect on nickel removal over 60 ℃. The optimum temperature is selected as 60 ℃.
4.4 Effect of time on NH3 removal
It is observed that the ammonia removal efficiency changes with time under the conditions: pH=11, airflow rate of 0.12 m3/h and 60 ℃. The results are shown in Fig.9. The results show that the residual ammonia concentration decreases fast with time prolonging, especially in the first 30 min; then the decreasing rate becomes slow, especially after 45 min. The residual ammonia concentration can be reduced to 3.80 mg/L after 75 min, with removal rate reaching 99.81%.

Fig.9 Effect of time on removal of ammonia
4.5 Absorption of tail gas
In this experiment, the tail gas was absorbed by 0.5 mol/L H2SO4 to avoid the secondary pollution. Under optimum conditions of pH=11, airflow rate of 0.12 m3/h and 60 ℃ for blowing 1.5 h and 2 h, residual ammonia concentration can be decreased to 25.81 mg/L and 5.02 mg/L, and residual nickel concentration to 0.23 mg/L and 0.25 mg/L, respectively, while the absorptivities of ammonia are 78.76% and 72.33%, respectively.
5 Conclusions
1) The wastewater containing nickel ammonia complex ion treated by air stripping meets the discharge standard of the effluent.
2) The ammonia removal rate increases with the increase in pH value, airflow rate, temperature and reaction time.
3) The ammonia removal rate can reach 96%, and the residual ammonia concentration is less than 15 mg/L under the conditions of pH=11-13, airflow rate of 0.06-0.14 m3/h and 50-80 ℃ for 1.5 h stripping.
4) The nickel removal performance is highly dependent on pH value. It can be decreased to less that 0.26 mg/L at pH over 11. Too high pH will promote irreversible hydrolysis of nickel.
References
[1] DENKHAUS E, SALNIKOw K. Nickel essentiality, toxicity, and carcinogenicity [J]. Oncology Hematology, 2002, 42: 35-56.
[2] GAVRILESEU M. Removal of heavy metals from the environment by biosorption [J]. England Life Science, 2004, 4(3): 219-232.
[3] AJMAL M, RAO R A K, AHMAD R, AHMAD J. Adsorption studies on Citrus reticulate (fruit peel of orange): Removal and recovery of Ni(Ⅱ) from electroplating wastewater [J]. Journal of Hazardous Materials, 2000, 79(1/2): 117-131.
[4] EOM T H, LEE C H, KIM J H, LEE C H. Development of an ion exchange system for plating wastewater treatment [J]. Desalination, 2005, 180(1/3): 163-172.
[5] CHAUDHARI S, TARE V. Analysis and evaluation of heavy metal uptake and release by insoluble starch xanthate in qqueous environment [J]. Wat Sci Tech, 1996, 34(10): 161-168.
[6] ZENG You-sheng. Technology of reclaiming ammonia in process of extraction nickel from electroplated nickel mud [J]. China Resources Comprehensive Utilization, 2004(4): 23-26. (in Chinese)
[7] FAN Jian-wei, SUN Yu, LIN Min-qing, YIN Lei. Treatment of metal-ammonia complex ion-containing high concentration ammonia nitrogen wastewater [J]. Industrial Water and Wastewater, 2007, 38(4): 54-57. (in Chinese)
[8] ZHANG Zi-li. Drainage engineering [M]. Beijing:China Architecture & Building Press, 2000. (in Chinese)
[9] LEI X H, SUGIURA N, FENG C P, MAEKAWA T. Pretreatment of anaerobic digestion effluent with ammonia stripping and biogas purification [J]. Journal of Hazardous Materials, 2007, 145(3): 391-397.
[10] ZHANG Xiang-lin, KANG Heng. Coordination chemistry [M]. Changsha: Central South University of Technology Press, 1986. (in Chinese)
[11] KOTRLY S, SUCHA L. Handbook of chemical equilibria in analytical chemistry [M]. Chichester: Ellis Horwood, 1985.
[12] YAO Yun-bin, XIE Tao, GAO Ying-min. Handbook of chemistry and physics [M]. Shanghai: Shanghai Scientific and Technical Publishers, 1985. (in Chinese)
[13] JOHN A D. Lange’s chemistry handbook [M]. SHANG Jiu-fang, trans. Beijing: Science Press, 1991: 9-1-9-25. (in Chinese)
[14] IHSAN B. Thermochemical data of pure substances [M]. CHENG Nai-liang. Beijing: Science Press, 2003. (in Chinese)
[15] HUANG Jiang-wei, SHAO Peng-cheng. Waste liquid treatment in electroless nickel deposition [J]. Corrosion & Protection, 2003, 24(9): 404-405. (in Chinese)
[16] ZHENG Xue-juan, XU Yue-en, CHEN Zeng-feng,HU Li-hua, ZHANG Hai-hua, NI Pei-lan. Blowing off the NH3-N from the landfill leachate [J]. Journal of Zhejiang Water Conservancy and Hydropower College, 2001, 13(2): 55-56. (in Chinese)
[17] AUGUST B, XAVIER F. Air stripping of ammonia from pig slurry: Characterization and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion [J]. Waste Management, 2003, 23: 261-272.
[18] MARTTINEN S K, KETTUNEN R H, SORMUNEN K M, SOIMASUO R M, RINTALA J A. Screening of physical-chemical methods for removal of organic material, nitrogen and toxicity from low strength landfill leachates [J]. Chemosphere, 2002, 46(6): 851-858.
Foundation item: Project(08SK1002) supported by the Major Project of Scientific and Technological Department of Hunan Province, China; Project(50508044) supported by the National Natural Science Foundation of China
Corresponding author: MIN Xiao-bo; Tel: +86-731-88830875; Fax: +86-731-88710171; E-mail: mxb@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60450-1


