Trans. Nonferrous Met. Soc. China 30(2020) 2188-2199
Preparation and electrochemical performance of nitrogen-doped carbon-coated Bi2Mn4O10 anode materials for lithium-ion batteries
Jing ZHAN1,2, Chang-fan XU1, Yi-yu LONG1, Qi-hou LI1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China
2. National Engineering Laboratory for High Efficiency Recovery of Refractory Nonferrous Metals Resources, Central South University, Changsha 410083, China
Received 1 January 2020; accepted 4 June 2020
Abstract:
To inhibit rapid capacity attenuation of Bi2Mn4O10 anode material in high-energy lithium-ion batteries, a novel high-purity anode composite material Bi2Mn4O10/ECP-N (ECP-N: N-doped Ketjen black) was prepared via an uncomplicated ball milling method. The as-synthesized Bi2Mn4O10/ECP-N composite demonstrated a great reversible specific capacity of 576.2 mA·h/g after 100 cycles at 0.2C with a large capacity retention of 75%. However, the capacity retention of individual Bi2Mn4O10 was only 27%. Even at 3C, a superior rate capacity of 236.1 mA·h/g was retained. Those remarkable electrochemical performances could give the credit to the introduction of ECP-N, which not only effectively improves the specific surface area to buffer volume expansion and enhances conductivity and wettability of composites but also accelerates the ion transfer and the reversible conversion reaction.
Key words:
Bi2Mn4O10 nanoparticles; N-doped Ketjen black; rate capability; lithium-ion batteries;
1 Introduction
Higher requirements have been put forward for the lithium-ion batteries (LIBs) with higher energy and power density, as well as more excellent cycle performance because of the rapid development of portable electronic equipment and electric vehicles and the limited theoretical specific capacity of commercial graphite anodes (372 mA·h/g) [1,2]. Among a multitude of anode materials, manganese- based oxides, such as MnO [3], Mn2O3 [4], MnO2 [5] have attracted tremendous research attention owing to their stable chemical states, environmental friendliness, low cost, and high specific capacities between 600 and 1000 mA·h/g [6]. Particularly, the metallic Bi can furnish a desirable volumetric capacity of 3760 mA·h/cm3. Bi-based materials, as anode materials for LIBs, have also exhibited distinctive characteristics of excellent volumetric density and specific capacity due to their tailor-made morphologies, microstructures, and multifarious composition [7-9]. For example, Bi2O3/rGO composite anode materials showed a specific capacity of 347.3 mA·h/g after 100 electro- chemical cycles at 1C [10]. However, most metal oxides generally display low conductivity, and their commercial application is severely impeded by dreadful capacity degradation caused by the extreme volume expansion, dramatic structure pulverization, and the loss of electronic connection during continuously repeated lithiation/delithiation processes [6,11]. Multicomponent metal oxides, such as ZnMn2O4 [12] and ZnCo2O4 [13] usually exhibit better electrochemical properties than single-component metal oxides on account of their abundant composition and synergistic effects among various metals [14,15].
Especially, mullite-type compound Bi2Mn4O10 with a considerable theoretical specific capacity of 873 mA·h/g has been investigated as an anode material for advanced Li-ion battery systems. SONG et al [16] prepared a Bi2Mn4O10 anode material via the sol-gel method, which displayed a high reversible specific capacity of 711.6 mA·h/g at 60 mA/g. Furthermore, our group synthesized Bi2Mn4O10 nanoparticles, which maintained a stable reversible specific capacity of 402.3 mA·h/g after 50 cycles at 0.2C [17]. It is essential to improve cycling lifespan and rate performances of Bi2Mn4O10. Downsizing the bulk material to nanoscale and integrating with highly conductive and excellent chemical stable carbon were considered as one of the most effective strategies for LIBs to circumvent capacity fading, inferior rate performance, and poor cyclic durability [18,19]. Reducing the size of particles can not only tailor the properties of surface interface and expose numerous active sites, but also ensure the adequate diffusion of electrolyte and ions [20]. As known, the carbon-based materials not only have high conductivity and stability but also can provide a larger reaction space to relieve the volume change during the continuous insertion/extraction of Li+, thereby reliably inhibiting the pulverization of electrode materials. In addition, doping elemental nitrogen (N) into the conductive carbon matrix is propitious to create extensive defects and active sites without causing lattice mismatch, which significantly strengthens the electrical conductivity and wettability, thus accelerating the faster transfer of ions and the highly reversible conversion reaction [21]. Hence, the economical and efficient preparation of Bi2Mn4O10/C anode composites using carbon materials with excellent electro- chemical properties is of great significance for improving the cycle stability, lithium storage capacity, and rate performance of Bi2Mn4O10 in portable electronic equipment and electric vehicles.
In this work, the N-doped Ketjen black (ECP-N) was prepared by a low-cost calcination reduction method. Subsequently, the Bi2Mn4O10/ ECP-N nanocomposites were prepared via a simple wet ball milling method. Most significantly, the Bi2Mn4O10/ECP-N composites serving as anodes for LIBs demonstrated higher cycling stability than bare Bi2Mn4O10. An attractive specific capacity of 576.2 mA·h/g was obtained at 0.2C after 100 cycles with prominent retention of 75%, while bare Bi2Mn4O10 only conveyed 169.5 mA·h/g with inferior retention of 27%. The ECP with N dopants not only suppresses the aggregation of Bi2Mn4O10 nanoparticles but also offers substantial defect sites to enhance ions transport and electronic conductivity.
2 Experimental
2.1 Material synthesis
All chemical reagents were used directly to prepare target samples without further purification. Bi2Mn4O10/ECP-N material was synthesized via a typical method. Firstly, the preparation of Bi2Mn4O10 powders was given in our previous report [17]. In this work, the ball milling time for preparing Bi2Mn4O10 powders was 18 h. ECP-N was synthesized via calcining Ketjen black (ECP600JD, Triquo Chemical Technology Co., Ltd.) at 800 °C under NH3 for 10 h. Then, 20 g Bi2Mn4O10 powders and 2 g ECP-N were added into a 250 mL ball mill tank for ball milling for 8 h to obtain the precursor, which was calcined at 300 °C in air for 3 h with a heating ramp of 2 °C/min, marked as Bi2Mn4O10/ECP-N.
2.2 Material characterization
The crystallographic structures of Bi2Mn4O10 and Bi2Mn4O10/ECP-N were investigated by an X-ray powder diffractometer (XRD, Rigaku- TTRIII) allocated with Cu Kα at 8 (°)/min. The morphologies of the as-prepared materials were observed by the scanning electron microscope (SEM, JSM-6360LV) and the transmission electron microscope (TEM, TEM, JEM-2100F). The Brunauer-Emmett-Teller (BET) specific surface areas and Barrett–Joyner–Halenda (BJH) pore size distribution of as-prepared materials were calculated according to N2 adsorption isotherms (ASAP2020). X-ray photoelectron spectroscopy analysis (XPS, Kratos Axis Ultra DLD) was used to testify the chemical composition of Bi2Mn4O10/ ECP-N.
2.3 Electrochemical test
In this work, the 2025-type coin cells were employed to measure the electrochemical properties of as-prepared materials. The assembling of cells was performed in the glove box filled with high purity argon, using lithium foil as the counter electrode. 1 mol/L LiPF6 in a mixture of ethylene carbonate (EC), dimethyl carbonate (DMC), and diethyl carbonate (DEC) with a volume ratio of 1:1:1 was employed as the electrolyte. Moreover, porous polypropylene membranes were applied as the separator. For obtaining the working electrode, the as-prepared electroactive materials were mixed with conductive carbon and polyvinylidene fluoride (PVDF) at a mass ratio of 7:2:1. The mixture was homogeneously painted on the copper foil and dried in a vacuum oven for 12 h at 80 °C, which was further cut into wafers with a diameter of 12.0 mm. The final mass of electroactive materials was approximately 1.2 mg/cm. The galvanostatic charge/discharge cycling characterization was performed on LANHE cell tester (CT2001A, Wuhan, China) between 0.05 and 3.0 V at room temperature. The electrochemical workstation (CHI 760e) was used to obtain the cyclic voltammogram (CV) between 0.05 and 3.0 V at a scan rate of 0.1 mV/s. Electrochemical impedance spectroscopy (EIS) measurements were also carried out on a CHI 760e system in the frequency range of 0.01-100000 Hz.
3 Result and discussion
3.1 Phase structure
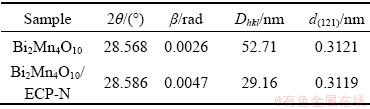
The crystalline nature of the as-prepared Bi2Mn4O10 nanoparticles and Bi2Mn4O10/ECP-N composites was investigated by XRD and the corresponding results were depicted in Fig. 1. As can be seen from Fig. 1, the ball-milled Bi2Mn4O10 nanoparticles and Bi2Mn4O10/ECP-N composites exhibit nearly the same XRD patterns, which perfectly match with the pure Bi2Mn4O10 phase (JCPDS No. 27-0048). However, there is a diffraction peak of Bi2O3 in Fig. 1(b), which might be attributable to the relatively short milling time. The bismuth and manganese cannot be mixed uniformly, resulting in the existence of the Bi2O3 phase. After the Bi2Mn4O10 powders and ECP-N were mixed by ball milling for 8 h, no peak of Bi2O3 was detected, indicating the higher purity of the as-obtained Bi2Mn4O10/ECP-N. Moreover, the peak intensities of Bi2Mn4O10/ECP-N composite become weaker than those of Bi2Mn4O10, which indicates that the crystallinity of different crystal faces declines and the grain size is smaller after ball milling with ECP-N. The interplanar spacing (d) and grains size (Dhkl) can be estimated through the Bragg equation 2dsin θ=nλ (n is the diffraction series, θ is the angle of incidence on a crystal plane and λ is the wavelength), and Scherrer equation Dhkl=Kλ/(βcos θ) (K=0.89, λ=0.154 nm, and β is the full width at half-maximum (FWHM)). Corresponding results are listed in Table 1. After ball milling with ECP-N, the interplanar spacing and grain size of Bi2Mn4O10 nanoparticles decreased obviously, which were of great significance to increase the specific surface area of the sample and release substantial active sites for Li+ storage. In addition, no obvious peak of ECP-N was detected on account of the higher peak intensity of Bi2Mn4O10, indicating that the low content of ECP-N had no obvious effect on the crystallization behavior of the Bi2Mn4O10.
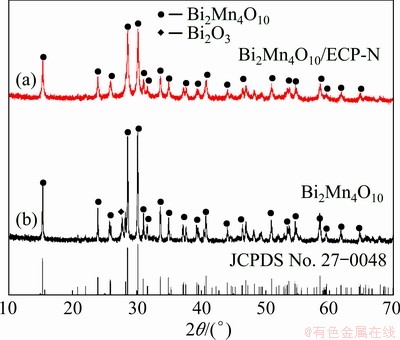
Fig. 1 XRD patterns of as-synthesized Bi2Mn4O10/ ECP-N (a) and bare Bi2Mn4O10 (b)
Table 1 Structural parameters of Bi2Mn4O10 and Bi2Mn4O10/ECP-N determined from XRD Rietveld refinement
3.2 SEM and TEM morphologies
The typical SEM and TEM images of as-synthesized materials were revealed in Fig. 2. As shown in Fig. 2(a), the Bi2Mn4O10/ECP-N composites were mainly composed of well- developed nanoparticles with particle sizes ranging from one hundred to several hundred nanometers. The morphology of Bi2Mn4O10/ECP-N nanoparticles demonstrated uniform distribution because the Bi2Mn4O10/ECP-N nanocrystals could be well separated and stabilized by the doped carbon matrix. Figure 2(c) displayed that the fine lattice fringes of Bi2Mn4O10/ECP-N nanoparticles with an interplanar spacing of approximately 0.265 nm, which corresponded to the (130) crystalline plane of the Bi2Mn4O10 phase. This result further demonstrated the Bi2Mn4O10/ECP-N nanocrystals with superior crystallization and purity. As can be seen from the SEM and TEM images of ECP-N in Figs. 2(b, d), ECP-N showed a nanoparticle structure with the particle size distribution between tens of nanometers and hundreds of nanometers, which could be conducive to uniform doping during the ball-milling.
To evidence elements distribution in Bi2Mn4O10/ ECP-N composites, the element mapping was measured via the energy-dispersive spectroscopy (EDS), as demonstrated in Fig. 3. The elemental mapping of Bi2Mn4O10/ECP-N nanoparticles indicated the existence of Bi, Mn, N, C and O elements (Figs. 3(a-f)). The uniform distribution of element C will improve more abundant reaction area and strengthen the electrical conductivity of Bi2Mn4O10, and the uniform distribution of element N provides substantial defects and active sites, which will improve the wettability of Bi2Mn4O10 in the electrolyte and the electrochemical properties of the Bi2Mn4O10 anode material.
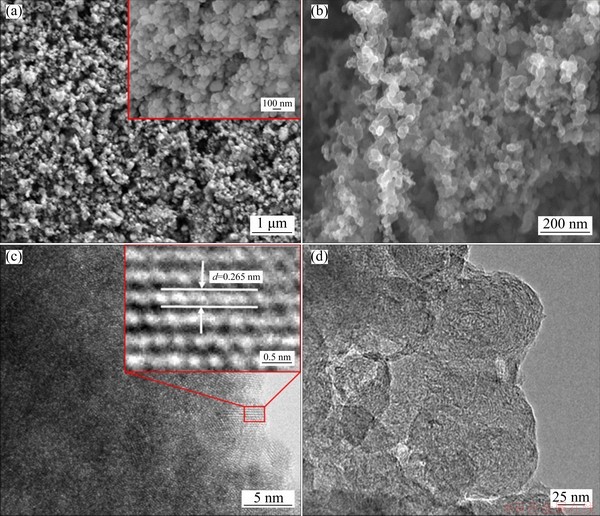
Fig. 2 Low and high magnification SEM images of Bi2Mn4O10/ECP-N (a), SEM image of ECP-N (b), high-resolution TEM image of Bi2Mn4O10/ECP-N (c) and TEM image of ECP-N (d)

Fig. 3 EDS image of Bi2Mn4O10/ECP-N (a), corresponding element mapping of Bi (b), Mn (c), N (d), C (e), O (f) and comparison of interface contact angle of Bi2Mn4O10 (g), ECP (h) and ECP-N (i)
In particular, the interaction among Li+ and Bi2Mn4O10, ECP, N-ECP was also measured via the contact angle test. As shown in Figs. 3(g-i), the three electrodes with Bi2Mn4O10, ECP, and ECP-N were adopted to characterize the contact angle, respectively, which used the same electrolytes (1 mol/L LiPF6 in a mixture of EC, DMC and DEC (1:1:1, volume ratio)). As shown in Fig. 3(i), ECP-N electrode manifested minimum contact angle of 1.36°, which is smaller than that on the Bi2Mn4O10 electrode (45.61°, Fig. 3(g)) and ECP electrode (2.34°, Fig. 3(h)), indicating good wettability for electrolyte after being N-doped. The enhanced affinity with electrolyte would promote Li+ transmission, thus increasing the lithium-ion diffusion rate on the surface of the electrode [22], which could be conducive to the improvement of the cyclicity and rate performance.
3.3 N2 adsorption/desorption isotherms
Generally, the specific surface area, total pore volume, and pore diameter have significant impact on the electrochemical performance of the electroactive materials, thus typical N2 adsorption/ desorption isotherm measurements were implemented to characterize the porous features and textural properties of the as-prepared Bi2Mn4O10/ ECP-N and Bi2Mn4O10 samples more clearly. As shown in Fig. 4, all N2 isotherms could be classified as typical type-IV characteristics with a type-H1 hysteresis loop [23,24], revealing mesoporous characteristics. Their BET specific surface area and total pore volume were computed to be 33.72 m2/g and 0.1538 cm3/g for Bi2Mn4O10/ECP-N, 11.87 m2/g and 0.0232 cm3/g for Bi2Mn4O10, respectively. The relatively large surface area and total pore volume of Bi2Mn4O10/ECP-N were beneficial to providing the electrochemical uptake and release of Li+ ions, and favoring enough room to accommodate volume expansion/ contraction during continuous cycling. Furthermore, the mesoporous characteristics could also be further testified by the Barrett-Joyner-Halenda (BJH) pore-size distribution (PSD) in the insets of Figs. 4(a, b), the pore distributions of Bi2Mn4O10/ ECP-N and Bi2Mn4O10 were mostly in the range of 2-20 nm, and their average pore sizes were approximately 18.24 and 7.83 nm respectively, which strongly demonstrated that the Bi2Mn4O10/ ECP-N contained a great number of mesoporous architectures. Indeed, the abundant mesoporous configuration not only promotes the contact between electroactive materials and electrolytes but also provides sufficient surfaces/interfaces to boost charge transfer and shorten path length of ion diffusion, thus strongly increasing the electrochemical performance of samples [23].
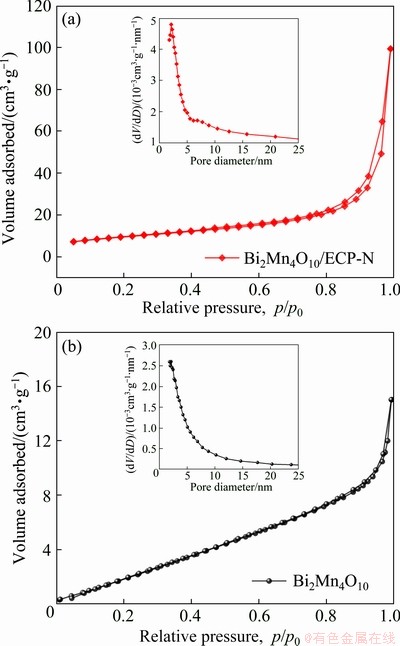
Fig. 4 N2 adsorption/desorption isotherms of Bi2Mn4O10/ ECP-N (a) and Bi2Mn4O10 (b) (Insets in (a, b) show corresponding BJH pore size distribution plots)
3.4 XPS spectra
The XPS was employed to characterize the chemical elements and the surface chemical valence states. The as-obtained XPS data and typically fitted curves by using the Gaussian fitting method were collected and shown in Fig. 5. The main peaks of Bi 4f, C 1s, O 1s, and Mn 2p were delivered in the survey spectrum of Bi2Mn4O10/ECP-N in Fig. 5(a), and another weak peak was situated at about 398 eV, which corresponded to the N 1s. As shown in the high-resolution spectrum of Bi 4f (Fig. 5(b)), the binding energies situated at 160.2 and 165.5 eV were contributed to Bi 4f5/2 and Bi 4f7/2, respectively [15,25]. As shown in Fig. 5(c), the high-resolution spectrum of Mn 2p exhibited two the characteristic peaks at 641.3 and 652.9 eV, which corresponded to Mn 2p3/2 and Mn 2p1/2, respectively. Moreover, the spectrum of Mn 2p could be separated into four peaks, in which 641.2 and 652.9 eV could characterize the existence of Mn3+, and the other two peaks at 642.5 and 654.1 eV could be assigned to Mn4+ species [23]. According to the high-resolution spectra of C 1s in Fig. 5(d), three peaks at 284.4, 285.6 and 288.6 eV were attributed to C—C, C—N, and C=O groups, which indicated that N atoms had been doped into the electroactive materials [26]. The existence of N species was further testified by N 1s peak in Fig. 5(e). The peaks located at 398.8, 399.6 and 401.4 eV could be assigned to pyridinic-N, pyrrolic-N and graphitic-N, respectively [22,27]. The total content of N was approximately 1.9%, which could enhance lithium-storage capability.
3.5 Electrocatalytic behavior
Subsequently, the Bi2Mn4O10/ECP-N was configured as an anode in a half cell, in which metallic lithium foil was utilized as the counter electrode, highlighting the application prospects of the material for high-performance LIBs and evaluating its electrochemical lithium-storage performance. The lithium-ion storage mechanism of Bi2Mn4O10/ECP-N composites was preliminarily investigated using cyclic voltammetry in the voltage range of 0.05-3.0 V (vs Li+/Li) at a scan rate of 0.1 mV/s. As shown in Fig. 6(a), the CV curves displayed multipeak characteristics, indicating that the reaction between Li+ and the Bi2Mn4O10/ECP-N was multistep. During the first negative scan, two irreversible cathodic peaks at about 1.19 and 0.73 V could be attributed to the decomposition of the electrolyte and the reduction of Mn3+, Mn4+ and Bi3+ to Mn0 and Bi0 with the production of Li2O, respectively [28,29]. The cathodic peak at around 0.38 V could be associated with the formation of SEI films and alloying reaction between Bi and Li, which could be considered as a multistep alloying reaction. However, the broad peak was divided into two small peaks at ~1.37 and ~1.06 V in subsequent cycles, which corresponded to the reduction of BiO to metallic Bi and MnO to metallic Mn, respectively [28,29]. During the positive scan, a strong anodic peak at around 1.12 V could be related to the dealloying processes of Li3Bi. Furthermore, two broad anodic peaks at about 1.4 and 1.7 V might have originated from the oxidation of Bi and Mn metal clusters. The aforementioned main reactions were shown in Table 2. From the CV curves, it was obvious that the reduction peaks and oxidation peaks exhibited apparent overlapped characteristics, which verified that the electrode exhibited excellent reversibility and stability during the electrochemical reaction.
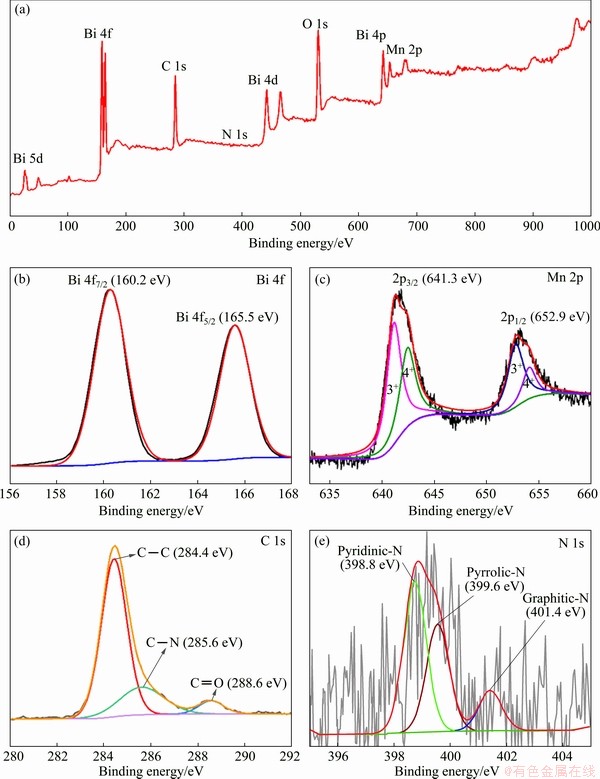
Fig. 5 Full XPS spectrum of Bi2Mn4O10/ECP-N (a), high-resolution XPS spectrum of Bi 4f (b), Mn 2p (c), C 1s (d) and N 1s (e)
Table 2 Chemical reactions during charge/discharge processes
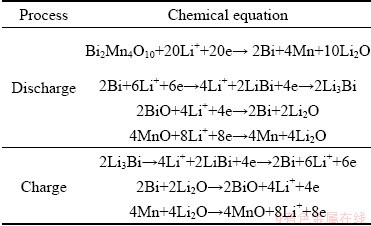
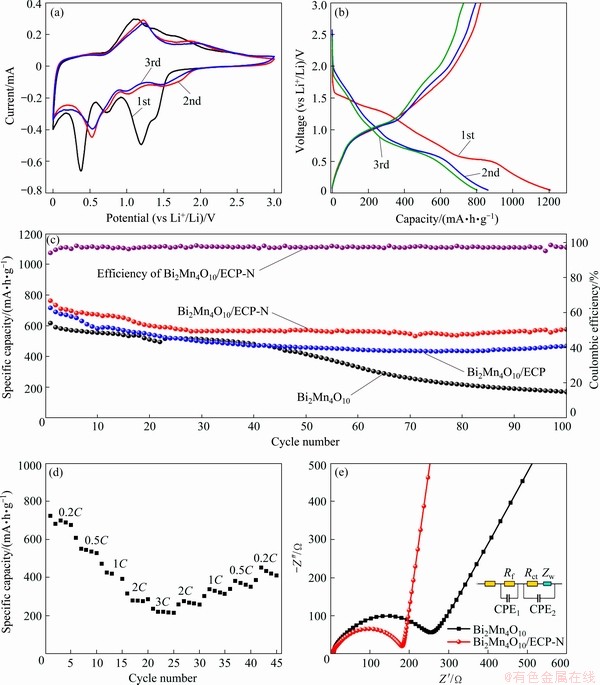
Fig. 6 CV curves of Bi2Mn4O10/ECP-N (a), charge/discharge curves of Bi2Mn4O10/ECP-N (b), cycling performance of Bi2Mn4O10, Bi2Mn4O10/ECP and Bi2Mn4O10/ECP-N at 0.2C (1C=800 mA/g) (c), rate performance curve of Bi2Mn4O10/ ECP-N (d) and EIS Nyquist curves of Bi2Mn4O10 and Bi2Mn4O10/ECP-N (e)
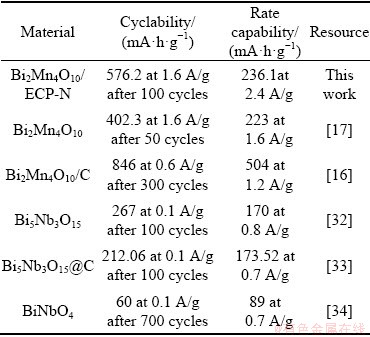
As illustrated in Fig. 6(b), the initial discharge specific capacity and charge specific capacity were 1207 and 823 mA·h/g, delivering Coulombic efficiency of 68%, the initial irreversible capacity loss could be attributed to the irreversible redox reaction and the formation of SEI layer on the anode electrode [30]. The formation of SEI layer is indispensable to conversion-type anode materials for large volume expansion [31]. Expectably, the stable redox processes could be obtained after the first cycle, resulting in high capacity retention, which could be clearly demonstrated in Fig. 6(c). As we could see the cycle performance at 0.2C in Fig. 6(c), it was obvious that the Bi2Mn4O10/ECP-N displayed a higher discharge specific capacity than the Bi2Mn4O10/ECP and Bi2Mn4O10. The specific capacity of Bi2Mn4O10/ECP-N showed a slight decrease and stabilized at 576.2 mA·h/g after 100 cycles, while the Bi2Mn4O10/ECP and Bi2Mn4O10 only demonstrated the specific capacity of 467.3 and 169.5 mA·h/g, respectively. It was obvious that the introduction of ECP-N could significantly strengthen the cycle stability, which could be ascribed to the protection of layered ECP-N for structural change during long-term continuous charge and discharge processes, and could be related to the better wettability for electrolyte after being nitrogen-doped. Figure 6(d) indicated that the Bi2Mn4O10/ECP-N composite showed superior rate performance and delivered reversible capacities of 723.1, 607.4, 470.5, 314.6 and 236.1 mA·h/g, at the current densities of 0.2C, 0.5C, 1C, 2C and 3C, respectively. These results are more satisfactory than previous work of bare Bi2Mn4O10 [17]. The electrochemical performance based on other Bi-M-O compounds anode materials for lithium-ion batteries was summarized in Table 3.
Table 3 Electrochemical performance comparison of Bi2Mn4O10/ECP-N with other reported Bi-M-O compound anode materials for lithium-ion batteries
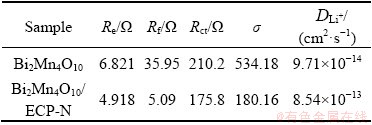
The heightened cyclic persistence and rate performance of Bi2Mn4O10/ECP-N could be closely related to their excellent conductivity owing to the existence of ECP-N, which could be evidenced by EIS characterization. The fitted Nyquist plots and relative equivalent circuit model were displayed in Fig. 6(e). The semicircle in high-medium frequency was characterized as the charge transfer resistance (Rct), which was ascribed to the electrochemical reactions between the interface of electrolyte and electrodes, the oblique line in low-frequency regions reflected Warburg impedance (Zw) for Li+ diffusion [35,36]. In addition, Re and Rf were ascribed to the resistances of the solvent electrolyte and SEI films, respectively. The resistance value of Rct+Re+Rf for the Bi2Mn4O10/ECP-N (185.808 Ω) was smaller than that of Bi2Mn4O10 (252.971 Ω), suggesting that Bi2Mn4O10/ECP-N composite anode material possessed a faster charge transfer than the bare Bi2Mn4O10 anode. Additionally, the linear slope of Bi2Mn4O10/ECP-N composite in low-frequency region was larger than that of bare Bi2Mn4O10, indicating the Li+ diffusion rate could be increased by using Bi2Mn4O10/ECP-N composite anode. Furthermore, the Li+ diffusion coefficient (DLi+) could be estimated from the low-frequency regions by the following formula: DLi+=0.5(RT)2/(An21F2Cσ)2, where R is the mole gas constant and R=8.314 J/(mol·K), T is the thermodynamic temperature and T=298 K, electrode surface area A=1.13 cm2, F is the Faraday constant and F=96500 C/mol, the concentration of Li+ C=0.001 mol/cm3, n1 is the electronic transfer number, and σ is the Warburg coefficient resulting from Z′=Rct+Re+Rf+σω-1/2, ω=2πf (Z', ω and f represented the real impedance, angular frequency and frequency, respectively). The EIS fitting statistic values and Li+ diffusion were presented in Table 4. The calculated DLi+ value of Bi2Mn4O10/ ECP-N (8.54×10-13 cm2/s) is much higher than that of pristine Bi2Mn4O10 (9.71×10-14 cm2/s), revealing the high Li+ diffusion coefficient and excellent reaction kinetics after the introduction of ECP-N. Bi2Mn4O10/ECP-N illuminated smaller charge transfer resistance and Warburg impedance than bare Bi2Mn4O10, reflecting that the introduction of ECP-N can not only advance Li+ diffusion and enhance the conductivity of the electroactive materials but also reduce the resistance of the charge transfer and enhance the reaction kinetics. Therefore, Bi2Mn4O10/ECP-N composite anode exhibited a superior cycle and rate performance.
Table 4 EIS fitting statistic values and Li+ diffusion coefficient
4 Conclusions
(1) The high-purity mesoporous architecture Bi2Mn4O10/ECP-N composite anode material with uniform distribution of C and N elements was designed via a convenient wet ball-milling method.
(2) Benefiting from the ECP-N layers, the Bi2Mn4O10/ECP-N composite anode demonstrates a high capacity of 576.2 mA·h/g after 100 cycles at 0.2C with a capacity retention of 75%, which is much higher than that of bare Bi2Mn4O10 (only 27%). Even at 3C, a superior rate capacity of 236.1 mA·h/g is retained.
(3) The N dopants make ECP have good wettability and electronic affinity, which can be beneficial to promoting the electronic conductivity of Bi2Mn4O10/ECP-N and enhance kinetics. In addition, using ECP-N as the buffer layer is a pretty effective technique to mitigate volume expansion and alleviate the rapid capacity decline. This work provides a potential electroactive material for lithium-ion batteries and a design strategy for large-scale synthesizing nanocomposite electrode materials.
References
[1] CHABI S, PENG C, HU D, ZHU Y Q. Ideal three-dimensional electrode structures for electrochemical energy storage [J]. Advanced Materials, 2014, 26(15): 2440-2445.
[2] PENG Ke, ZHANG Zhi-jian, ZHAO Ze-jun, YANG Chao, TIAN Zhong-liang, LAI Yan-qing. Performance of carbon-coated nano-ZnO prepared by carbonizing gel precursor as anodic material for secondary alkaline Zn batteries [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(10): 2151-2159.
[3] GUO J C, LIU Q, WANG C S, ZACHARIAH M R. Interdispersed amorphous MnOx-carbon nanocomposites with superior electrochemical performance as lithium- storage material [J]. Advanced Functional Materials, 2012, 22(4): 803-811.
[4] QIU Yong-cai, XU Gui-liang, YAN Ke-you, SUN Hui, XIAO Jun-wu, YANG Shi-he, SUN Shi-gang, JIN Li-min, DENG Hong. Morphology-conserved transformation: Synthesis of hierarchical mesoporous nanostructures of Mn2O3 and the nanostructural effects on Li-ion insertion/ deinsertion properties [J]. Journal of Materials Chemistry, 2011, 21(17): 6346-6353.
[5] KUNDU M, NG C C A, PETROVYKH D Y, LIU L F. Nickel foam supported mesoporous MnO2 nanosheet arrays with superior lithium storage performance [J]. Chemical Communications, 2013, 49(76): 8459-8461.
[6] REDDY M V, RAO G V S, CHOWDARI B V R. Metal oxides and oxysalts as anode materials for Li ion batteries [J]. Chemical Reviews, 2013, 113(7): 5364-5457.
[7] ZHAO Yang, LIU Ting-ting, XIA Hui, ZHANG Ling, JIANG Jia-xing, SHEN Ming, NI Jiang-feng, GAO Li-jun. Branch-structured Bi2S3-CNT hybrids with improved lithium storage capability [J]. Journal of Materials Chemistry A, 2014, 2(34): 13854-13858.
[8] CHEN Jun, ZHAN Jing, LI Qi-hou. Exploration and crystal phase engineering from bismuthinite ore to visible-light responsive photocatalyst of Bi2O3 [J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 103375.
[9] CHEN Jun, ZHAN Jing, ZHANG Yu-meng, TANG Yi-wei. Construction of a novel ZnCo2O4/Bi2O3 heterojunction photocatalyst with enhanced visible light photocatalytic activity [J]. Chinese Chemical Letters, 2019, 30(3): 735-738.
[10] DENG Zhuo, LIU Ting-ting, CHEN Tao, JIANG Jia-xiang, YANG Wan-li, GUO Jun, ZHAO Jian-qing, WANG Hai-bo, GAO Li-jun. Enhanced electrochemical performances of Bi2O3/rGO nanocomposite via chemical bonding as anode materials for lithium ion batteries [J]. ACS Applied Materials & Interfaces, 2017, 9(14): 12469-12477.
[11] CABANA J, MONCONDUIT L, LARCHER D, PALACIN M R. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions [J]. Advanced Materials, 2010, 22(35): E170-E192.
[12] GAO Qi-li, YUAN Zhao-xiao, DONG Lin-xi, WANG Gao-feng, YU Xue-bin. Reduced graphene oxide wrapped ZnMn2O4/carbon nanofibers for long-life lithium-ion batteries [J]. Electrochimica Acta, 2018, 270: 417-425.
[13] ZHAO Rui-zheng, LI Qun, WANG Cheng-xiang, YIN Long-wei. Highly ordered mesoporous spinel ZnCo2O4 as a high-performance anode material for lithium-ion batteries [J]. Electrochimica Acta, 2016, 197: 58-67.
[14] SHEN Lai-fa, YU Le, YU Xin-yao, ZHANG Xiao-gang, LOU Xiong-wen. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors [J]. Angewandte Chemie: International Edition, 2015, 54(6): 1868-1872.
[15] ZHANG Yang, ZHAO Gang-gang, GE Peng, WU Tian-jing, LI Lin, CAI Peng, LIU Cheng, ZOU Guo-qiang, HOU Hong-shuai, JI Xiao-bo. Bi2MoO6 microsphere with double-polyaniline layers toward ultrastable lithium energy storage by reinforced structure [J]. Inorganic Chemistry, 2019, 58(9): 6410-6421.
[16] SONG Zi-han, ZHANG Hong-zhang, FENG Kai, WANG Huai-qing, LI Xian-feng, ZHANG Hua-min. Bi2Mn4O10: A new mullite-type anode material for lithium-ion batteries [J]. Dalton Transactions, 2018, 47(23): 7739-7746.
[17] ZHAN Jing, LONG Yi-yu. Synthesis of Bi2Mn4O10 nanoparticles and its anode properties for LIB [J]. Ceramics International, 2018, 44(12): 14891-14895.
[18] NI Jiang-feng, ZHAO Yang, LIU Ting-ting, ZHENG Hong-he, GAO Li-jun, YAN Cheng-lin, LI Liang. Strongly coupled Bi2S3@CNT hybrids for robust lithium storage [J]. Advanced Energy Materials, 2014, 4(16): 1400798.
[19] PAN Cheng-chi, YANG Ying-chang, HOU Hong-shuai, JING Ming-jun, ZHU Yi-rong, SONG Wei-xin, JI Xiao-bo. Effect of lithium content on electrochemical property of Li1+x(Mn0.6Ni0.2Co0.2)1-xO2(0≤x≤0.3) composite cathode materials for rechargeable lithium-ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(1): 145-150.
[20] GE Peng, LI Si-jie, SHUAI Hong-lei, XU Wei, TIAN Ye, YANG Li, ZOU Guo-qiang, HOU Hong-shuai, JI Xiao-bo. Engineering 1D chain-like architecture with conducting polymer towards ultra-fast and high-capacity energy storage by reinforced pseudo-capacitance [J]. Nano Energy, 2018, 54: 26-38.
[21] XU Chang-fan, ZHANG Kai-wen, ZHANG Da, CHANG Shi-lei, LIANG Feng, YAN Peng-fei, YAO Yao-chun, QU Tao, ZHAN Jing, MA Wen-hui, YANG Bing, DAI Yong-nian, SUN Xue-liang. Reversible hybrid sodium-CO2 batteries with low charging voltage and long-life [J]. Nano Energy, 2020, 68: 104318.
[22] DONG Qing-yuan, HONG Bo, FAN Hai-lin, GAO Chun-hui, HONG Shu, LAI Yan-qing. Electron-rich functional doping carbon host as dendrite-free lithium metal anode [J]. Electrochimica Acta, 2018, 284: 376-381.
[23] ZHANG Long-hai, ZHU Si-qi, CAO Hui, HOU Lin-rui, YUAN Chang-zhou. Hierarchical porous ZnMn2O4 hollow nanotubes with enhanced lithium storage toward lithium-ion batteries [J]. Chemistry—A European Journal, 2015, 21(30): 10771-10777.
[24] JU Zhi-cheng, MA Guang-yao, ZHAO Yu-long, XING Zheng, QIANG Ying-huai, QIAN Yi-tai. A facile method for synthesis of porous NiCo2O4 nanorods as a high-performance anode material for Li-ion batteries [J]. Particle & Particle Systems Characterization, 2015, 32(11): 1012-1019.
[25] CHEN Rui, SHEN Zhu-rui, WANG Hu, ZHOU Hui-jing, LIU Yu-ping, DING Da-tong, CHEN Tie-hong. Fabrication of mesh-like bismuth oxide single crystalline nanoflakes and their visible light photocatalytic activity [J]. Journal of Alloys and Compounds, 2011, 509(5): 2588-2596.
[26] HONG Wan-wan, GE Peng, JIANG Yun-ling, YANG Li, TIAN Ye, ZOU Guo-qiang, CAO Xiao-yu, HOU Hong-shuai, JI Xiao-bo. Yolk-shell-structured bismuth@N-doped carbon anode for lithium-ion battery with high volumetric capacity [J]. ACS Applied Materials & Interfaces, 2019, 11(11): 10829-10840.
[27] YUAN Zhao-xia, DONG Lin-xi, GAO Qi-li, HUANG Zhen-guo, WANG Lu-wen, WANG Gao-feng, YU Xue-bin. SnSb alloy nanoparticles embedded in N-doped porous carbon nanofibers as a high-capacity anode material for lithium-ion batteries [J]. Journal of Alloys and Compounds, 2019, 777: 775-783.
[28] ZHENG Yang, ZHOU Teng-fei, ZHAO Xu-dong, PANG Wei-kong, GAO Hong, LI Se-an, ZHOU Zhen, LIU Hua-kun, GUO Zai-ping. Atomic interface engineering and electric-field effect in ultrathin Bi2MoO6 nanosheets for superior lithium ion storage [J]. Advanced Materials, 2017, 29(26): 1700396.
[29] CHEN Hong-bin, DING Liang-xin, XIAO Kang, DAI Sheng, WANG Su-qing, WANG Hai-hui. Highly ordered ZnMnO3 nanotube arrays from a “self-sacrificial” ZnO template as high-performance electrodes for lithium ion batteries [J]. Journal of Materials Chemistry A, 2016, 4: 16318-16323.
[30] SHARMA Y, SHARMA N, RAO G V S, CHOWDARI B V R. Nanophase ZnCo2O4 as a high performance anode material for Li-ion batteries [J]. Advanced Functional Materials, 2007, 17(15): 2855-2861.
[31] HU Ling-ling, QU Bai-hua, LI Cheng-chao, CHEN Yue-jiao, MEI Lin, LEI Dan-ni, CHEN Li-bao, LI Qiu-hong, WANG Tai-hong. Facile synthesis of uniform mesoporous ZnCo2O4 microspheres as a high-performance anode material for Li-ion batteries [J]. Journal of Materials Chemistry A, 2013, 1: 5596-5602.
[32] LI Yu-hang, ZHENG Run-tian, YU Hao-xiang, CHENG Xing, LIU Ting-ting, PENG Na, ZHANG Jun-dong, SHUI Miao, SHU Jie. Fabrication of one-dimensional architecture Bi5Nb3O15 nanowires by electrospinning for lithium-ion batteries with enhanced electrochemical performance [J]. Electrochimica Acta, 2019, 299: 894-901.
[33] LI Yu-hang, ZHENG Run-tian, YU Hao-xiang, CHENG Xing, ZHU Hao-jie, BAI Ying, LIU Ting-ting, SHUI Miao, SHU Jie. Carbon-coated Bi5Nb3O15 as anode material in rechargeable batteries for enhanced lithium storage [J]. Ceramics International, 2018, 44(10): 11505-11511.
[34] TANG Guang-xia, ZHU Hao-jie, YU Hao-xiang, CHENG Xing, ZHENG Run-tian, LIU Ting-ting, ZHANG Jun-dong, SHUI Miao, SHU Jie. Ultra-long BiNbO4 nanowires with hierarchical architecture exhibiting reversible lithium storage [J]. Journal of Electroanalytical Chemistry, 2018, 823: 245-252.
[35] ZHOU Qian, LIU Li, HUANG Zhi-feng, YI Ling-guang, WANG Xian-you, CAO Guo-zhong. Co3S4@polyaniline nanotubes as high-performance anode materials for sodium ion batteries [J]. Journal of Materials Chemistry A, 2016, 4(15): 5505-5516.
[36] ZHANG Jia-feng, ZHANG Jian-yong, OU Xing, WANG Chun-hui, PENG Chun-li, ZHANG Bao. Enhancing high-voltage performance of Ni-rich cathode by surface modification of self-assembled NASICON fast ionic conductor LiZr2(PO4)3 [J]. ACS Applied Materials & Interfaces, 2019, 11(17): 15507-15516.
氮掺杂碳包覆Bi2Mn4O10锂离子电池负极材料的制备及其电化学性能
湛 菁1,2,徐昌藩1,龙怡宇1,李启厚1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 难冶有色金属资源高效利用国家工程实验室,长沙 410083
摘 要:为抑制高能锂离子电池负极材料Bi2Mn4O10容量的快速衰减,通过简单球磨法制备新型高纯Bi2Mn4O10/ECP-N(ECP-N为氮掺杂科琴黑)负极复合材料。所合成的Bi2Mn4O10/ECP-N复合材料在 0.2C倍率下 循环100次后可保持576.2 mA·h/g的比容量,容量保持率为75%,而纯Bi2Mn4O10的容量保持率仅为27%。3C倍率下Bi2Mn4O10/ECP-N复合材料的放电容量仍保持在236.1 mA·h/g。引入氮掺杂的科琴黑ECP-N不仅可以有效地提高比表面积以缓冲体积膨胀,增强材料的电导率和可湿性,而且还可以促进离子传输和可逆转化反应。
关键词:Bi2Mn4O10纳米颗粒;氮掺杂科琴黑;倍率性能;锂离子电池
(Edited by Wei-ping CHEN)
Foundation item: Project (2019zzts502) supported by the Fundamental Research Funds for the Central Universities of Central South University, China; Project (2018GK4001) supported by the Scientific and Technological Breakthrough and Major Achievements Transformation of Strategic Emerging Industries of Hunan Province, China
Corresponding author: Jing ZHAN, Tel: +86-13975147556, E-mail: zhanjing@csu.edu.cn;
Qi-hou LI, E-mail: li_qihou@126.com
DOI: 10.1016/S1003-6326(20)65371-7
Abstract: To inhibit rapid capacity attenuation of Bi2Mn4O10 anode material in high-energy lithium-ion batteries, a novel high-purity anode composite material Bi2Mn4O10/ECP-N (ECP-N: N-doped Ketjen black) was prepared via an uncomplicated ball milling method. The as-synthesized Bi2Mn4O10/ECP-N composite demonstrated a great reversible specific capacity of 576.2 mA·h/g after 100 cycles at 0.2C with a large capacity retention of 75%. However, the capacity retention of individual Bi2Mn4O10 was only 27%. Even at 3C, a superior rate capacity of 236.1 mA·h/g was retained. Those remarkable electrochemical performances could give the credit to the introduction of ECP-N, which not only effectively improves the specific surface area to buffer volume expansion and enhances conductivity and wettability of composites but also accelerates the ion transfer and the reversible conversion reaction.


