
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1582-1587
High temperature oxidation behavior of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho eutectic alloy
WANG Zhen-sheng1,2, XIE Yi2,3, GUO Jian-ting2, ZHOU Lan-zhang2, HU Zhuang-qi2,
ZHANG Guang-ye1, CHEN Zhi-gang1
1. College of Electromechanical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
2. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China;
3. Hunan Electric Power Corporation Research Institute, Changsha 410007, China
Received 23 September 2011; accepted 5 January 2012
Abstract:
The isothermal oxidation behavior of NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho directional eutectic alloy was investigated with the help of scanning electron microscopy and X-ray diffraction. The results revealed that a continuous Al2O3 scale was formed and owned excellent oxidation resistance in the temperature range of 900-1100 ℃. When the temperature was up to 1150 ℃, the continuous Al2O3 oxide film ruptured. Trace rare earth element Ho distributed uniformly in the alloy and relatively high level of Al in Cr(Mo) phase are beneficial to the formation of continuous and compact Al2O3 scale. During the oxidation, a phase transformation from θ-Al2O3 to α-Al2O3 existed on the surface of oxidation film. It resulted in the abnormal oxidation mass gain happening when the alloy was oxidized at 1000 ℃ or 1050 ℃.
Key words:
intermetallic compounds; NiAl; high temperature oxidation; directional eutectic alloy;
1 Introduction
Ordered intermetallics NiAl is a kind of great potential high-temperature materials for aircraft engine because of its high melting point, low density, good thermal conductivity and excellent oxidation resistance. Over the years, scholars at home and abroad have taken up with the industrial application of NiAl and have achieved some results [1-4], one of which is the directionally solidified NiAl-31Cr-3Mo eutectic alloy with good overall performance. According to the reports of National Aeronautics and Space Administration (NASA), toughness of directionally solidified NiAl-31Cr-3Mo alloy at room temperature is up to 17-22.1 MPa·m1/2, and its high temperature strength is further improved by micro-alloying. Therefore, its overall performance can be comparable with that of NiAl-28Cr-6Mo eutectic alloy [5]. But its chemical stability in the service environment must be considered. Due to the complex preparation and high cost of directional solidification, usually the regularly cast NiAl-Cr(Mo) eutectic alloy is studied to speculate the oxidation of equiaxed directionally solidified eutectic alloy at high temperature. The results showed that the addition of a large number of alloying elements severely reduced the oxidation resistance of NiAl alloy [6]. At higher temperatures, Cr2O3 and Mo oxides are unstable and volatilizable, which undermines the continuity and compactness of oxide film and reduces its productivity. However, trace amount of rare earth element, such as Ce, Nd, Ho and Dy, is useful to improve the oxidation resistance of NiAl-Cr(Mo) eutectic alloy. Because trace rare earth element can change mass transfer mechanism of Al and Cr in the oxidation process, namely, outward transmission of Al and Cr is changed to inward transmission of O. It results in elimination of interspace in the oxide film and the significant reduction of oxidation mass gain [7-9]. Directionally solidified NiAl-28Cr-6Mo eutectic alloy has superior oxidation resistance compared with ordinarily cast NiAl-28Cr-6Mo eutectic alloy. The rapid cooling rate of directional solidification could refine eutectic microstructure and increase Al element content in Cr(Mo) phase, which is conducive to formation of a continuous Al2O3 oxide film [10].
In the present work, NiAl-31Cr-3Mo eutectic alloy is directionally solidified by liquid metal cooling (LMC) process to study the high temperature oxidation behavior and its mechanism. Meanwhile, in order to both strengthen high temperature strength of the directionally solidified eutectic alloy and also its antioxidant properties, trace Hf and rare earth element Ho are added according to Refs. [6-10].
2 Experimental
A vacuum induction melted and drop cast ingot with nominal composition of NiAl-31Cr-2.9Mo- 0.1Hf-0.05Ho (mole fraction, %) alloy was directionally solidified in a Al2O3-SiO2 ceramic mold under Ar atmosphere by liquid metal (Sn) cooling technique. Based on the technical parameters of this furnace, the thermal gradient (G) at the solid/liquid interface is about 150 K/cm. Specimens of 15.0 mm in diameter and 210 mm in length were obtained with withdrawal rate of 3 mm/min.
Oxide samples having a gauge section of 10 mm×10 mm×2 mm were cut from the directionally solidified alloy by an electron discharge machine (EDM). Their surfaces were removed to 800 grit by abrasive paper. Then oxide samples were ultrasonic cleaned with acetone and ethanol.
Thermal oxidation tests were carried out in an ordinary muffle furnace for 100 h. The experimental temperature range was 900-1150 ℃. To prevent oxidation film from brittle and splashing out during cooling, the samples were put into two overlap Al2O3 crucibles with constant mass. Every a certain amount of time, the oxidation mass gain of sample was measured by electronic balance in the sense of 2×10-5 g. Oxidation mass gain was the average mass gain of three. S-3400N scanning electron microscope (SEM) equipped with energy dispersive X-ray spectroscopy (EDX) and Rigaku D/max-2500CC X-ray diffraction was used to analyze the microstructure and phases of the oxidation samples.
3 Results and discussion
3.1 Microstructure
Figure 1(a) shows the microstructure of directionally solidified Ni-33Al-31Cr-2.9Mo-0.1Hf- 0.05Ho eutectic alloy. It was composed of black dendritic primary NiAl, NiAl/Cr(Mo) eutectic cell and white phase which was identified as Hf solid solution (Hfss) with the help of EDX (Table 1). Most of Hfss distributed along the eutectic cell boundaries (see Fig. 1(b)). The intercellular spacing of directionally solidified eutectic alloy is 1-10 μm, less than that of ordinarily cast eutectic alloy [11].
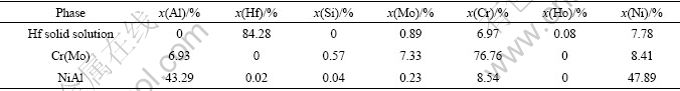
The compositions of the observed phases obtained by energy dispersive spectroscopy (EDS) are listed in Table 1. Al content in Cr(Mo) phase is similar between directionally solidified (DS) NiAl-Cr(Mo)-Hf eutectic alloy and ordinarily cast NiAl-Cr(Mo)-Hf eutectic alloy [11]. However, Cr content in NiAl phase of DS NiAl-Cr(Mo)-Hf eutectic alloy is higher than that of ordinarily cast NiAl-Cr(Mo)-Hf eutectic alloy. Ni2Al3Ho phase was detected by TEM at the NiAl grain boundary[12], but any Ho compounds were not found in Cr(Mo) and Hf solid solution phases. In addition, Si is an impurity element which was from the reaction between SiO2 mold shell materials and liquid NiAl-Cr(Mo)-Hf eutectic alloy in the process of directional solidification.
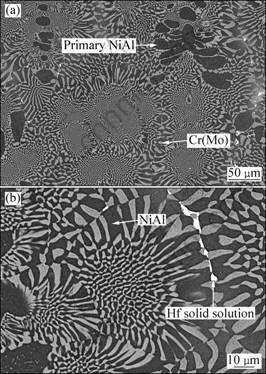
Fig. 1 SEM-BSE images showing microstructure of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho eutectic alloy: (a) Transverse section; (b) Locally high magnified image
Table 1 Compositions of phase in directionally solidified eutectic alloys by EPMA
3.2 Oxidation kinetics
Figure 2 shows the oxidation kinetics curves of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf- 0.05Ho eutectic alloy at 900-1150 ℃. Oxidation mass gain increased with increasing temperature except 1100 ℃. Such an anomaly phenomenon was related with phase transition on surface of oxidation film. When the samples were oxidized for 100 h at 900, 1000, 1050, 1100 and 1150 ℃, oxidation mass gain values were 0.15, 0.29, 0.34, 0.27 and 0.92 mg/cm2, respectively. Compared with NiAl, the oxidation mass gain of NiAl-Cr(Mo)-Hf eutectic alloy is similar [13]. But at 1050-1150 ℃, the mass gain is slightly lower than that of NiAl. Some Cr and Mo oxides in the oxidation film of eutectic alloy are volatile at high temperature. It can be seen from Fig. 2(b), compared with commonly cast NiAl-30.9Cr-3Mo-0.1Dy and NiAl-30.75Cr-3Mo-0.25Ho, the oxidation mass gain of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf- 0.05Ho eutectic alloy is about 1/2 and 1/3 respectively in the temperature range of 1050-1100 ℃ [14,15]. Although the influence of different rare earth elements on the oxidation mechanism of NiAl-28Cr-6Mo eutectic alloy exits, it can be sure that directional solidification process can improve the oxidation resistance of NiAl-31Cr-3Mo eutectic alloy.
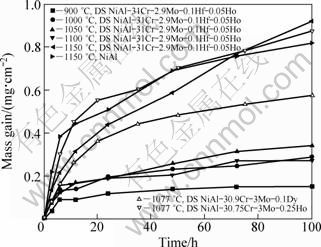
Fig. 2 Oxidation kinetics curves of NiAl and directionally solidified (DS) NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho eutectic alloy at different temperatures in air
3.3 Surface morphology and phase composition of oxidation products
Figure 3 shows the surface oxide film morphology at different temperatures. There is a good relationship between the oxide film surface morphology and the morphology of the matrix alloy under low magnification (Fig. 3(a)).
At the initial oxidation stage, the oxidation was not serious. The mainly product of directionally solidified eutectic alloy was fine needle or flake θ-Al2O3 and tilting tumor (Fig. 3(b)) which was identified as Cr2O3 with the help of EDS.
With increasing the oxidation time, fine equiaxed α-Al2O3 began to appear on the surface of DS eutectic alloy (Fig. 3(c)), which indicated the transformation of θ-Al2O3→α-Al2O3. Moreover, the transformation time decreased with increasing the temperature. At the same time, Cr2O3 tilting tumor presented honeycomb morphology (Fig. 3(d)).
Based on the results of X-ray diffraction analysis (Fig. 4), when the oxidation temperature was 900 ℃, oxide film was mainly composed of flake θ-Al2O3 and Cr2O3 tumor (Fig. 3(e)). When the temperature was 1000 ℃, there existed a small amount of fine equiaxed α-Al2O3 in surface film (Fig. 3(f)), and the quantity of α-Al2O3 increased with increasing the oxidation temperature. As the temperature was up to 1150 ℃, oxide film was mainly composed of small and dense α-Al2O3 (Fig. 3(g)). Because of the easy formation of gaseous volatile CrO3, Cr2O3 tumor morphology gradually transformed from dense to loose honeycomb with increasing the temperature and oxidation time. During oxidation at 1150 ℃ for 100 h, Cr2O3 tumor completely disappeared, and the oxide film spalled seriously (Fig. 3(h)). The spalling reason was that poor adhesive Al2O3 oxide grew laterally and folded or cracked during cooling [11]. So, eutectic alloy oxidation mass gain increased dramatically at 1150 ℃. There were not any Mo and Ho oxides in the film detected by X-ray diffraction, which is due to highly volatile Mo oxides and lower level of Ho content.
Figure 5 shows the fracture surface of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho eutectic alloy after 100 h oxidation at different temperatures. Directionally solidified eutectic alloy did not oxidize seriously at 900 ℃, a continuous oxide film with thickness around 1 μm formed on the surface. When the oxidization temperature reached 1000 ℃, a continuous and loose oxide film formed on surface, some larger block Cr2O3 existed in the oxide film, and a few holes distributed along the interface between matrix and oxide film. Moreover, internal oxidation along the NiAl / Cr (Mo) phase was serious. From Fig. 5(a), EDS analysis shows that small Ho oxides formed at the interface between oxide film and alloy substrate, and the small oxides had a pinning effect on oxide film [15] (Fig. 5(a)). Similar to cases at 1050 ℃ and 1000 ℃, a continuous and dense oxide film formed on surface at 1100 ℃ (Fig. 5(b)). There was a Cr-rich layer between oxide film and alloy substrate. Characteristic X-ray surface scanning results show that the black oxide film was rich in Al and O elements. It could be identified as Al2O3 according to X-ray diffraction analysis.
There was phase transformation of θ-Al2O3→α-Al2O3 in the oxidation process. At a lower temperature or initial oxidation stage, the metastable θ-Al2O3 grew rapidly. But at a high temperature or late oxidation stage, the needle metastable θ-Al2O3 transformed into equiaxed α-Al2O3. The mainly oxidized products of directionally solidified eutectic alloy are loose and continuous θ-Al2O3 oxide film at 1000 ℃ and 1050 ℃. Because of the faster growth speed and less protectiveness of θ-Al2O3 [6], serious internal oxidation occurred. A continuous and dense α-Al2O3 oxide film formed at 1100 ℃, which enhanced the oxidation resistance. Therefore, oxidation kinetics of directionally solidified eutectic alloy appeared anomaly at 1000 ℃ and 1050 ℃ [16].
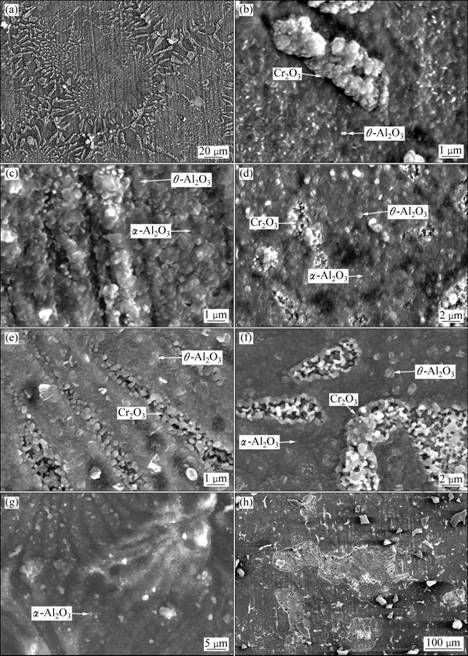
Fig. 3 Surface morphologies of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho eutectic alloy oxidized in air: (a) 1000 ℃ for 1 h; (b) 1000 ℃ for 1 h; (c) 1050 ℃ for 12 h; (d) 1150 ℃ for 3 h; (e) 900 ℃ for 100 h; (f) 1000 ℃ for 100 h; (g) 1150 ℃ for 100 h; (h) 1150 ℃ for 100 h
3.4 Discussion
The oxidation experimental (higher than 900 ℃) result of cast NiAl-28Cr-5Mo-1Hf eutectic alloy was reported in Ref. [6]. The higher content of Al in NiAl matrix would form a single Al2O3, and the Al2O3 film could be quickly formed along the substrate surface in oxidation process. Meanwhile, the Cr(Mo) phase and Heusler phase of eutectic alloy were exposed to air and oxidation occurred. Because the growth of Al2O3 was slower than that of Cr2O3, Al2O3 film could not quickly cover the Cr (Mo) phase, resulting in the formation of nodular Cr(Mo)-rich oxide along the Cr(Mo) phase. In addition, because of the higher thermodynamic stability of Al2O3 phase than HfO2 phase, HfO2 oxide was formed on the surface of Heusler phase. Although the oxidation resistance of Cr2O3 was good at low temperature, the unstable Cr2O3 would become volatile CrO3 at high temperature. The CrO3 evaporation increased with increasing the oxidation temperature and time. In addition, the Mo oxides volatilize more at high temperature. Therefore, it undermined the continuity and compactness of oxide film and reduced the protection. The oxidation results of binary NiAl alloys showed that a continuous, dense Al2O3 oxide film with good oxidation resistance was formed in the oxidation process[13].
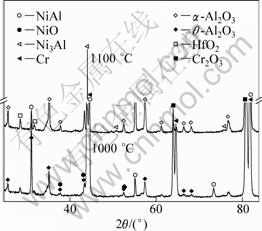
Fig. 4 XRD patterns of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho eutectic alloy after oxidation in air for 100 h at 1000 ℃ and 1100 ℃ respectively
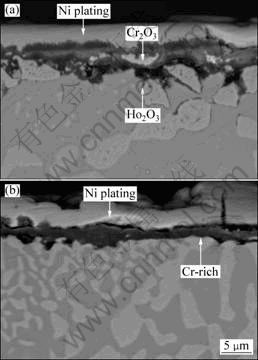
Fig. 5 SEM-BSE cross-sectional morphologies of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho eutectic alloy after oxidation in air for 100 h at 1000 ℃ (a) and 1100 ℃ (b)
According to the experimental results, a continuous Al2O3 oxide film was formed on the surface of directionally solidified NiAl-31Cr-2.9Mo-0.1Hf- 0.05Ho eutectic alloy at 900-1100 ℃, which played a controlling role in oxidation and had a good antioxidant performance. It may be due to the following reasons.
1) Trace rare earth element Ho changed mass transfer mechanism of alloying elements in the oxidation process, namely, outward transmission of Al and Cr is changed to inward transmission of O. It results in elimination of interspace in the oxide film and the significant reduction of oxidation mass gain. Ho oxide could pin the oxidization film and promote the adhesion of oxidization film [7,15].
2) Liquid metal cooling process with large thermal gradient gave rise to the microstructure and phase composition change of directionally solidified eutectic alloy. Based on the constitutional supercooling theory, cooling rate was proportional to the product of thermal gradient and withdrawal rate. As the cooling rate became higher, the corresponding cooling time was less, the diffusion time of solid solution atoms was shorter. At the same time, higher cooling rate could decrease the diffusion of atoms and shorten the diffusion distance, which could refine the directionally solidified eutectic alloy and increase the content of alloying elements in solid solution phase. For example, compared with cast NiAl-30.75Cr-3Mo-0.25Ho alloy, the directional solidification process refined the eutectic alloy and increased Cr content in NiAl phase. The refinement of microstructure may promote the Al2O3 film to cover the Cr(Mo) phase on the surface as soon as possible. Of cause, the Cr-rich layer was formed easily between oxide film and alloy substrate, which would reduce the internal stress during alternation of hot and cold and improve adhesion of oxide film [7].
4 Conclusions
1) A continuous Al2O3 oxide film formed on the surface of DS NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho eutectic alloy at 900-1100 ℃, which resulted in the good oxidation resistance. However, Al2O3 oxide grew laterally and folded or cracked during cooling at 1150 ℃, and the adhesion of oxide film with the matrix was poor. So the oxidation resistance became bad.
2) Directional solidification process with faster solidification speed restrained the diffusion to alloying element and increased the Cr content in NiAl phase, which was conducive to the formation of continuous Al2O3 oxide film on the surface of Cr(Mo) phase and Cr-rich layer between oxide film and alloy substrate.
3) Trace rare earth element Ho changed mass transfer mechanism of alloying elements in the oxidation process, namely, outward transmission of Al and Cr is changed to inward transmission of O. It results in elimination of interspace in the oxide film and the significant reduction of oxidation mass gain. Ho oxide could pin the oxidization film and promote the adhesion of oxidization film.
4) There existed phase transformation from θ-Al2O3 to α-Al2O3 in the oxidation process, which was the reason of abnormal oxidation mass gain of DS eutectic alloy at 1000 ℃ and 1050 ℃.
References
[1] GUHA S, MONROE P R, BAKER I. Room temperature deformation behavior of multiphase Ni-20at.%Al-30at.%Fe and its constituent phases [J]. Materials Science and Engineering A, 1991, 131: 27-37.
[2] KUMAR K S, DAROLIA R, LAHRMAN D F, MANNAN S K. Tensile creep response of an NiAl-TiB2 particulate composite [J]. Scripta Metallurgica et Materialia, 1992, 26: 1001-1006.
[3] DAROLIA R, WALSTON W S. Effect of specimen surface preparation on room temperature tensile ductility of an Fe-containing NiAl single crystal alloy [J]. Intermetallics, 1996, 4: 505-516.
[4] JOHNSON D R, CHEN X F, OLIVER B F, NOEBE R D, WHITTENBERGER J D. Processing and mechanical properties of in-situ composites from the NiAl-Cr and the NiAl-(Cr,Mo) eutectic systems [J]. Intermetallics, 1995, 3: 99-113.
[5] WHITTENBERGER J D, RAJ S V, LOCCI I E, SALEM J A. Effect of growth rate on elevated temperature plastic flow and room temperature fracture toughness of directionally solidified NiAl-31Cr-3Mo [J]. Intermetallics, 1999, 7: 1159–1168.
[6] XU Chun-mei, GUO Jian-ting, YANG Fu-bao. High temperature oxidation on behavior of NiAl-28Cr-5Mo-1Hf alloy [J]. Acta Metallurgica Sinica, 2001, 37(8): 857-860. (in Chinese)
[7] ZHANG Shi-zhen, GUO Jian-ting, REN Wei-li, ZHOU Wen-long. Effects of rare earth elements on high temperature oxidation resistance of NiAl/CrMo(Hf) alloy [J]. Journal of Chinese Society for Corrosion and Protection, 2005, 25(2): 100-114. (in Chinese)
[8] ZHANG Guang-ye. Effect of rare-earth-elements on the microstructure and mechanical and chemical properties of NiAl- based eutectic alloy [D]. shenyang: Institute of Metal Research, Chinese Academy of Sciences, 2004: 86-98. (in Chinese)
[9] ZHANG Guang-ye, ZHANG Hua, WANG Zhen-sheng, GUO Jian-ting, ZHOU Lan-zhang. Oxidation behavior of NiAl-30.9Cr-3Mo-0.1Dy alloy at high temperature [J]. Rare Metal Materials and engineering, 2006, 35(5): 719-723. (in Chinese)
[10] WANG Zhen-sheng, ZHOU Lan-zhang, GUO Jian-ting, LIANG Yong-chun, HU Zhuang-qi. High temperature oxidation behavior of directional solidification NiAl-28Cr-5.94Mo-0.05Hf-0.01Ho eutectic alloy [J]. Chinese Journal of Materials Research, 2010, 24(4): 585-591. (in Chinese)
[11] GUO Jian-ting. Ordered intermetallic compound NiAl alloy [M]. Beijing: Science Press, 2003: 80-100. (in Chinese)
[12] SHENG Li-yuan. Effects of Ho alloying addition, rapid solidification and strong magnetice field treatment on the microstructures and mechanical properties of NiAl-based eutectic alloys [D]. Shenyang: Institute of Metal Research, Chinese Academy of Sciences, 2009: 39-42. (in Chinese)
[13] LAI Wan-hui, WANG Shu-he, SUN Chao, TAN Ming-hui, LI Hui, GUO Jian-ting. Oxidation resistance behavior of three kinds NiAl-based eutectic alloys [J]. Corrosion Science and Protection Technique, 1993, 5(3): 213-217. (in Chinese)
[14] ZHANG Guang-ye, GUO Jian-ting, YE Heng-qiang. The microstructure and oxidation behavior for NiAl-30.9Cr-3Mo-0.1Dy alloy at high temperature [J]. Journal of Aeronautical Materials, 2005, 25(2): 6-11. (in Chinese)
[15] ZHANG Guang-ye, GUO Jian-ting, YE Heng-qiang. Oxidation Behavior of NiAl-30.75Cr-3Mo -0.25Ho alloy at high temperature [J]. Journal of the Chinese Rare Earth Society, 2005, 23(1): 75-80. (in Chinese)
[16] XU Chun-mei. Investigation on the directionally solidified microstructure and mechanical and high temperature oxidation properties of NiAl-based eutectic alloy [D]. Shenyang: Institute of Metal Research, Chinese Academy of Sciences, 2003: 89-102. (in Chinese)
NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho定向共晶合金的高温氧化行为
王振生1,2,谢 亿2,3,郭建亭2,周兰章2,胡壮麒2,张光业1,陈志钢1
1. 湖南科技大学 机电工程学院,湘潭 411201;
2. 中国科学院 金属研究所,沈阳 110016;
3. 湖南省电力公司 科学研究院,长沙 410007
摘 要:采用SEM和XRD等分析手段对NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho定向共晶合金的高温氧化行为进行研究。结果表明,在900~1100 ℃下合金表面生成连续的Al2O3氧化膜,从而使合金具有良好的抗氧化性能;在1150 ℃下合金表面的Al2O3氧化膜破裂,氧化增重升高。定向凝固工艺细化合金的组织以及微量稀土元素Ho的加入,均有利于在合金表面形成连续的Al2O3氧化膜。在氧化过程中,表面氧化膜存在着θ-Al2O3→α-Al2O3的相变过程,从而导致1000 ℃和1050 ℃氧化增重反常现象的出现。
关键词:金属间化合物;NiAl;高温氧化;定向共晶合金
(Edited by YANG Hua)
Foundation item: Project (51101055) supported by the National Natural Science Foundation of China
Corresponding author: GUO Jian-ting; Tel: +86-24-23971907; Fax: +86-24-23971907; E-mail: jtguo@imr.ac.cn
DOI: 10.1016/S1003-6326(11)61359-9
Abstract: The isothermal oxidation behavior of NiAl-31Cr-2.9Mo-0.1Hf-0.05Ho directional eutectic alloy was investigated with the help of scanning electron microscopy and X-ray diffraction. The results revealed that a continuous Al2O3 scale was formed and owned excellent oxidation resistance in the temperature range of 900-1100 ℃. When the temperature was up to 1150 ℃, the continuous Al2O3 oxide film ruptured. Trace rare earth element Ho distributed uniformly in the alloy and relatively high level of Al in Cr(Mo) phase are beneficial to the formation of continuous and compact Al2O3 scale. During the oxidation, a phase transformation from θ-Al2O3 to α-Al2O3 existed on the surface of oxidation film. It resulted in the abnormal oxidation mass gain happening when the alloy was oxidized at 1000 ℃ or 1050 ℃.


