J. Cent. South Univ. (2017) 24: 2468-2474
DOI: https://doi.org/10.1007/s11771-017-3658-7
Differences in leaf functional traits between exotic and native Compositae plant species
WANG Cong-yan(王从彦)1, 2, LIU Jun(刘君)1, ZHOU Jia-wei(周嘉伟)1, XIAO Hong-guang(肖鸿光)1
1. School of the Environment and Safety Engineering, Jiangsu University, Zhenjiang 212013, China;
2. State Key Laboratory of Soil and Sustainable Agriculture (Institute of Soil Science,Chinese Academy of Sciences), Nanjing 210008, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract:
This study aims to determine the differences in leaf functional traits and phenotypic plasticity of leaf functional traits between exotic and native Compositae plant species. Leaf width of exotic plants was significantly lower than that of native species. Leaf length, specific leaf area (SLA), single-leaf wet and dry weights, leaf moisture, and leaf thickness of exotic plants were also lower than those of native species but not significantly. The leaf shape index of exotic plants was higher than that of native species but not significantly. This implies that the relatively low leaf construction cost for exotic plants may play an important role in the success of their invasions. The higher leaf shape index and lower leaf width of exotic plants can enhance the efficiency of resource capture (especially sunlight capture) via adjustments to leaf shape and size, thereby increasing the survival of exotic plants. The plasticity indices of single-leaf wet weight and leaf thickness of exotic plants were significantly lower than those of native species. The lower phenotypic plasticity of single-leaf wet weight and leaf thickness of exotic plants may be the result of a cost to plasticity. That is, if the plasticity is too high, the fitness of plant species might be reduced sharply under unfavorable environments. Thus, lower plasticity of leaf functional traits may compensate for the negative impact of adverse environments and stabilize leaf construction costs for exotic plants. Moreover, reduced phenotypic plasticity might be one of the key competitive strategies by which exotic plants successfully invade new habitats. Overall, exotic plants did not always exhibit higher values of leaf functional traits or increased phenotypic plasticity of leaf functional traits compared with native species.
Key words:
leaf functional traits; specific leaf area (SLA); phenotypic plasticity; exotic plants; Compositae;
1 Introduction
Leaves are one of the most important plant organs as they capture the sunlight required for plant development, growth, and survival [1–3]. Thus, plastic responses of leaf functional traits to changes in environmental factors can enable sessile plant species to inhabit a wide variety of habitats. Individual plants can thus employ successful ecological strategies throughout their lifespan despite their leaves being exposed to multivariate environments and highly sensitive to environmental changes [4–7]. Leaves can exhibit phenotypic plasticity in response to environmental changes to enhance their fitness; through phenotypic plasticity, plants can utilize successful ecological strategies across abrupt environmental changes [4, 8]. As one of the most important leaf functional traits, specific leaf area (SLA is defined as the physical investment per unit of light capture surface; i.e., leaf area divided by dry leaf mass) can characterize the resource-use strategy of plant species, namely, SLA controls and balances the dynamics between light capture and leaf construction costs [4, 6, 9, 10]. Normally, high SLA indicates high resource acquisition and use efficiency with low investment in leaf construction and protective tissues [4, 9, 10]. Meanwhile, leaf size, leaf thickness, leaf shape index (calculated as the ratio of leaf length to leaf width), single-leaf wet and dry weights, and leaf moisture are also important indices; these factors have been shown to be good indicators of resource-use strategies of plant species [4–7]. Thus, understanding leaf functional traits as well as phenotypic plasticity of those traits is important to elucidate the mechanisms underlying successful ecological strategies of plant species.
At present, biological invasions have imposed serious damage to ecosystems by specifically changing the structure and/or functions of the ecosystems in which these invasions have occurred [11–13]. Numerous studies have suggested that certain plant species can successfully invade certain environments because the leaf functional traits (such as higher SLA) of many exotic plants can enable them to outcompete native species for resources [14–18]. Meanwhile, differences in leaf functional traits between exotic and native plant species are believed to be strongly linked with the mechanisms underlying the relative success of exotic plants because co-occurring exotic and native plant species experience similar environmental selective pressures [14, 18]. Thus, determination of the differences in leaf functional traits between exotic and native plant species is vital for illuminating the mechanisms supporting the success of exotic species [1, 18]. The growth and development of plants is limited by their ability to acquire resources, especially by their ability to capture sunlight, which is perhaps the most important ecological factor affecting plant establishment, growth, and survival [2, 3].
Eight exotic plant species [Solidago canadensis L., Conyza canadensis (L.) Cronq., Erigeron annuus (L.) Pers., Erigeron sumatrensis Retz., Sonchus oleraceus L., Eclipta prostrata (L.) L., Helianthus tuberosus L., and Symphyotrichum subulatum (Michaux.) G. L. Nesom)] and four native species [Hemistepta lyrata (Bunge) Fisch. and C. A. Mey., Lactuca indica L., Cirsium arvense (L.) Scop. var. integrifolium, and Xanthium strumarium L.] were chosen for this study because these are amongst the most common plant species in the study area, Zhenjiang, China. More importantly, those species all belong to the Compositae family, which comprises the largest number of exotic plants in China at the family level [19, 20]. This study aims to address the differences in leaf functional traits and the phenotypic plasticity of leaf functional traits between exotic and native plant species and then to assess the role each factor may have played in the invasion process. This study presents the following hypotheses. 1) SLA of exotic plants may be likely to be higher than that of native species because exotic plants must invest more in leaf surface area (i.e., sunlight capture) rather than leaf structures per unit area in order to grow at higher rates relative to native species [14–18]. Higher SLA often correlates with a growth advantage for exotic plant species over native plant species [21–23]. 2) The plasticity index of exotic species may is likely to be higher than that of native plant species because plant species that exhibit higher phenotypic plasticity in response to changes in environmental factors can improve their fitness [14, 15]. This is supported by the positive correlation shown between phenotypic plasticity and competitiveness in some exotic plant species [8, 14, 24]. 3) Leaf thickness and single-leaf wet and dry weights may be likely to be negatively correlated with SLA; in contrast, leaf size, and leaf moisture may be positively correlated with SLA because leaves with high SLAs invest substantially in leaf structure per unit area, but leaves with low SLAs are able to invest more overall biomass on leaf structures [4, 5, 10].
2 Materials and methods
2.1 Experimental design
From mid-May 2015 to mid-September 2015, the samples of eight exotic plants, namely, S. canadensis, C. canadensis, E. annuus, E. sumatrensis, S. oleraceus, E. prostrata, H. tuberosus, and S. subulatum, and four native species, namely, H. lyrata, L. indica, C. arvense, and X. strumarium, were collected in Zhenjiang, China, which has a warm temperate climate. The annual mean temperature of the area is approximately 13.8 °C, and monthly mean temperature reaches a maximum of 27.2 °C in July and decreases to a minimum of -3.2 °C in January. Annual precipitation is approximately 614 mm, and monthly mean precipitation reaches a maximum of 196 mm in July and decreases to a minimum of 7 mm in January. Sixteen individuals of each species were collected randomly from different sites. Five mature, intact leaves of each plant were selected randomly for measurement of leaf functional traits. All samples were stored in sealed bags and immediately transported back to the laboratory to measure indices.
2.2 Determination of plant characteristics
The leaf functional traits were determined using variation in leaves. Leaf shape index was calculated as the ratio of leaf length to leaf width [5, 6, 25, 26]. Leaf length is the maximum value along the midrib, while leaf width is the maximum value perpendicular to the midrib [26]. Leaf length and leaf width were measured using a ruler [5–7, 18].
SLA was computed using the ratio of leaf area to leaf dry weight (cm2·g–1), following previous studies [5–7, 9, 27].
Leaf moisture was calculated by subtracting leaf dry weight from leaf wet weight and then dividing the difference by the leaf wet weight [5, 6, 18]. Single-leaf wet weight was determined using an electronic balance. Single-leaf dry weight was obtained by initially oven-drying the samples at 60 °C for 24 h until a constant weight was reached; final single-leaf dry weight was then determined using an electronic balance with an accuracy of 0.001 g [5, 6, 18].
Leaf thickness was calculated by overlaying five leaves and measuring their combined thickness using a Vernier caliper with an accuracy of 0.01 mm [5–7, 18].
The plasticity index [ranging from zero (no plasticity) to one (maximum plasticity)] of characteristics of those plant species was calculated with the following equation [15, 27]:
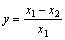
where y is the plasticity index; x1 is the maximum value; x2 is the minimum value.
2.3 Statistical analysis
Data were evaluated to determine any deviations from normality and homogeneity of variance before data analysis. Differences among various dependent variables were assessed using an analysis of variance. Statistical significance was set at P values equal to or lower than 0.05. Correlation analysis was performed using Pearson product-moment correlation coefficient to determine the patterns among various dependent variables. All statistical analyses were performed using SPSS Statistics (version 22.0; IBM, Armonk, NY, USA).
3 Results
3.1 Differences in leaf functional traits between exotic and native plant species
Differences were observed in leaf functional traits between exotic and native plant species (Table 1). In particular, leaf width of exotic plants was significantly lower than that of native species (P<0.05). Leaf length, SLA, single-leaf wet and dry weights, leaf moisture, and leaf thickness of exotic plants were also lower than those of native species, but not significantly (P>0.05). The leaf shape index of exotic plants was higher than that of native species, but not substantially (P>0.05).
3.2 Differences in plasticity indices of leaf functional traits between exotic and native plant species
The plasticity indices of single-leaf wet weight and leaf thickness of exotic plants were significantly lower than those of native species (Table 2, P<0.05). Meanwhile, the plasticity indices of leaf length, leaf width, and SLA of exotic plants were also lower than those of native species, but not significantly (P>0.05). The plasticity indices of leaf shape index and leaf moisture of exotic plants were higher than those of native species, but not significantly (P>0.05).
3.3 Relationships between indices of leaf functional traits
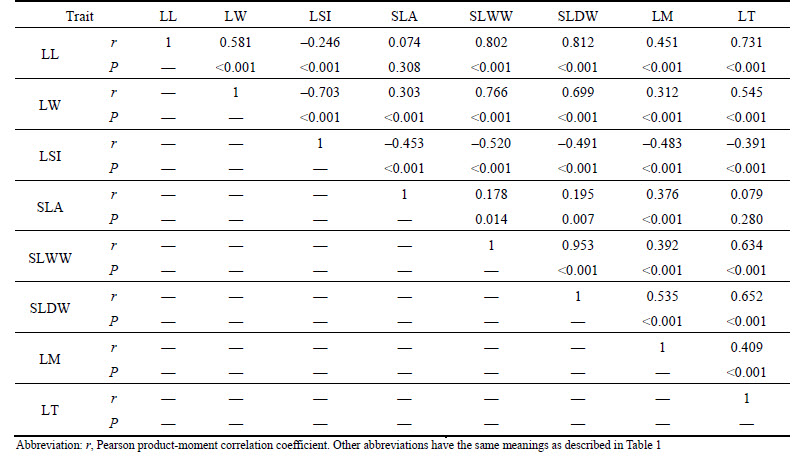
Correlation patterns among leaf functional traits of the twelve plant species were assessed via correlation analysis (Table 3). Many significant correlations were revealed. In particular, leaf length was positively correlated with leaf width, single-leaf wet and dry weights, leaf moisture, and leaf thickness but negatively correlated with leaf shape index (P<0.001). Leaf width was positively correlated with SLA, single-leaf wet and dry weights, leaf moisture, and leaf thickness (P<0.001), but negatively correlated with leaf shape index (P<0.001). Leaf shape index was negatively correlated with SLA, single-leaf wet and dry weights, leaf moisture, and leaf thickness (P<0.001). SLA was positively correlated with leaf moisture (P<0.001). Single-leaf wet and dry weights were positively correlated with leaf moisture, and leaf thickness (P<0.001). Leaf moisture was positively correlated with leaf thickness (P<0.001).
4 Discussion
Functional trait differences between exotic and native plant species are believed to explain the ability of the former species to successfully invade the habitats of the latter [14, 18, 19]. Previous studies have shown that exotic plants invest more biomass on leaf growth rather than leaf structures per unit area, thus enabling a higher growth rate [14–17, 28] and that exotic plant SLA is often positively correlated with a growth advantages over native species [8, 22, 23]. It was therefore expected that exotic plants may display higher SLA than native species. This study rejected the first hypothesis by showing that SLA of exotic plants was actually slightly lower than that of native species but not substantially. This indicates that exotic plants do not show higher or even slightly lower resource capture ability than native species, suggesting that this functional trait may not necessarily contribute to successful survival of the exotic plants in this study. Previous investigators also showed that some leaf traits are not responsible for increasing the competitiveness of exotic species [29]. Other studies have also reported some inconsistent results regarding the difference in SLA between exotic and native plant species, i.e., exotic plants may display higher [8, 14–18, 30], similar [31, 32], or even lower [33, 34] SLA values compared with native species. Accordingly, the relationship between relative growth rate and SLA requires special attention or even re-examination because these conflicting results, namely, the observed relationship between relative growth rate and SLA of plant species that were positive [4, 35], negative [36, 37], or variable amongst habitats [38] and growth periods [39]. Thus, the relationship between relative growth rate and SLA is somewhat species specific.
Table 1 Differences in leaf functional traits between exotic and native plant species

Table 2 Differences in plasticity indices of leaf functional traits between exotic and native plant species
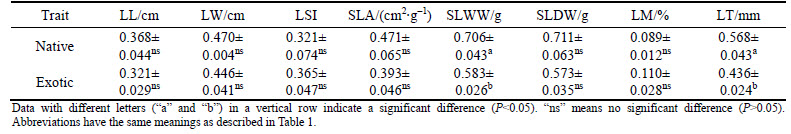
Table 3 Relationship among leaf functional traits of twelve plant species
Additionally, single-leaf wet and dry weights, and leaf thickness of exotic plants were lower than those of native species but not substantially. To some extent, this implies that leaf construction costs of exotic plants were lower than those of native species [8, 40, 41]. Generally, low leaf construction costs are closely related to efficient energy use, which consequently facilitates plant growth [40, 42]. Thus, the relatively low leaf construction costs of exotic plants may play an important role in the success of their invasions. Moreover, the higher leaf shape index and lower leaf width of exotic plants can enhance the efficiency of resource acquisition (especially of sunlight) via adaptive or plastic changes in leaf shape and leaf size, thereby enhancing the survival of exotic plants.
Because any functional trait that enhances the fitness of plants in their habitats will be selected and may therefore adapt, phenotypic plasticity itself is a potential target for selection [4, 43]. Phenotypic plasticity can enable plant species to optimally utilize or partition niches in plant communities and thus enhance biodiversity in both the short and long term [44, 45]. As such, the increased phenotypic plasticity of plant species for any functional traits related to their performance and fitness may play a central role in the success of their invasions [4, 14, 43]. All leaf functional traits of the species examined in this study displayed phenotypic plasticity. This indicates that those functional traits that displayed some degree of phenotypic plasticity may be involved in local adaptation of plants to their various habitats. Phenotypic plasticity of leaf functional traits in response to environment changes may yield ecological strategies by which plant species are able to live in competitive environments. Hence, the phenotypic plasticity of leaf functional traits of exotic plants may be higher than that of native species. Additionally, phenotypic plasticity of exotic plants may play an important role in their successful invasions [24, 30].
Contrary to expectations, the phenotypic plasticity of single-leaf wet weight and leaf thickness of exotic plants were significantly lower than those of native species, which was not consistent with the second hypothesis. The lower phenotypic plasticity of single-leaf wet weight and leaf thickness of exotic plants may be attributed to a fitness cost for plastic plant species under unfavorable environments. Lower plasticity of functional traits can actually compensate for the negative effects of such adverse environments and thus facilitate an invasion [46, 47]. For example, the lower phenotypic plasticity of single-leaf wet weight and leaf thickness of exotic plants can stabilize their leaf construction costs. Thus, the lower phenotypic plasticity of some traits may be one of the competitive strategies by which exotic plants successfully invade new habitats [47].
However, other indices of leaf functional traits did not significantly differ between exotic and native plant species. Similarly, as previously indicated, exotic plants did not always exhibit a higher range of phenotypic plasticity of functional traits compared with native species. Phenotypic plasticity is supposed to be important for plant species as it allows them to thrive in a wide range of environments; however, numerous studies have showed that the relationship between phenotypic plasticity and the competitiveness of exotic plants is inconsistent. Some studies have shown that exotic plants may exhibit higher plasticity than native species and that the higher phenotypic plasticity of exotic plants is closely related to their competitiveness [8, 14, 15]. Other studies have also demonstrated that exotic plants may possess similar [48, 49] or even reduced [46, 47, 50] phenotypic plasticity compared with native species. Overall, phenotypic plasticity may play an important role in the successful survival of some exotic plants, but not all species.
In general, leaves with higher SLAs invest less biomass into leaf construction to achieve high resource use efficiency and thereby exhibit lower leaf thickness and single-leaf wet and dry weights but higher leaf size and leaf moisture [4, 10, 43]. It was therefore expected that SLA may be positively correlated with leaf size and leaf moisture but negatively correlated with leaf thickness and single-leaf wet and dry weights. This would be consistent with high SLA leaves bearing low leaf construction costs, while low SLA leaves invest substantial biomass in leaf structures [4, 5, 10]. This study showed that SLA was positively correlated with leaf width and leaf moisture but negatively correlated with leaf shape index, while SLA and single-leaf wet and dry weights did not show a significant relationship. Thus, results obtained in this study are only partly consistent with the third hypothesis considered.
5 Conclusions
Leaf width of exotic plants was significantly lower than that of native species, while the plasticity indices of single-leaf wet weight and leaf thickness of exotic plants were also significantly lower than those of native species. Thus, exotic plants did not always exhibit higher values of leaf functional traits or increased phenotypic plasticity of leaf functional traits compared with native species.
References
[1] VILE D, GARNIER E, SHIPLEY B, LAURENT G, NAVAS M L, ROUMET C, LAVOREL S,  S, HODGSON J G, LLORET F, MIDGLEY G F, POORTER H, RUTHERFORD M C, WILSON P J, WRIGHT I J. Specific leaf area and dry matter content estimate thickness in laminar leaves [J]. Annals of Botany, 2005, 96: 1129–1136.
S, HODGSON J G, LLORET F, MIDGLEY G F, POORTER H, RUTHERFORD M C, WILSON P J, WRIGHT I J. Specific leaf area and dry matter content estimate thickness in laminar leaves [J]. Annals of Botany, 2005, 96: 1129–1136.
[2] LIU F D, YANG W J, WANG Z S, XU Z, LIU H, ZHANG M, LIU Y H, AN S Q, SUN S C. Plant size effects on the relationships among specific leaf area, leaf nutrient content, and photosynthetic capacity in tropical woody species [J]. Acta Oecologica-International Journal of Ecology, 2010, 36: 149–159.
[3] MENG F Q, CAO R, YANG D M, NIKLAS K J, SUN S C. Trade-offs between light interception and leaf water shedding: A comparison of shade- and sun-adapted species in a subtropical rainforest [J]. Oecologia, 2014, 174: 13–22.
[4] POORTER H, NIINEMETS U, POORTER L, WRIGHT I J, VILLAR R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis [J]. New Phytologist, 2009, 182: 565–588.
[5] XIAO H G, WANG C Y, LIU J, WANG L, DU D L. Insights into the differences in leaf functional traits of heterophyllous Syringa oblata under different light intensities [J]. Journal of Forestry Research, 2015, 26: 613–621.
[6] WANG C Y, LIU J, XIAO H G, Du D L. Response of leaf functional traits of Cerasus yedoensis (Mats.) Yü li to serious insect attack [J]. Polish Journal of Environmental Studies, 2016, 25: 333–339.
[7] WANG C Y, XIAO H G, LIU J, ZHOU J W, DU D L. Insights into the effects of simulated nitrogen deposition on leaf functional traits of Rhus typhina [J]. Polish Journal of Environmental Studies, 2016, 25: 1279–1284.
[8] HUANG Q Q, SHEN Y D, LI X X, LI S L, FAN Z W. Invasive Eupatorium catarium and Ageratum conyzoides benefit more than does a common native plant from nutrient addition in both competitive and non-competitive environments [J]. Ecological Research, 2016, 31: 145–152.
[9] SCHEEPENS J F, FREI E S,  J. Genotypic and environmental variation in specific leaf area in a widespread Alpine plant after transplantation to different altitudes [J]. Oecologia, 2010, 164: 141–150.
J. Genotypic and environmental variation in specific leaf area in a widespread Alpine plant after transplantation to different altitudes [J]. Oecologia, 2010, 164: 141–150.
[10] PIETSCH K A, OGLE K, CORNELISSEN J H C, CORNWELL W K,  G, CRAINE J M, JACKSON B G, KATTGE J, PELTZER D A, PENUELAS J, REICH P B, WARDLE D A, WEEDON J T, WRIGHT I J, ZANNE A E, WIRTH C. Global relationship of wood and leaf litter decomposability: The role of functional traits within and across plant organs [J]. Global Ecology and Biogeography, 2014, 23: 1046–1057.
G, CRAINE J M, JACKSON B G, KATTGE J, PELTZER D A, PENUELAS J, REICH P B, WARDLE D A, WEEDON J T, WRIGHT I J, ZANNE A E, WIRTH C. Global relationship of wood and leaf litter decomposability: The role of functional traits within and across plant organs [J]. Global Ecology and Biogeography, 2014, 23: 1046–1057.
[11] POWELL K I, CHASE J M, KNIGHT T M. Invasive plants have scale-dependent effects on diversity by altering species-area relationships [J]. Science, 2013, 339: 316–318.
[12] WANG C Y, XIAO H G, WANG L, LIU J, DU D L. Insights into ecological effects of invasive plants on soil nitrogen cycling [J]. American Journal of Plant Sciences, 2015, 6: 34–46.
[13] WANG C Y, XIAO H G, ZHAO L L, LIU J, WANG L, ZHANG F, SHI Y C, DU D L. The allelopathic effects of invasive plant Solidago canadensis on seed germination and growth of Lactuca sativa enhanced by different types of acid deposition [J]. Ecotoxicology, 2016, 25: 555–562.
[14] VAN KLEUNEN M, WEBER E, FISCHER M. A meta-analysis of trait differences between invasive and non-invasive plant species [J]. Ecology Letters, 2010, 13: 235–245.
[15] CHEN L Y, TIU C J, PENG S L, SIEMANN E. Conspecific plasticity and invasion: invasive populations of Chinese tallow (Triadica sebifera) have performance advantage over native populations only in low soil salinity [J]. PLoS ONE, 2013, 8: e74961.
[16] SHEPPARD C S, BURNS B R. Effects of interspecific alien versus intraspecific native competition on growth of native woody plants [J]. Plant Ecology, 2014, 215: 1527–1538.
[17] TE BEEST M, ESLER K J, RICHARDSON D M. Linking functional traits to impacts of invasive plant species: A case study [J]. Plant Ecology, 2015, 216: 293–305.
[18] GAO X M, ZHAO Y J, YANG X J, SUN S C. Linking trait differences to community dynamics: Evidence from Eupatorium adenophorum and co-occurring native species during a three-year succession [J]. PLoS ONE, 2013, 8: e50247.
[19] YAN X L, LIU Q R, SHOU H Y, ZENG X F, ZHANG Y, CHEN L, LIU Y, MA HY, QI S Y, MA J S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants [J]. Biodiversity Science, 2014, 22(5): 667–676. (in Chinese)
[20] WANG C Y, LIU J, XIAO H G, ZHOU J W, DU D L. Floristic characteristics of alien invasive seed plant species in China [J]. Anais da Academia Brasileira de Ciências, 2016, 88(3): 1791–1797.
[21] GLEASON S M, ARES A. Photosynthesis, carbohydrate storage and survival of a native and an introduced tree species in relation to light and defoliation [J]. Tree Physiology, 2004, 24: 1087–1097.
[22] LEISHMAN M R, HASLEHURST T, ARES A, BARUCH Z. Leaf trait relationships of native and invasive plants: community- and global-scale comparisons [J]. New Phytologist, 2007, 176: 635–643.
[23] HULME P. Phenotypic plasticity and plant invasions: is it all Jack? [J] Functional Ecology, 2008, 22: 3–7.
[24] JEONG N, MOON J K, KIM H S, KIM C G, JEONG S C. Fine genetic mapping of the genomic region controlling leaflet shape and number of seeds per pod in the soybean [J]. Theoretical and Applied Genetics, 2011, 122: 865–874.
[25] WANG Z, ZHANG L. Leaf shape alters the coefficients of leaf area estimation models for Saussurea stoliczkai in central Tibet [J]. Photosynthetica, 2012, 50: 337–342.
[26] KARDEL F, WUYTS K, BABANEZHAD M, VITHARANA U W A, WUYTACK T, POTTERS G, SAMSON R. Assessing urban habitat quality based on specific leaf area and stomatal characteristics of Plantago lanceolata L. [J]. Environmental Pollution, 2010, 158: 788–794.
[27] LAMARQUE L J, LORTIE C J,  A J, DELZON S. Genetic differentiation and phenotypic plasticity in life-history traits between native and introduced populations of invasive maple trees [J]. Biological Invasions, 2015, 17: 1109–1122.
A J, DELZON S. Genetic differentiation and phenotypic plasticity in life-history traits between native and introduced populations of invasive maple trees [J]. Biological Invasions, 2015, 17: 1109–1122.
[28] LAMARQUE L J,  A J, EYMERIC C, LASNIER J B, LORTIE C J, DELZON S. A test for pre-adapted phenotypic plasticity in the invasive tree Acer negundo L. [J]. PLoS ONE, 2013, 8: e74239.
A J, EYMERIC C, LASNIER J B, LORTIE C J, DELZON S. A test for pre-adapted phenotypic plasticity in the invasive tree Acer negundo L. [J]. PLoS ONE, 2013, 8: e74239.
[29] GROTKOPP E, ERSKINE-OGDEN J,  M. Assessing potential invasiveness of woody horticultural plant species using seedling growth rate traits [J]. Journal of Applied Ecology, 2010, 47: 1320–1328.
M. Assessing potential invasiveness of woody horticultural plant species using seedling growth rate traits [J]. Journal of Applied Ecology, 2010, 47: 1320–1328.
[30] WANG C Y, ZHOU J W, XIAO H G, LIU J, WANG L. Variations in leaf functional traits among plant species grouped by growth and leaf types in Zhenjiang, China [J]. Journal of Forestry Research, 2017, 28(2): 241–248.
[31] ORDONEZ A. Global meta-analysis of trait consistency of non-native plants between their native and introduced areas [J]. Global Ecology and Biogeography, 2014, 23: 264–273.
[32] FAN S F, LIU C H, YU D, XIE D. Differences in leaf nitrogen content, photosynthesis, and resource-use efficiency between Eichhornia crassipes and a native plant Monochoria vaginalis in response to altered sediment nutrient levels [J]. Hydrobiologia, 2013, 711: 129–137.
[33] GENG X Y, JIANG S, LI B, PAN X Y. Do higher resource capture ability and utilization efficiency facilitate the successful invasion of exotic plant? A case study of Alternanthera philoxeroides [J]. American Journal of Plant Sciences, 2013, 4: 1839–1845.
[34] TIAN D S, PAN Q M, SIMMONS M, CHAOLU H, DU B H, BAI Y F, WANG H, HAN X G. Hierarchical reproductive allocation and allometry within a perennial bunchgrass after 11 years of nutrient addition [J]. PLoS ONE, 2012, 7: e42833.
[35] SHIPLEY B. Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance [J]. Functional Ecology, 2002, 16: 682–689.
[36] POSSEN B J H M, ANTTONEN M J, OKSANEN E, ROUSI M, HEINONEN J, KOSTIAINEN K, KONTUNEN-SOPPELA S, HEISKANEN J, VAPAAVUORI E M. Variation in 13 leaf morphological and physiological traits within a silver birch (Betula pendula) stand and their relation to growth [J]. Canadian Journal of Forestry Research, 2014, 44: 657–665.
[37] LEBEL P, BRADLEY R L, THIFFAULT N. The relative importance of nitrogen vs. moisture stress may drive intraspecific variations in the SLA-RGR relationship: The case of Picea mariana seedlings [J]. American Journal of Plant Sciences, 2013, 4: 1278–1284.
[38] IIDA Y, KOHYAMA T S, SWENSON N G, SU S H, CHEN C T, CHIANG J M, SUN I F. Linking functional traits and demographic rates in a subtropical tree community: the importance of size dependency [J]. Journal of Ecology, 2014, 102: 641–650.
[39] NAGEL J M, GRIFFIN K L. Construction cost and invasive potential: comparing Lythrum salcaria (Lythraceae) with co-occurring native species along pond banks [J]. American Journal of Botany, 2001, 88: 2252–2258.
[40] SONG L Y, NI G Y, CHEN B M, PENG S L. Energetic cost of leaf construction in the invasive weed Mikania micrantha HBK and its co-occurring species: Implications for invasiveness [J]. Botanical Studies, 2007, 48: 331–338.
[41] HOU Q Q, CHEN B M, PENG S L, CHEN L Y. Effects of extreme temperature on seedling establishment of nonnative invasive plants [J]. Biological Invasions, 2014, 16: 2049–2061.
[42] MCINTYRE P J, STRAUSS S Y. Phenotypic and transgenerational plasticity promote local adaptation to sun and shade environments [J]. Ecology and Evolution, 2014, 28: 229–246.
[43] ASHTON I W, MILLER A E, BOWMAN W D, SUDING K N. Niche complementarity due to plasticity in resource use: Plant partitioning of chemical N forms [J]. Ecology, 2010, 91: 3252–3260.
[44] ZUPPINGER-DINGLEY D, SCHMID B, PETERMANN J S, YADAV V, DE DEYN G B, FLYNN D F. Selection for niche differentiation in plant communities increases biodiversity effects [J]. Nature, 2014, 515: 108–111.
[45] MARON J L, ELMENDORF S C,  M. Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum [J]. Evolution, 2007, 61: 1912–1924.
M. Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum [J]. Evolution, 2007, 61: 1912–1924.
[46] QUAN G M, MAO D J, ZHANG J E, XIE J F, XU H Q, AN M. Response of invasive Chromolaena odorata and two coexisting weeds to contrasting irradiance and nitrogen [J]. Photosynthetica, 2015, 53: 419–429.
[47]  K, GIANOLI E. Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis [J]. Oikos, 2011, 120: 1393–1401.
K, GIANOLI E. Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis [J]. Oikos, 2011, 120: 1393–1401.
[48] MATZEK V. Trait values, not trait plasticity, best explain invasive species performance in a changing environment [J]. PLoS ONE, 2012, 7: e48821.
[49] WILLIAMS J L, AUGE H, MARON J L. Different gardens, different results: Native and introduced populations exhibit contrasting phenotypes across common gardens [J]. Oecologia, 2008, 157: 239–248.
(Edited by YANG Hua)
Cite this article as:
WANG Cong-yan, LIU Jun, ZHOU Jia-wei, XIAO Hong-guang. Differences in leaf functional traits between exotic and native Compositae plant species [J]. Journal of Central South University, 2017, 24(10): 2468–2474.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-017-3658-7Foundation item: Project(31300343) supported by the National Natural Science Foundation of China; Project(Y20160023) supported by Open Science Research Fund of State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, China; Project supported by Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment, China
Received date: 2016-04-18; Accepted date: 2016-10-06
Corresponding author: WANG Cong-yan, PhD; Tel: +86–511–88790955; E-mail: liuyuexue623@163.com
Abstract: This study aims to determine the differences in leaf functional traits and phenotypic plasticity of leaf functional traits between exotic and native Compositae plant species. Leaf width of exotic plants was significantly lower than that of native species. Leaf length, specific leaf area (SLA), single-leaf wet and dry weights, leaf moisture, and leaf thickness of exotic plants were also lower than those of native species but not significantly. The leaf shape index of exotic plants was higher than that of native species but not significantly. This implies that the relatively low leaf construction cost for exotic plants may play an important role in the success of their invasions. The higher leaf shape index and lower leaf width of exotic plants can enhance the efficiency of resource capture (especially sunlight capture) via adjustments to leaf shape and size, thereby increasing the survival of exotic plants. The plasticity indices of single-leaf wet weight and leaf thickness of exotic plants were significantly lower than those of native species. The lower phenotypic plasticity of single-leaf wet weight and leaf thickness of exotic plants may be the result of a cost to plasticity. That is, if the plasticity is too high, the fitness of plant species might be reduced sharply under unfavorable environments. Thus, lower plasticity of leaf functional traits may compensate for the negative impact of adverse environments and stabilize leaf construction costs for exotic plants. Moreover, reduced phenotypic plasticity might be one of the key competitive strategies by which exotic plants successfully invade new habitats. Overall, exotic plants did not always exhibit higher values of leaf functional traits or increased phenotypic plasticity of leaf functional traits compared with native species.


