网络首发时间: 2015-11-06 15:10
包覆型P507浸渍树脂的制备及吸附铟(Ⅲ)的性能
鲁东大学化学与材料科学学院
摘 要:
以2-乙基己基磷酸单-2-乙基己酯(P507)为萃取剂,苯乙烯-二乙烯基苯大孔非极性吸附树脂(HZ818)为载体,聚丙烯酸(PAA)为包覆材料,N,N-亚甲基双丙烯酰胺为交联剂,制备了包覆型P507浸渍树脂。红外光谱(FTIR)表征表明包覆后树脂上的萃取剂未受影响,热重分析(TGA)表明制备的包覆型浸渍树脂具有较好的热稳定性。分别以静态和动态实验法考察包覆型浸渍树脂在盐酸介质中吸附铟(Ⅲ)的性能。静态实验表明,该树脂在盐酸体系中对铟(Ⅲ)的最佳吸附pH为0.8,吸附平衡时间为10 h,饱和吸附量在288,298,308 K时分别为29.4,32.7,35.3 mg·g-1。等温吸附实验表明,Langmuir等温吸附模型能较好地反映包覆型浸渍树脂的吸附过程,此吸附过程为吸热反应。动态实验表明,与未包覆的P507浸渍树脂相比,包覆树脂的稳定性更高。
关键词:
中图分类号: TF843.1;TQ424.3
作者简介:陈晓亮(1988-),男,山东烟台人,硕士研究生,研究方向:稀有金属分离与回收;E-mail:ldu_chexl@163.com;;刘军深,教授;E-mail:liujunshen@163.com;电话:0535-6697537;
收稿日期:2015-02-02
基金:国家自然科学基金项目(21171085);山东省自然科学基金项目(ZR2010BM027)资助;
Preparation of Coated Impregnated Resin Containing P507 and Its Adsorption Properties for Indium(Ⅲ)
Chen Xiaoliang Liu Junshen Liu Qingqing Xu Cuiping
School of Chemistry & Materials Science,Ludong University
Abstract:
A coated impregnated resin was prepared with 2-ethylhexyl phosphoric acid mono( 2-ethylhexyl) ester( P507) as the extractant,styrene pinylbenzene macroreticular nonpolarity adsorption resin( HZ818) as the carrier,polyacrylic acid( PAA) as the coating film and N,N'-methylenebis( 2-propenamide) as crosslinked agent. Fourier transform infrared spectroscopy( FTIR) result showed that there was no influence on the nature of extractant in the coating procedure. Thermogravimetry analysis( TGA) indicated that the prepared coated impregnated resin had good thermal stability. The adsorption properties for indium( Ⅲ) with this resin from hydrochloric acid medium were investigated by batch method and column method. Batch experiments showed that the optimum adsorption p H was 0. 8,the adsorption equilibrium time was 10 h,and the saturated adsorption capacities were 29. 4,32. 7,35. 3 mg·g-1at288,298,308 K,respectively. Langmuir adsorption isotherm was suitable for simulating the adsorption process with this coated resin,and this process was shown as an endothermic reaction. Compared with the uncoated impregnated resin prepared by conventional method,the stability of the coated impregnated resin was better in the column experiments.
Keyword:
solvent impregnated resins; indium(Ⅲ); adsorption; coated; indium;
Received: 2015-02-02
具有特殊物理化学性能的金属铟被广泛应用在微电子、半导体材料、液晶材料、高性能合金和核工业等领域。稀散金属铟基本不具有独立开采价值的金属矿床,具有开采价值的铟通常以类质同象的形式存在于相伴生的矿物中,如闪锌矿、方铅矿、黄铁矿等
1 实验
1.1 主要仪器与试剂
仪器:ZD-85气浴恒温振荡器、DF-Ⅱ集热式恒温磁力搅拌器(金坛市恒丰仪器厂),梅特勒LE204电子天平,梅特勒托利多SEVENMULTI p H/电导率/离子综合测定仪(梅特勒托利多仪器(上海)有限公司),DGL-200电热鼓风干燥箱(山东龙口先科仪器公司),UV-2550紫外—可见光分光光度计(FTIR,日本岛津公司),JSM-5610LV扫描电子显微镜(SEM,日本JEOL公司),Netzsch 209热重分析仪(TGA,德国Netzsch公司)。
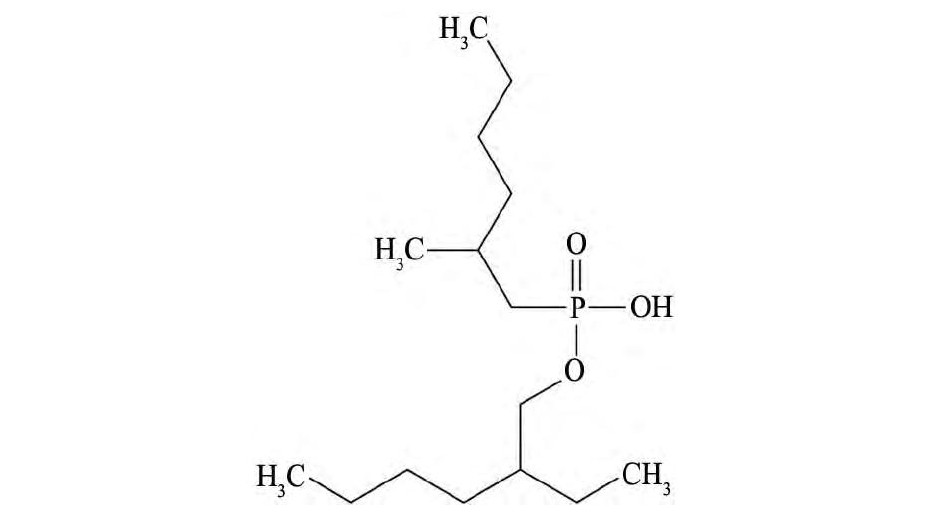
试剂:上海莱雅仕化工有限公司生产的纯度95%的P507萃取剂,结构式如图1。载体为HZ818苯乙烯-二乙烯基苯大孔非极性吸附树脂,上海华震聚合物有限公司生产,金属铟(5N)购自于国药集团。丙烯酸,异丙醇,过硫酸铵,N,N-亚甲基双丙烯酰胺均为分析纯。
1.2 包覆型树脂的制备
(1)称取一定量已纯化的大孔树脂,加入到一定比例的P507/乙醇溶液中。在温度为40℃条件下振荡48 h,洗涤干燥得到P507浸渍树脂(SIR)。
(2)向四颈瓶中加入100 ml蒸馏水和1 g过硫酸铵,溶解后加入5 g丙烯酸单体和8 g异丙醇。
图1 2-乙基己基磷酸单-2-乙基己酯(P507)萃取剂结构式Fig.1Structure of 2-ethyl hexylphosphic mono-2-ethyl-hexy-lester(P507)
搅拌加热,使反应瓶内温度达到65~70℃。45 g的丙烯酸单体与2 g过硫酸铵水溶液,分别由两个滴液漏斗0.5 h内滴入瓶内。在94℃继续回流1 h,得到聚丙烯酸溶液。
(3)称取一定量的浸渍树脂浸泡在一定浓度的聚丙烯酸水溶液中,恒温振荡22 h,将树脂从溶液中分离出来后,浸于N,N-亚甲基双丙烯酰胺溶液中8 h以使其完全交联。随后真空抽滤并洗涤得到PAA包覆型浸渍树脂,差减法计算可知萃取剂含量为2.22 mmol·L-1。
1.2.1 静态吸附方法
碘量瓶中加入定量包覆型浸渍树脂,再配置一定组分及酸度的溶液于100 ml的碘量瓶中,放入恒温振荡器中在一定温度下振荡至吸附平衡。测量吸附完成后溶液的浓度按式(1),(2)计算铟(Ⅲ)的分配比及吸附量。

式中:Co,Ce分别为水相中铟(Ⅲ)的起始浓度和平衡浓度,mg·L-1;Ct为水相中铟(III)的任意时刻的浓度,mg·g-1;V为吸附液体积,ml;W为树脂的质量,g。qt为树脂在任意时刻的吸附量,mg·g-1;qe为树脂达到吸附平衡时的平衡吸附量,mg·g-1。
1.2.2 动态吸附方法
用500 ml的p H=0.5的盐酸溶液进行预处理12 h后,将定量的包覆型浸渍树脂湿法装柱,再将p H=0.5的盐酸铟试液通过恒流泵以一定流速过柱,然后测出吸附流出液中金属离子的浓度。
2 结果与讨论
2.1 包覆型树脂的表征
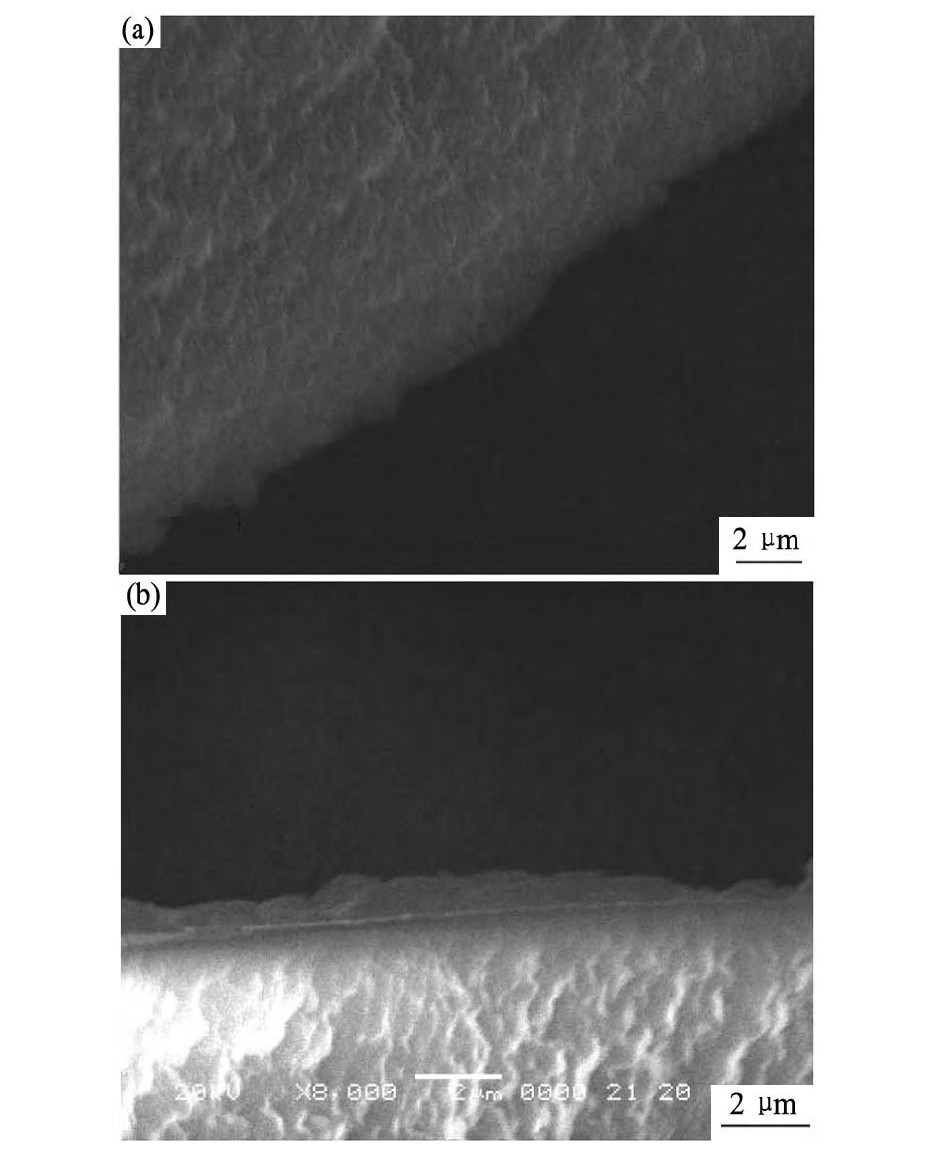
采用SEM手段对制备的稳定前后的P507浸渍树脂进行了结构观察(图2)。SEM扫描的结果表明,两种树脂颗粒均为规则小圆球,其外观和结构与大孔离子交换树脂基本相同。稳定后的P507浸渍树脂粒径略比稳定前的浸渍树脂的粒径大些,其外表面被覆盖了一层厚约1μm的膜,使其中的萃取剂得以保护,稳定性得到加强。与文献
使用红外光谱对改性前后树脂进行表征,结果如图3,改性前出现在1190 cm-1处的是P=O键的特征吸收峰,(P-O)-C特征吸收峰出现在1039 cm-1处,P-(O-H)键的特征峰出现在988cm-1,改性后这些萃取剂特征峰的位置与改性前几乎一致,说明改性后树脂上的萃取剂未受影响。改性后的树脂在1708 cm-1处出现C=O伸缩振动吸收峰,这表明聚丙烯酸成功浸渍附着在浸渍树脂上。
图2 包覆前后两种树脂的扫描电镜图像Fig.2 SEM images of two kinds of resins before and after coating
(a)SIR;(b)CSIR
图3 载体及包覆前后两种树脂的红外图谱Fig.3FTIR spectra of carrier and two kinds of resins before and after coating
(1)HZ818;(2)SIR;(3)CSIR
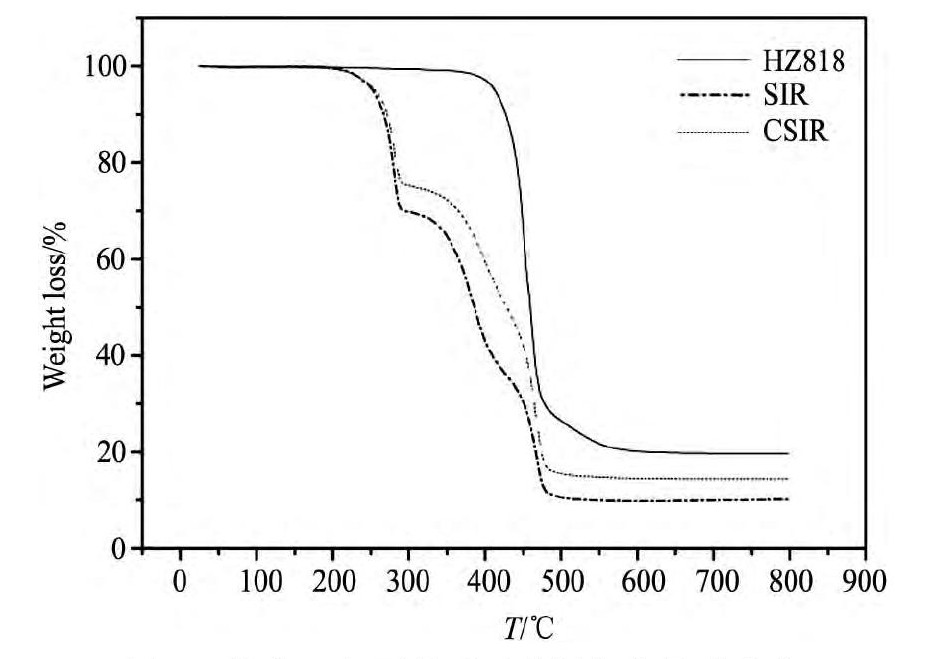
热重曲线可以反映出所制备材料的热稳定性,图4为树脂及浸渍包覆前后两种树脂的失重曲线。由于树脂HZ818在350℃开始失重,证明此载体具有较好的热稳定性,SIR和CSIR同时在200℃开始失重,这是由于树脂所负载的P507萃取剂分解及羟基缩水所致,但CSIR在此失重相对较少,这是因为包覆的PAA薄膜增加了树脂质量,使得萃取剂含量降低,同时也证明了聚丙烯酸成功浸渍附着在浸渍树脂上。
2.2 p H值对吸附的影响
在温度为25℃,铟(Ⅲ)浓度为50 mg·L-1的条件下,在盐酸介质中采用静态吸附法测定在不同p H值的溶液中浸渍树脂对铟(Ⅲ)吸附量的变化,实验结果如图5所示。由图5可以看出,树脂对铟(Ⅲ)的吸附先增大后降低,在p H=0.8的时候达到最大,p H值较小时,过多的H+抑制了萃取剂与铟(III)的离子交换作用,吸附量减小。随后,随p H值增大,铟(Ⅲ)离子水解程度增大,吸附量随之降低。因此,在实验条件下,吸附铟(Ⅲ)的最佳p H为0.8,本文以下的研究中采用的溶液p H值都为0.8。
图4 载体及包覆前后两种树脂的热重曲线Fig.4TGA curves of carrier and two kinds of resins before and after coating
(a)HZ818;(b)SIR;(c)CSIR
2.3 包覆树脂对铟的吸附等温线
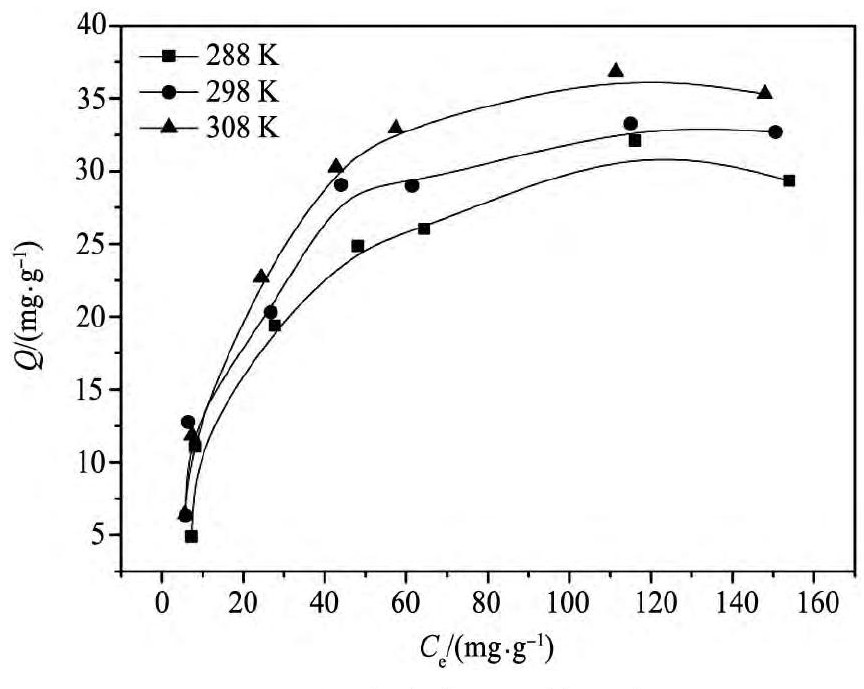
保持固液比(25 mg/25 ml)不变,以相同质量的浸渍树脂分别吸附浓度为10~200 mg·L-1的铟(Ⅲ)溶液,得到浸渍树脂对铟(Ⅲ)的等温吸附曲线如图6所示。由图6可知,浸渍树脂对铟(Ⅲ)的吸附量随着平衡浓度的增加而逐渐增加,后慢慢达到饱和,饱和吸附量在288,298,308 K时分别为29.4,32.7,35.3 mg·g-1。在铟(Ⅲ)原液浓度相同的条件下,随着温度的升高树脂的吸附量增加,说明浸渍树脂对铟(Ⅲ)的吸萃过程为吸热反应。
图5 溶液p H对CSIR吸附铟(Ⅲ)的影响Fig.5 Effect of initial solution p H on adsorption of In(Ⅲ)on-to impregnated resin
图6 铟(Ⅲ)的吸附等温线Fig.6 Adsorption isotherms of In(III)with CSIR
分别以Langmuir和Freundlich等温吸附模型对等温吸附实验数据进行拟合:
Langmnir等温吸附模型:

Freundlich等温吸附模型:

式中Q为吸附剂对铟(Ⅲ)的平衡吸附量,mg·g-1;Ce为溶液中铟(Ⅲ)平衡浓度,mg·g-1;Qo为饱和吸附量,mg·g-1;KL为Langmuir等温吸附方程的常数,L·mg-1;n为Freundlich等温方程的常数,Kf为结合能常数。结果见表1。由表1可见,浸渍树脂对铟(Ⅲ)等温吸附的Langmnir拟合曲线线性关系好于Freundlich拟合曲线,理论最大吸附量与实验结果相近。吸附发生在树脂的内控表面,属于单分子层吸附。
2.4 包覆树脂对铟的吸附动力学
由图7可知,因为浸渍树脂包膜的影响,CSIR初始吸附速率较未包膜浸渍树脂大
表1 浸渍树脂吸萃铟(Ⅲ)的Freundlich和Langmuir拟合参数Table 1 Freundlich and Langmuir fitted constants for ad-sorption of In(Ⅲ)with impregnated resin 下载原图
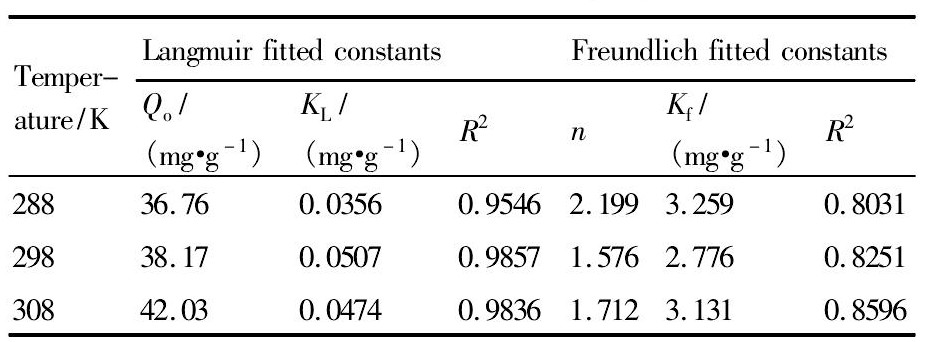
表1 浸渍树脂吸萃铟(Ⅲ)的Freundlich和Langmuir拟合参数Table 1 Freundlich and Langmuir fitted constants for ad-sorption of In(Ⅲ)with impregnated resin
图7 CSIR对铟(III)的吸附动力学曲线Fig.7 Adsorption kinetics of In(III)with CSIR
2.5 包覆树脂的动态吸附性能
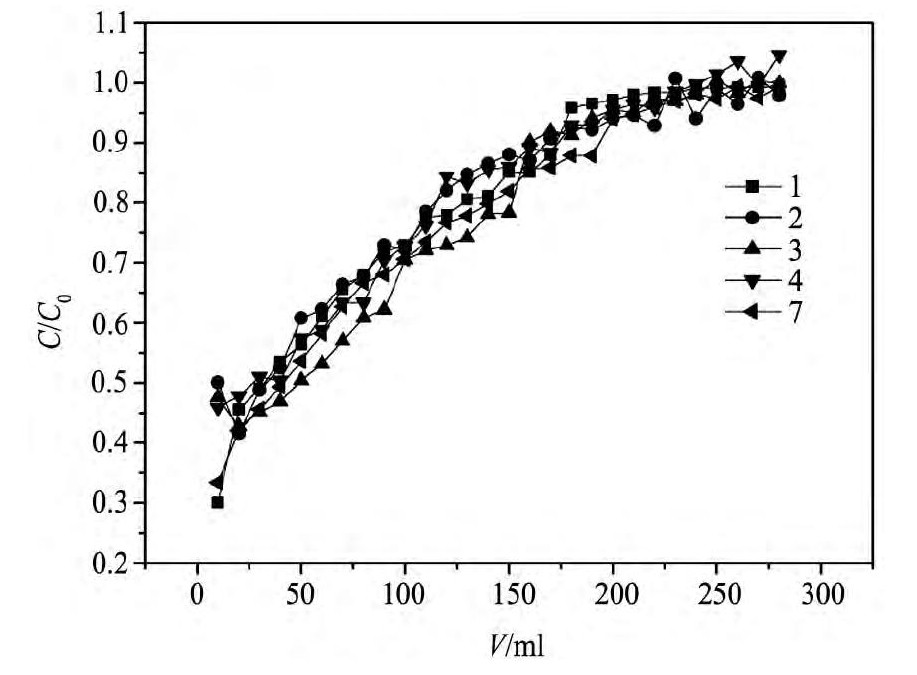
称取200 mg包覆型浸渍树脂装入玻璃柱中,在温度(室温)、料液浓度(40mg·L-1)、p H值(p H=0.8)一定的情况下,将铟(Ⅲ)溶液通过用p H值为0.8的盐酸溶液平衡好的树脂柱中,对包覆后的浸渍树脂进行了动态吸附稳定性的研究,绘制出包覆树脂的动态吸附曲线,如图8。
图8显示的是7次动态吸附时,吸附浓度与流出液体积关系。从图8可知,在料液浓度,p H不变的条件下,随着循环次数的增加,动态吸附循环7次吸附得到的吸附曲线变化趋势基本相同,相比未包覆的浸渍树脂
图8 包覆型浸渍树脂的循环使用性能Fig.8 Comparison of breakthrough curves of CSIR after sever-al sorption-elution cycles
3 结论
以丙烯酸为单体合成低分子量的聚丙烯酸,再以聚丙烯酸为膜材料,N,N-亚甲基双丙烯酰胺为交联剂,包覆HZ818浸渍P507萃取剂的浸渍树脂,制得包覆型P507浸渍树脂。
在该树脂吸萃铟(Ⅲ)的过程中,最佳p H为0.8,吸附在10 h的时候达到平衡,对铟的吸附动力学符合Langmuir等温吸附方程,属于单分子层吸附,并且此吸附过程是一个吸热过程。动态实验表明包覆型树脂能有效地增加浸渍树脂的稳定性。
参考文献
[1] Alfantazi A M,Moskalyk R R.Processing of indium:a review[J].Minerals Engineering,2003,16(8):687.