J. Cent. South Univ. (2013) 20: 488–494
DOI: 10.1007/s11771-013-1510-2
A system combining microbial fuel cell with photobioreactor for continuous domestic wastewater treatment and bioelectricity generation
Jiang Hai-ming(蒋海明)1, 2, 3, Luo Sheng-jun(罗生军)1, Shi Xiao-shuang(师晓爽)1, Dai Meng(戴萌)1, Guo Rong-bo(郭荣波)1
1. Key Laboratory of Biofuels Qingdao Institute of Bioenergy and Bioprocess Technology,
Chinese Academy of Sciences, Qingdao 266101, China;
2. School of Mathematics, Physics and Biological Engineering, Inner Mongolia
University of Science and Technology, Baotou 014010, China
3. Graduate University, Chinese Academy of Sciences, Beijing 100049, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Abstract:
a coupled system consisting of an upflow membrane-less microbial fuel cell (upflow ML-MFC) and a photobioreactor was developed, and its effectiveness for continuous wastewater treatment and electricity production was evaluated. Wastewater was fed to the upflow ML-MFC to remove chemical oxygen demand (COD), phosphorus and nitrogen with simultaneous electricity generation. The effluent from the cathode compartment of the upflow ML-MFC was then continuously fed to an external photobioreactor for removing the remaining phosphorus and nitrogen using microalgae. Alone, the upflow ML-MFC produces a maximum power density of 481 mW/m3, and obtains 77.9% COD, 23.5% total phosphorus (TP) and 97.6% NH4+-N removals. When combined with the photobioreactor, the system achieves 99.3% TP and 99.0% NH4+-N total removal. These results show both the effectiveness and the potential application of the coupled system to continuously treat domestic wastewater and simultaneously generate electricity and biomass.
Key words:
wastewater treatment; microbial fuel cell; photobioreactor; microalgae; bioelectricity;
1 Introduction
A microbial fuel cell (MFC) is a device that uses bacteria as catalysts to oxidize organic matters and generate current [1]. MFC is an emerging innovative technique for wastewater treatment and simultaneous power generation, which is especially important in offsetting the operational costs of wastewater treatment plants [2–3]. Besides energy recovery as electricity, MFCs can reduce as much as 50% of the electricity usage in the conventional biological treatment processes, and produce 50%–90% less solids than the conventional biological treatment processes [4]. In previous reports, various types of wastewater, including domestic and industrial wastewaters [5–6] have been successfully tested in MFCs with effective electricity generation. It has been reported that up to 80%–99% COD (chenical oxygen demand) removal could be achieved with MFCs [5–8].
High COD removal can be obtained using MFCs; however, this technology alone is not effective in removing nitrogen and phosphorus. To improve nitrogen removal, systems combining a MFC with other nitrogen removal process have been developed [7–9]. Although these coupled systems have been proved to increase nitrogen removal, it is noteworthy that they have either not been able to completely remove the nitrogen or too complex to be used in practice. Besides, they did not resolve the removal of phosphorus. Up to now, it has not been reported that there are effective techniques for removing phosphorus from wastewater using MFCs.
Meanwhile, many researches have shown that microalgae are effective in removing nitrogen and phosphorus from wastewater [10–11]. Microalgae assimilate nitrogen and phosphorus in wastewater for synthesizing biomass in the process of photosynthesis as a result of removing nitrogen and phosphorus and purifying the wastewater.
Upflow mode membrane-less MFC (ML-MFC) inspired by the idea of in situ marine sediment MFC works in continuous upflow mode [12–14]. It omits ion exchange membrane and is simple in structure and easy in scalizing, thus more cost-effective.
The aim of this work was to develop an upflow ML-MFC and photobioreactor coupled system and evaluate the effectiveness of the coupled system for wastewater treatment, electricity generation and biomass production. Wastewater was first treated with the upflow ML-MFC to remove COD, phosphorus and nitrogen, and to produce electricity. Then, microalgae were cultivated using the MFC-treated wastewater to further remove the residual phosphorus and nitrogen and simultaneously produce biomass.
2 Materials and methods
2.1 Wastewater
Domestic wastewater was collected from the effluent of primary clarifier of Qingdao Tuandao Wastewater Treatment Plant (Qingdao, China), centrifuged (8×103 g for 10 min) to remove the particles with a centrifuge (CR22GII, Hitachi, Ltd., Japan), and stored in a refrigerator at –20°C before use. The pH, COD, NH4+-N and TP (total phosphorus) of the centrifuged wastewater were 7.37, (238.7±7.6) mg/L, (52.56±0.78) mg/L and (5.87±0.02) mg/L (standard deviation, n=3), respectively. The centrifuged wastewater was used for all MFC tests without any additional treatment.
2.2 Configuration of upflow ML-MFC and photobio-reactor coupled system
Figure 1 shows the schematic diagram and photograph of the upflow ML-MFC and photobioreactor coupled system used in this work. The upflow ML-MFC was mainly consisted of a polymethylmethacrylate (PMMA) plastic cylinder (65 cm long and 8.0 cm in inner diameter) with one end sealed, a carbon fiber brush anode, a carbon fiber brush cathode, and a glass wool layer and glass bead layer separator. The PMMA plastic cylinder was divided into anode compartment and cathode compartment by glass wool layer and glass bead layer. The anode compartment (24 cm in depth) was at the bottom, and the cathode compartment (31 cm in depth) was at the top of the PMMA plastic cylinder. Glass wool layer (4 cm in depth) and glass bead layer (4 cm in depth) were successively placed on the upper of the anode compartment, supported by a perforated PMMA plastic sheet (uniformly drilled with 3 mm holes and 2 mm hole spacing). An influent port was set at the bottom of the PMMA plastic cylinder for feeding substrate and an effluent port on the side near top of the PMMA plastic cylinder. Carbon fiber (Dalian Xingke Carbon Fiber Co., Ltd, China, with elastic modulus of 220 GPa) brush electrodes were constructed as described by LOGAN et al [15]. The brush anodes (22 cm long and 3.0 cm in diameter) were first acid treated with a solution of ammonium peroxydisulfate and sulfuric acid, then heat treated as previously described by FENG et al [16], and finally washed three times with deionized water before use in the MFC. The brush cathodes (24 cm long and 3.0 cm in diameter) were placed on a ceramic plate in a pre-heated furnace (SX2-10-13, Longkou City Xianke Instrument Co., China) at 400°C for approximately 30 min to remove the organic binder from the surface of carbon fiber. Four brush anodes were uniformly fixed on the bottom of the anode compartment and four brush cathodes were placed on the upper of the glass bead layer. The distance between the top of the carbon brush anode electrode and the bottom of the carbon brush cathode electrode was approximately 10.5 cm. Copper wire was used to connect the circuit containing a 100 Ω load, unless stated otherwise. A round aeration tube was placed on the top of the glass bead layer. An external column photobioreactor (1.5 L) was coupled with the upflow ML-MFC.
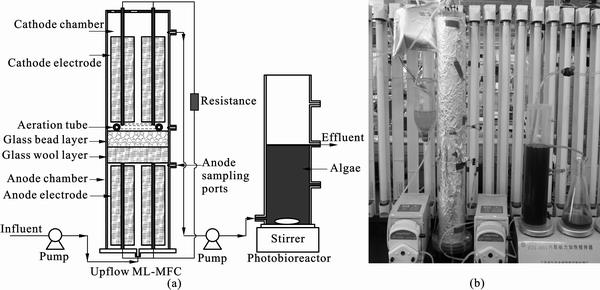
Fig. 1 Schematic diagram (a) and photograph (b) of upflow ML-MFC and photobioreactor coupled system
2.3 Operation of upflow ML-MFC
The anode compartment was inoculated with a mixture of activated sludge and effluent of the primary clarifier (with a volume ratio of 1:7). The pH and volatile suspended solid concentration of the activated sludge were 6.39 and 1.6% (w/w), respectively. Besides, the pH and volatile suspended solid concentration of the inoculum were 7.02 and 0.3% (w/w), respectively. The centrifuged wastewater was purged with CO2 and N2 (with a volume ratio of 15:85) mixture gas to remove the dissolved oxygen, sealed and then sterilized (121°C for 20 min) before use. The sterilized wastewater was continuously fed into the anode compartment from the bottom at a constant flow rate of 0.29 mL/min with a peristaltic pump (BT100-1J, Baoding Longer Precision Pump Co., Ltd., China), and the effluent exited through the cathode compartment on the side near the top. The cathode compartment was aerated with air at a rate of 120 mL/min using the aeration tube via an air pump (CX-0088, CHUANGXING Electrical appliances Co., Ltd., China). A polyester bag (1 L) filled with pure nitrogen gas was connected to the sterilized wastewater container through a silicon pipe and a disposable syringe filterc pore size 0.02 mm to balance the pressure of the container. The voltage over the external resistance (Rext) was recorded every 1 min to a desk personal computer via a data acquisition system (BC6040, Beijing BAOTRON Technology Co., Ltd., China). The upflow ML-MFC was covered with aluminum foil to exclude light. All experiments were operated at ambient temperature ((23±3)°C). When a maximum voltage was obtained, the anode was considered fully enriched with electro-active microbes. Then, the upflow ML-MFC was used for wastewater treatment. The voltage over the Rext was recorded. Water samples (10 mL) were taken from the upper of anode chamber and effluent of cathode chamber every 24 h, respectively. The concentrations of COD, TP and NH4+-N in the supernatant were measured after the water sample was centrifuged (8×103g for 10 min).
2.4 Cultivation of microalgae
After the upflow ML-MFC voltage output stabilized, the effluent from the cathode compartment of the upflow ML-MFC was continuously pumped into a column photobioreactor (with a 7 cm inner diameter, a 40 cm column height and 850 ml of working volume) at a rate of 0.29 mL/min. A mixed culture of microalgae with an optical density of 0.331 (UV759S, Shanghai Precision & Scientific Instrument Co., Ltd., China), obtained from domestic wastewater, was used as the inoculum. Mixture gas consisting of CO2 and air (with a volume ratio of 2:98) was purged into the photobioreactor at a rate of 100 mL/min from the bottom of the photobioreactor with an aeration stone. The height of the aerated culture fluid was 24 cm. The culture, unless otherwise stated, was grown under a light intensity of 135.8 mmol/(m2×s) (LI-250A Light Meter, LI-COR, USA), with continuous illumination. After the OD750 of the culture fluid stabilized, samples of the culture fluid (30 mL) were taken from the photobioreactor every 24 h during the cultivation, centrifuged (8×103g for 10 min) with a centrifuge (Allegra X-22R, Beckman Coulter, Inc., USA), and the concentrations of nitrogen and phosphorus in the supernatant were measured.
2.5 Analysis
The power density, P (W/m3), was obtained according to the equation P =IU/V, where I (A) is the current, U (V) is the voltage over Rext, and V (m3) is the volume of the anode compartment. The polarization curve was obtained by cutting off the circuit and establishing constant voltage, and then varying the Rext over a range of 5 000–25 Ω, and the voltage over Rext at 5 min intervals per resistor was recorded. The COD removal efficiency and Coulombic efficiency (CE) for a MFC running in continuous mode were calculated as described by LOGAN et al [1]. The polarization characteristics of anode and cathode were examined at different Rext (5 000–25 Ω) by placing a reference electrode (Ag/AgCl) into the anode and cathode chambers, respectively.
The concentrations of COD, TP and ammonia nitrogen were measured by a multi-parameter COD Tachometer (5B-3(B), Lanzhou Environmental Protection Technology Co., Ltd., China). The concentrations of ammonia nitrogen and TP were determined by Nessler’s reagent (HgCl2-KI-KOH) colorimetric method at 420 nm wavelength [17] and ammonium molybdate spectrophotometric method at 710 nm wavelength [18], respectively. After the samples were digested at 120°C for 30 min with potassium persulfate solution in sealed glass tubes, the TP concentrations of the samples were determined. COD was analyzed using fast digestion-spectrophotometric method [19].
3 Results and discussion
3.1 Enrichment of electro-active microbes
An up flow ML-MFC was used to enrich electrochemically active microbes using activated sludge and effluent of primary clarifier as inoculum and domestic wastewater as substrate. As shown in Fig. 2, during the electrochemically active microbe enrichment,the voltage output of the upflow ML-MFC slowly increases. After approximately 40 d, the voltage output becomes stable and reaches an average value of 0.200 V, showing that the anode is fully enriched with electro-active microbe.
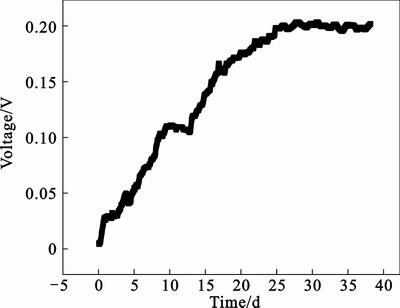
Fig. 2 Voltage output as function of time during start-up of upflow ML-MFC (Anode flow: 0.29 mL/min; Air flow: 120 mL/min; Rext: 100 Ω; Ambient temperature: (23±3)°C)
3.2 Performance of upflow ML-MFC
The performance of an MFC is often evaluated in terms of maximum power density, internal resistance (Rint), and CE. The maximum power density and Rint can be obtained from the power and polarization curves. As shown in Fig. 3, a maximum volumetric power density of 481 mW/m3 (1 293.4 mA/m3) with respect to the anode chamber volume is achieved at a cell potential of 0.372 V and optimal Rext of 250 Ω. The maximum volumetric power density obtained is higher than the values (22.4–451 mW/m3) reported in literatures for treating wastewater with similar MFC configuration [12–14], though the COD concentrations and ionic strengths of the wastewater used in the reported literatures are higher than those of the wastewater used in present work.
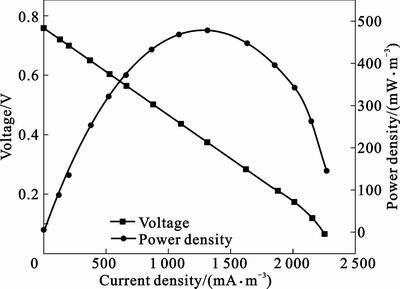
Fig. 3 Voltage and power density generated as function of current density of upflow ML-MFC
The high power density achieved in this work might be attributed to the use of carbon fiber brush electrodes, which have large surface areas compared to the electrodes used in the reported upflow ML-MFCs [12–13] and provide more sites for electrochemically active microbes adhesion and oxygen reduction, respectively. It has been proved that the power output of a MFC can be improved by increasing the surface area of electrode [15, 20]. LOGAN et al [15] achieved up to 2 300 mW/m3 in an air-cathode MFC containing carbon brush anode electrode using wastewater, versus 965 mW/m3 with a plain carbon paper anode electrode.
The Rint of an MFC is another important parameter to evaluate the MFC performance. The distribution and precise value of Rint are generally determined by impedance spectroscopy. For a linear polarization curve, the Rint of a MFC can also be obtained from the polarization curve as it is equal to the slope [1]. As shown in Fig. 2, the polarization curve of the SMFC is linear, so the Rint of the SMFC can be represented by the slope of polarization curve. The Rint of the MFC is approximately 256 Ω in this case.
CE demonstrates the ability of a MFC capturing electrons from the substrate as current and is an other important parameter in the evaluation of MFC performance. The CE of the upflow ML-MFC is 14%, a value that is consistent with the value (<20%) reported in the literatures for wastewater treatment also with upflow ML-MFCs [12, 14]. ML-MFCs simplify the reactor structure and reduce the manufacturing and operating costs, but at the expense of somewhat reduced CE. Low CE indicates that there is substantial COD that is not associated with power generation. COD removal in the absence of power generation may be as a result of oxygen transferring across the glass bead and glass wool layers on top of the anode, and a loss of COD using other electron acceptors (such as nitrate and sulfate) presented in the wastewater, and biomass production. The effect of oxygen diffusion from the cathode chamber into the anode chamber on CE is significant [21], leading to aerobic degradation of the substrate, resulting in less organic matter available for electricity production and consequently lowering the overall CE, as evidenced by low CE in previous study [3].
The changes in anode and cathode potential with the increase of current density are shown in Fig. 4. It can be seen that the cathode potential essentially is unchanged (from 0.362 V to 0.350 V) with the current density increasing. However, the anodic potential increases rapidly (from –0.351 V to 0.295 V) with the increase of current density, which indicates that severe polarization occurs in the anode. These results demonstrate that the upflow ML-MFC performance is mainly affected by the anode, which may include the anodic mass transfer, the rate of microbe oxidizing the substrate, and the rate of electrons transferring from microbes to anode electrode.
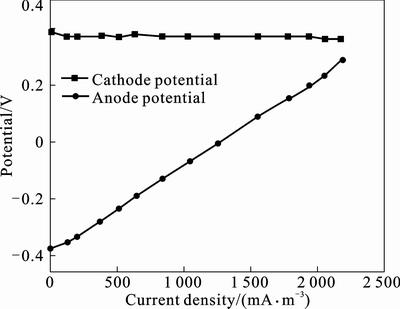
Fig. 4 polarization characteristics of anode and cathode as a function of current density
3.3 COD, phosphorus and nitrogen removals in upflow ML-MFC
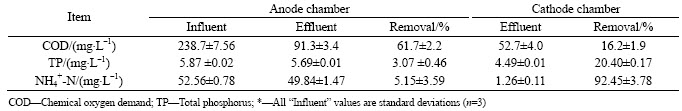
The changes in the removal and concentration of COD, TP and NH4+-N in the upflow ML-MFC are presented in Table 1. The COD of the influent is reduced from 238.7 mg/L to 91.3 mg/L in the anode chamber, and the COD in the effluent of anode chamber further decreases to less than 53 mg/L by feeding the anode effluent to the aerobic cathode. The average concentration of COD in MFC-treated wastewater meets the first level criteria (Class B) specified in discharge standard of pollutants for municipal wastewater treatment plant of China (GB18918—2002). A total COD removal of 77.9% is obtained with the upflow ML-MFC, and approximate 61.7% of influent COD is removed in the anodic compartment. These results demonstrate that the cathode chamber contributes to the total COD removal. High COD removal achieved in this work is due to the cathode process acting as an additional aerobic treatment step following the anodic treatment. LIU et al [5] demonstrated that MFC anodes did not have the ability to remove slowly biodegradable COD thoroughly. By feeding the effluent of anode to the aerobic cathode chamber directly, aerobic heterotrophic microorganisms growing in aerobic cathode chamber can use oxygen to hydrolyze and oxidize slowly biodegradable COD, thus enhancing COD removal.
As given in Table 1, the concentration of TP is reduced from 5.87 mg/L to 4.49 mg/L, with a total TP removal of 23.5%. Only 3.1% TP is removed in anode chamber, and the remaining TP is removed in the cathode chamber. These results demonstrate that the upflow ML-MFC anode alone is not effective in phosphorus removal, and the subsequent aerobic carbon-consumed phase following the anodic treatment
promotes the TP removal. The removal of phosphorus is achieved in the upflow ML-MFC possibly in the following two ways: 1) conversion into biomass as part of microorganism growth and 2) excessive accumulation in microorganisms as polyphosphate. Microorganisms in the upflow ML-MFC successively go through an anaerobic process and an aerobic process, which is similar to the anaerobic/aerobic alternating enhanced biological phosphorus removal process (EBPRP), and is expected to favor phosphorus removal as EBPRP does. However, the upflow ML-MFC lacks the activated sludge that forms in the EBPRP and is rich in phosphorus-accumulating microorganisms, which inhibits the over-uptake of phosphorus by phosphorus-accumulating microorganisms. As a result, phosphorus can not be excessively accumulated in microorganisms as polyphosphate. Thus, phosphorus in the upflow ML-MFC is mainly removed by synthesizing new biomass in aerobic phase.
The concentration of NH4+-N is reduced from 52.56 mg/L to 1.26 mg/L, and its total removal is as high as 97.6% (Table 1). Only 5.15% of influent NH4+-N is removed by the anode chamber, and the cathode compartment accounts for approximately 92.45% NH4+-N removal. These results show that most of the NH4+-N is removed in the cathode chamber. A majority (68%–82%) of ammonia was oxidized and removed in the cathodic compartment through nitrification using a membrane-aerated MFC [8]. These results also show that conventional MFCs, only anode responsible for nitrogen removal, are not effective in removing NH4+-N. In the upflow ML-MFC, the effluent of anode is directly fed to the aerated cathode. NH4+-N and other nutrients can then be utilized by aerobic heterotrophic microorganism growth in the cathode chamber to synthesize new biomass. Alternatively, NH4+-N is converted to nitrate by nitrifying bacteria in the cathode chamber through aerobic nitrification, thereby reducing the NH4+-N concentration. Therefore, the upflow ML-MFC, having a sequential anode-cathode configuration, is more effective in removing nitrogen for wastewater treatment than conventional MFCs. The removal of NH4+-N in MFC is a consequence of complex biological and physiochemical processes, which need deeper investigation in further work for better understanding of the mechanism for nitrogen removal and transformation in MFC.
Table 1 Upflow ML-MFC performance for removals of COD, TP and NH4+-N
3.4 Phosphorus and nitrogen removals in photobioreactor using microalgae
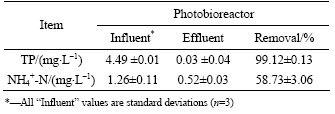
The effluent from the cathode compartment of the upflow ML-MFC was continuously pumped into a column photobioreactor to cultivate microalgae, which further removed the remaining phosphorus and nitrogen. The TP and NH4+-N in the influent and effluent of the photobioreactor were measured and the results are presented in Table 2. The TP concentration is reduced from 4.49 to 0.03 mg/L, and 99% TP is removed at a hydraulic retention time (HRT) of 48.9 h. Meanwhile, NH4+-N concentration is reduced from 1.26 to 0.52 mg/L, and a removal of 58.7% NH4+-N is achieved. The majority of phosphorous and nitrogen present in the liquid medium is removed by the microalgae, which utilize these compounds to synthesize new biomass. Besides, a part of NH4+-N might be removed by nitrification occurring in the reactor, as evidenced by concentration of NOx--N increased in a photobioreactor with algal-based immobilization process to treat the effluent from a secondary wastewater treatment plant [22]. A total removal of 99.3% TP and 99.0% NH4+-N are achieved with the coupled system. After treated with the coupled system, the average concentrations of TP and NH4+-N in treated wastewater meet the first level criteria (Class A) specified in discharge standard of pollutants for municipal wastewater treatment plant of China (GB18918—2002). To completely remove phosphorous and nitrogen, however, the algal biomass should be removed from the wastewater to prevent the microalgae from settling and subsequently releasing their stored phosphorus [23]. Simultaneously, the recovered biomass can be used as an energy source in the form of either biodiesel [24] or biogas [25–26].
Table 2 Micoalgae performance for removals of TP and NH4+-N
4 Conclusions
1) A coupled system, consisting of an upflow ML-MFC and a photobioreactor, has been shown to efficiently achieve continuous domestic wastewater treatment and simultaneous electricity and biomass production. The coupled system is shown to improve phosphorus and nitrogen removal compared to wastewater treatment using only a MFC. It is also demonstrated that the coupled system can serve as a potential way to enhance pollutants removal and recover energy from wastewater treatment, providing a promising candidate for wastewater treatment and bioenergy generation. Furthermore, this novel coupled system for wastewater treatment and bioenergy generation has only been preliminarily explored and verified
2) The additional studies are needed to scale up and optimize the process in further, which includes the design of a more effective coupled system, the isolation of more effective microalgae, the effect of photobioreactor’s and MFC’s HRT on microalgae growth and pollutants removal and the recovery of biomass from the effluent.
References
[1] LOGAN B E, HAMELERS B, ROZENDAL R A, SCHRORDER U, KELLER J, FREGUIA S, AELTERMAN P, VERSTRAETE W, RABAEY K. Microbial fuel cells: Methodology and technology [J]. Environmental Science & Technology, 2006, 40(17): 5181–5192.
[2] JADHAV G S, GHANGREKAR M M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration [J]. Bioresource Technology, 2009, 100(2): 717–723.
[3] LIU H, LOGAN B E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane [J]. Environmental Science & Technology, 2004, 38(14): 4040–4046.
[4] HOLZMAN D C. Microbe power![J]. Environmental Health Perspectives, 2005, 113(11): 754–757.
[5] LIU H, RAMNARAYANAN R, LOGAN B E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell [J]. Environmental Science & Technology, 2004, 38(7): 2281–2285.
[6] LU Na, ZHOU Shun-gui, ZHUANG Li, ZHANG Jin-tao, NI Jin-ren. Electricity generation from starch processing wastewater using microbial fuel cell technology [J]. Biochemical Engineering Journal, 2009, 43(3): 246–251.
[7] XIE Shan, LIANG Peng, CHEN Yang, XIA Xue, HUANG Xia. Simultaneous carbon and nitrogen removal using an oxic/anoxic-biocathode microbial fuel cells coupled system [J]. Bioresource Technology, 2011, 102(1): 348–354.
[8] YU Chang-ping, LIANG Zhi-hua, DAS A, HU Zhi-qiang. Nitrogen removal from wastewater using membrane aerated microbial fuel cell techniques [J]. Water Research, 2011, 45(3): 1157–1164.
[9] VIRDIS B, RABAEY K, YUAN Z, KELLER J. Microbial fuel cells for simultaneous carbon and nitrogen removal [J]. Water Research, 2008, 42(12): 3013–3024.
[10] MARTINEZ M E, SANCHEZ S, JIMENEZ J M, EL YOUSFI F, MUNOZ L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus [J]. Bioresource Technology, 2000, 73(3): 263–272.
[11] DI TERMINI I, PRASSONE A, CATTANEO C, ROVATTI M. On the nitrogen and phosphorus removal in algal photobioreactors [J]. Ecological Engineering, 2011, 37(6): 976–980.
[12] DU Zhu-wei, LI Qing-hai, TONG Meng, LI Shao-hua, LI Hao-ran. Electricity generation using membrane-less microbial fuel cell during wastewater treatment [J]. Chinese Journal Of Chemical Engineering, 2008, 16(5): 772–777.
[13] GHANGREKAR M M, SHINDE V B. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production [J]. Bioresource Technology, 2007, 98(15): 2879–2885.
[14] JANG J K, PHAM T H, CHANG I S, KANG K H, MOON H, CHO K S, KIM B H. Construction and operation of a novel mediator- and membrane-less microbial fuel cell [J]. Process Biochemistry, 2004, 39(8): 1007–1012.
[15] LOGAN B E, CHENG S, WATSON V, ESTADT G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells [J]. Environmental Science & Technology, 2007, 41(9): 3341–3346.
[16] FENG Yu-jie, YANG Qiao, WANG Xin, LOGAN B E. Treatment of carbon fiber brush anodes for improving power generation in air-cathode microbial fuel cells [J]. Journal Of Power Sources, 2010, 195(7): 1841–1844.
[17] GB 7479—87. Water quality-Determination of ammonium-Nessler’s reagent colorimetric method [S]. (in Chinese)
[18] WEI Fu-sheng. Water and wastewater monitoring and analysis methods [M]. Beijing: China Environmental Science Press, 2002: 243–245. (in Chinese)
[19] HJ/T 399–2007. Water quality-Determination of the chemical oxygen demand-Fast digestion-Spectrophotometric method [S]. (in Chinese)
[20] OH S, MIN B, LOGAN B E. Cathode performance as a factor in electricity generation in microbial fuel cells [J]. Environmental Science & Technology, 2004, 38(18): 4900–904.
[21] LIU H, CHENG S A, LOGAN B E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration [J]. Environmental Science & Technology, 2005, 39(14): 5488–5493.
[22] HE Sheng-bing, XUE Gang. Algal-based immobilization process to treat the effluent from a secondary wastewater treatment plant (WWTP) [J]. Journal Of Hazardous Materials, 2010, 178(1/2/3): 895–899.
[23] POWELL N, SHILTON A N, PRATT S, CHISTI Y. Luxury uptake of phosphorus by microalgae in full-scale waste stabilisation ponds [J]. Water Science & Technology, 2011, 63(4): 704–709.
[24] CHISTI Y. Biodiesel from microalgae [J]. Biotechnology Advances, 2007, 25(3): 294–306.
[25] YUAN Xian-zheng, SHI Xiao-shuang, ZHANG Da-lei, QIU Yan-ling, GUO Rong-bo, WANG Li-sheng. Biogas production and microcystin biodegradation in anaerobic digestion of blue algae [J]. Energy & Environmental Science, 2011, 4(4): 1511–1515.
[26] MUSSGNUG J H, KLASSEN V, SCHLUETER A, KRUSE O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept [J]. Journal of Biotechnology, 2010, 150(1): 51–56.
(Edited by HE Yun-bin)
Foundation item: Projects(2009GG10005004, 2010GHY10504) supported by the Scientific and Technological Foundation of Shandong Province, China; Project(2011GHY11531) supported by the Science and Technology Development Program of Shandong Province, China; Project (ZR2009BM015) supported by the Natural Science Foundation of Shandong Province, China
Received date: 2011–12–22; Accepted date: 2012–04–24
Corresponding author: GUO Rong-bo, Professor, PhD; Tel: +86–532–80662708; Fax: +86–532–80662708; E-mail: guorb@qibebt.ac.cn
Abstract: a coupled system consisting of an upflow membrane-less microbial fuel cell (upflow ML-MFC) and a photobioreactor was developed, and its effectiveness for continuous wastewater treatment and electricity production was evaluated. Wastewater was fed to the upflow ML-MFC to remove chemical oxygen demand (COD), phosphorus and nitrogen with simultaneous electricity generation. The effluent from the cathode compartment of the upflow ML-MFC was then continuously fed to an external photobioreactor for removing the remaining phosphorus and nitrogen using microalgae. Alone, the upflow ML-MFC produces a maximum power density of 481 mW/m3, and obtains 77.9% COD, 23.5% total phosphorus (TP) and 97.6% NH4+-N removals. When combined with the photobioreactor, the system achieves 99.3% TP and 99.0% NH4+-N total removal. These results show both the effectiveness and the potential application of the coupled system to continuously treat domestic wastewater and simultaneously generate electricity and biomass.


