
J. Cent. South Univ. (2021) 28: 1627-1636
DOI: https://doi.org/10.1007/s11771-021-4722-x
Influence of particle size and surfactants on uniformity and quantity of silicon carbide particles in electrodeposited nickel-silicon carbide coatings
KAN Hong-min(阚洪敏)1, 2, MENG Yuan-yuan(孟媛媛)1, 2, Ramana G REDDY3
1. School of Mechanical Engineering, Shenyang University, Shenyang 110044, China;
2. Key Laboratory of Research and Application of Multiple Hard Films of Liaoning Province,Shenyang University, Shenyang 110044, China;
3. Department of Metallurgical and Materials Engineering, the University of Alabama, Tuscaloosa,AL 35487, USA
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
The electrodeposition of nickel-silicon carbide coatings on a copper electrode was done by mixing SiC particles in the nickel electrodeposition solution. The influence of surfactants and silicon carbide particle size on uniformity and quantity of silicon carbide particles in nickel-silicon carbide composite coatings was investigated. It was found that particle size affects the nucleation overpotential, with 40 nm silicon carbide nanoparticles more effective in promoting nickel nucleation than 500 nm particles due to an increase in active nucleation sites. In terms of surfactants, anionic surfactant sodium dodecyl sulfate (SDS) produced better dispersion of 40 nm silicon carbide particles than cationic surfactant cetyltrimethyl ammonium bromide (CTAB), but little difference was found between the two when 500 nm silicon carbide particles were used. Thus, although the suspension of silicon carbide particles can be improved and their co-deposition can be promoted with a cationic surfactant CTAB, it is less effective than an anionic surfactant SDS in terms of surface finish.
Key words:
Cite this article as:
KAN Hong-min, MENG Yuan-yuan, Ramana G REDDY. Influence of particle size and surfactants on uniformity and quantity of silicon carbide particles in electrodeposited nickel-silicon carbide coatings [J]. Journal of Central South University, 2021, 28(6): 1627-1636.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4722-x1 Introduction
Nickel and nickel based alloy coatings prepared by electrodeposition are used extensively as protective coatings on an industrial scale. However, pure nickel has low strength and hardness. The electrodeposition of micrometer and nanometer sized particles into different matrices can be used to create nano-composite materials with superior properties compared to pure metal coatings [1-4]. Silicon carbide particles are broadly used as second phase particles in composite coatings to enhance deposit properties because they are commercially available, hard and cheap. These coatings can improve the heat and corrosion resistance, wear resistance and micro hardness for various applications. These coatings can be used in cutting tools, cylinder walls for aluminum internal combustion engines, micro-electronic components and aerospace engine components with complex shapes [5-8]. Of the various composites that have been developed, nickel-silicon carbide composite coatings have attracted considerable attention because of the high micro-hardness, corrosion resistance and wear resistance when used for industrial applications [9-11]. The composite coatings obtained by using nanoparticles with unique physical and chemical properties exhibit better performance than those obtained by using micro-sized particles [12]. The properties of such coatings are also greatly affected by the quantity and uniformity of silicon carbide particles they contain [13-15], as the added nanoparticles have a refining effect on the crystal grains of the matrix metal. This results in an increase in nanocrystal formation during co-deposition with the yield strength and hardness increased by about 5 to 10 times when the particle size is less than 100 nm [16]. The downside of this, however, is that the agglomerate of fine nanoparticles will occur in the electrolyte cell because their surface free energy is high. This creates an uneven dispersion of particles in the electrodeposition bath and changes the static electricity, intermolecular and chemisorption forces, which in turn affects the properties of the coating. In addition, the adsorption and desorption around cathode area will be affected because of the presence of silicon carbide particles during electrocrystallization. Nanoparticles make the electrodeposition process more complex, but increasing their distribution is crucial to improve the properties of the coating [17-21]. The properties of composition coatings mainly depend on the matrix phases, the uniformity and quantity of particles in composite coatings. High dispersion of silicon carbide particles in the bath is helpful to incorporate higher and more uniform particles into the deposit. Surfactants play an important role in reducing particle agglomeration, improving suspension stability and enhancing particles codeposition. The proper surfactants can enhance the deposit properties.
The preparation and performance of various composite coatings have been previously studied [22-26], and the mechanisms of co-deposition and crystal deposition kinetics have also been investigated using electrochemical techniques [27-30]. From this, it is clear that understanding the electrochemical behavior of composite coatings is helpful to understand the role of solid particles in electrically driven crystallization, as well as the final microstructure and properties of the composite coating. Therefore, the present study aims to determine the crystallization behavior of nickel-silicon carbide composite coatings and how silicon carbide particles affect nickel crystal nucleation by forming coating with nano- and micrometer-sized silicon carbide particles under the same electrodeposition conditions. In addition to silicon carbide particle size, the effects of different surfactants (SDS and CTAB) on nucleation are also discussed.
2 Materials and methods
Nickel-silicon carbide composite coatings were prepared using a modified Watts bath containing SiC particles in the electrolyte. The bath composition and the deposition parameters are shown in Table 1. For this study, the mean diameter of silicon carbide particles is 40 or 500 nm. The silicon carbide particles were gray-black powder, which was prone to agglomeration. Surface morphologies of SiC with different mean diameters are shown in Figure 1. As shown in Figure 1, the 40 nm silicon carbide particles were round, evenly distributed and uniform in size, but also exhibited a greater tendency for agglomeration. The 500 nm SiC particles exhibited irregular shapes and different particle sizes. Each bath was added surfactant either sodium dodecyl sulfate (SDS) 4-7 g or cetyltrimethy ammonium bromide (CTAB) 0.3-0.6 g. To disperse silicon carbide particles uniformly and suspend it in the bath, the electrolyte containing silicon carbide was ultrasonically dispersed for 30 min and then stirred with a magnetic stirrer for 2 h prior to electrodeposition. The anode was a pure nickel plate. The cathode was copper plate sealed with insulated tapes to leave a 10 mm×10 mm exposed area. The electrodes were polished with SiC abrasive papers of different grit sizes, and then ultrasonically cleaned in the distilled water for 5 min before plating. The electrodes were immersed vertically in the bath and the distance between them was 25 mm. Electroplating of nickel-silicon carbide onto a 1 cm2 Cu disk was then conducted at a temperature of about 40 °C and pH=4. A current density 30 mA/cm2 was applied for 75 min. During electrodeposition, the bath was agitated using magnetic stirrer to assist with silicon carbide deposition.
Table 1 Chemicals composition of electrodeposition bath and electrodeposition parameters


Figure 1 (a) SEM image of 40 nm SiC; (b) TEM image of 40 nm SiC; (c) SEM image of 500 nm SiC
Electrochemical experiments were performed using a potentiostat by IVIUM with a three-electrode electrochemical cell. The reference electrode and working electrode were high purity Pt wires (99.999%) with diameter of 1 mm, while the counter electrode was a Pt plate with 1 cm2. The Pt electrodes were washed to remove residue before measurement in a mixture containing 98%H2SO4 (25%), 85%H3PO4 (70%) and 52.5% HNO3 (5%) for 15 min at room temperature.
After electrodeposition, the coatings were ultrasonically cleaned in the distilled water for 5 min to remove loosely adsorbed particles from the cathode surface and dried at room temperature in the air. The surface and cross-sectional morphologies of each coating were observed with scanning electron microscope (SEM, Hitachi S-4800). Specimens for cross-sectional images were prepared by embedding coats in resins and polishing with a polisher. The cross-sectional SEM image indicated the thickness of the final composite coating was about 300 μm. The weight percentage of silicon in the coatings was measured with energy dispersive X-ray spectroscope (EDS) coupled to the SEM to determine the weight percentage of silicon carbide.
3 Results and discussion
3.1 Effect of different surfactants with 40 nm silicon carbide particles
The addition of SiC particles increases the instability of the system and directly affects the discharge nucleation behavior of Ni, as follows:
Ni2++2e= Ni
The adsorption of SiC particles shields the surface of the working electrode. The cyclic voltammetry curves in Figure 2 reveal that the current from the electrolyte with SDS is higher than that from the electrolyte containing CTAB when the electrodepositions were carried out. This indicates a greater co-deposition of silicon carbide nanoparticles in the electrolyte containing CTAB. This can be attributed to a more adsorption content of silicon carbide nanoparticles on the electrode surface as the electrolyte contains surfactant CTAB, which reduces the active surface area and inhibits cathodic reactions. This in turn results in a lower deposition current.
The surface morphology of nickel-silicon carbide deposits created with 40 nm silicon carbide is showed in Figure 3. It can be seen that better dispersion is achieved using SDS as a surfactant than using CTAB. CTAB increased the number of 40 nm silicon carbide nanoparticles in the nickel-silicon carbide plating, but it has poor uniformity due to agglomeration. The EDS spectra in Figures 3(b) and (d) also show that more 40 nm silicon carbide nanoparticles are present in the nickel-silicon carbide plating when CTAB is contained in the electrolyte. This is the reason that cations ionized from CTAB attaching to the surface of silicon carbide particles make it easier for them to move to the cathode surface under an applied electric field and promotes the co-deposition of nickel and silicon carbide. Cationic surfactant CTAB is beneficial to increase the suspension property of SiC particles. Then CTAB surfactant adsorbs more silicon carbide nanoparticles and reduces the active surface area. This agrees with the lower deposition current observed in the cyclic voltammograms. Surface morphology and element distribution diagrams of Ni-SiC (40 nm SiC) deposits are shown in Figure 4.
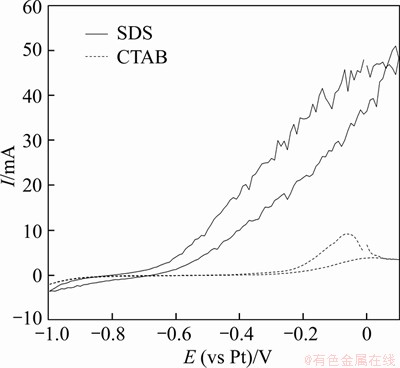
Figure 2 Cyclic voltametry curves of Ni-SiC (40 nm SiC) electrolytes
3.2 Effect of different surfactants with 500 nm silicon carbide particles
The cyclic voltammetry curves in Figure 5 obtaining 500 nm silicon carbide particles reveal similar electrochemical behavior. The curves have similar deposition current and beginning of the cathodic reaction potential in the electrolyte with SDS and CTAB. It shows that the adsorptions of SiC particles on the electrode surface are almost the same in the electrolyte with surfactant SDS and surfactant CTAB. The effective reaction area of Ni2+ is almost the same accordingly, resulting in the similar discharge current. This indicates that choice of surfactant has little effect on uniformity and quantity of 500 nm silicon carbide particles in nickel-silicon carbide coatings.
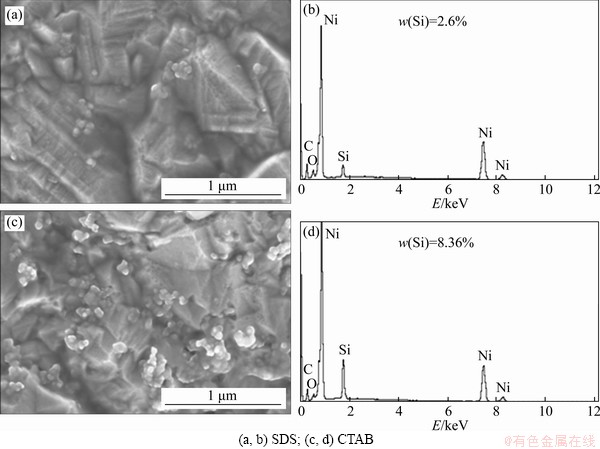
Figure 3 Surface morphology and EDS spectroscopy of Ni-SiC (40 nm SiC) deposits for two different surfactants:

Figure 4 Surface morphology and element distribution diagrams of Ni-SiC (40 nm SiC) deposits

Figure 5 Cyclic voltametry curves of Ni-SiC (500 nm SiC) electrolytes containing SDS and CTAB
The surface morphology of nickel-silicon carbide coatings in Figure 6 demonstrates that the dispersion of 500 nm silicon carbide particles in nickel-silicon carbide composite coating is similar, regardless of whether the electrolyte contains SDS or CTAB, with both producing a uniform distribution of particles. This agrees well with the similarity in their respective cyclic voltammograms.
Though the different surfactants produced similar results with 500 nm silicon carbide particles, the dispersion of 40 nm silicon carbide was improved in the nickel-silicon carbide composite coating by using SDS rather than using CTAB in the electrolyte. That is, the use of CTAB resulted in more 40 nm silicon carbide particles in the composite coating. However, SDS produced a smoother surface. This difference can be attributed to cations ionized from CTAB attaching to the surface of silicon carbide particles, which makes easier for them to move to the cathode surface under an applied electric field and promotes the co-deposition of nickel and silicon carbide. However, as silicon carbide is adsorbed onto the coating by surface-active cations from the CTAB, the coating is made hydrophobic and therefore relatively dull and rough.

Figure 6 Surface morphology of Ni-SiC (500 nm SiC) deposits with SDS (a) and CTAB (b)
3.3 Effect of silicon carbide particle size on matrix metal refinement with SDS surfactant
When subjected to various forces such as static electricity, intermolecular force and chemical adsorption in an electrolyte, any silicon carbide particles remaining stable on the cathode surface will increase the irregularity of its surface. These irregularities can provide a substrate for the heterogeneous nucleation of nickel, thereby reducing its nucleation potential. The potentiostatic curves for the nucleation and growth of nickel-silicon carbide by electrodeposition in an electrolyte containing SDS are presented in Figure 7. The potential applied was -1.0, -1.1, -1.2 and -1.3 V for the electrolyte containing 500 nm silicon carbide (Figure 7(a)), but this was changed to -0.9, -1.0, -1.1 and -1.2 V for the 40 nm silicon carbide (Figures 7(b)). During each constant potential step, the current first increases and then decreases due to the charge of the electric double layer. The current then gradually rises again to a maximum due to nucleation and growth of a new phase. Soon after this, the current gradually decreases again. Thus, the nature of the electrode surface is controlled by diffusion.
The shape of the curve in Figure 7 appears to depend on the overpotential. That is, the current follows an abrupt change because of the charge of the double layer when potential reaches a constant value (beyond –1.0 V in Figure 7(a) and -0.9 V in Figure 7(b)) [14]. As a result, the current begins to decrease with a decrease in the charge current. When the potential is -1.1 V in Figure 7(a) (or -1.0 V in Figure 7(b)), these transients reveal the typical nucleation characteristics. The increase in current when the potential is -1.2 V in Figure 7(a) (or -1.1 V in Figure 7 (b)) is caused by an increase in effective electrode area due to either the growth of individual nuclei, or an increase in the number of nuclei increases [14, 23, 24]. The current reaches a maximum value (Im) when the diffusion zones of the nuclei growing begin to overlap, and then begins to decrease.

Figure 7 Potentiostatic curves for nucleation and growth of Ni-SiC electrodeposition:
It is evident from Figure 7 that the time which it takes to reach the maximum current depends on the overpotential, i.e., a more negative potential reduces the time needed. The reason for this could be that the number of active nucleation sites on the electrode surface increases with overpotential, resulting in an earlier peak current of electric crystal nucleation.
The above analyses show that the current begins to increase at 1.0 V due to crystal nucleation and growth with 40 nm silicon carbide particles, but a voltage of 1.1 V is needed with 500 nm silicon carbide particles. This indicates that the silicon carbide particles around the electrode affect the nucleation overpotential during composite electrodeposition, and that 40 nm silicon carbide particles are more effective in promoting nickel crystal nucleation than 500 nm particles. This may be explained by the fact that 40 nm silicon carbide nanoparticles on the electrode surface provide more active nucleation sites and then inhibit grain growth, as shown by the SEM images in Figure 8. As the SiC particle size decreases, the grain size of the coating decreases overall. The smaller the SiC particles are, the more obvious the effect of grain refinement is. This is because the smaller the size of the second phase particles is, the easier it is to become the nucleation point of the matrix, providing more growth centers on the cathode surface during the deposition process. At the same time, the growth rate is reduced and the growth of matrix crystals is inhibited, and then the matrix crystal grain size of the composite coating is effectively reduced. The smaller the particle size is, the easier it is for SiC to be transported to the electrode surface, and then the adsorption efficiency is improved. The amount of SiC in the coating increases, and the microstructure of the coating becomes denser and smoother.
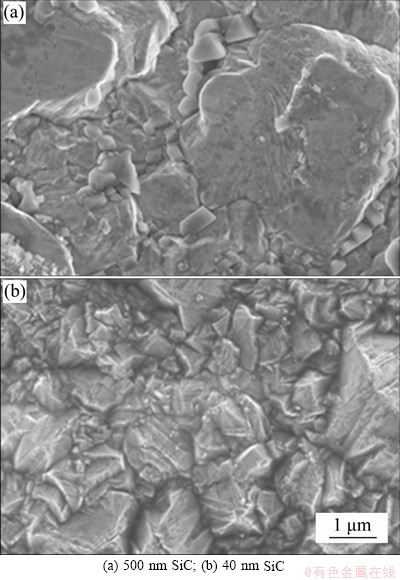
Figure 8 Surface morphology of Ni-SiC electrodeposition in electrolyte containing SDS:
3.4 Effect of silicon carbide particle size on uniformity with SDS surfactant
The morphology of the electrodeposited nickel-silicon carbide coating in Figure 8 shows that uniform dispersion was achieved with both 40 and 500 nm silicon carbide particles, which refines the grain size of the final composite. The reason of the more pronounced refinement achieved with 40 nm silicon carbide is that the finer nanoparticles provide more growth centers that can reduce the overpotential needed for nickel nucleation, as well as inhibit crystal growth more effectively than the 500 nm microparticles. Furthermore, although the adhesion of anions from SDS increases the electric force acting on the silicon carbide particles, larger particles are more affected by gravity and therefore more difficult to deposit.
Increasing the hardness of the composite coating relies on not only controlling the quantity of silicon carbide particles, but also reducing the grain size (the Hall-Petch relationship). A combination of fine nanoparticles and grain strengthening will improve the microstructure and micro-hardness of the coating, and is conducive to improving its tribological properties.
3.5 Effect of silicon carbide particle size on uniformity with CTAB surfactant
Figure 9 shows the cyclic voltammetry curves of the electrodeposition of nickel-silicon carbide coating in the electrolyte with CTAB. The sizes of silicon carbide particles are 40 and 500 nm, respectively.

Figure 9 Cyclic voltametry curves of Ni-SiC electrodeposition in electrolyte containing CTAB
The cyclic voltammetry curves in Figure 9 clearly demonstrate that when using CTAB as a surfactant, the deposition current when using 40 nm silicon carbide particles is lower than that using 500 nm silicon carbide particles, i.e., the former has a higher potential than the latter. This is related to the greater amount of silicon carbide that is co-deposited with electrolytes containing 40 nm silicon carbide, and will be attributed to the decrease of the active surface area caused by a greater adsorption of silicon carbide onto the cathode surface.
The surface morphology of nickel-silicon carbide coating electrodeposited in an electrolyte with CTAB is shown in Figure 10, which reveals that using 40 nm silicon carbide results in far more particles content in the nickel-silicon carbide composite plating than 500 nm particles are used. This may be due to the higher adsorption of silicon carbide onto the electrode surface, which results in more silicon carbide particulates embedded in the coating. This coincides with the cyclic voltammetry results.

Figure 10 Surface morphology of Ni-SiC electrodeposition in electrolyte containing CTAB:
The increase in the amount of codeposited silicon carbide with decreasing particle size can be attributed to two main aspects: 1) the density of silicon carbide is greater than that of the electrolyte, and so an increase in particle diameter increases the sedimentation rate and decreases the number of silicon carbide particles suspended in the electrolytic cell. As a result, the content of silicon carbide particles embedded in the composite coating is reduced; 2) It takes longer time to completely cover large particles during electrodeposition, and so only fine particles are firmly embedded in the coating while others are returned to the bath. This ultimately means that it is easier to obtain a uniform suspension with smaller particles, and these are also more readily covered and incorporated into the final coating. This dispersion of fine particles provides a strengthening effect that helps to improve the hardness of the coating when compared to using coarser particles.
4 Conclusions
1) The silicon carbide particle size affects the nucleation overpotential during composite electrodeposition, with 40 nm particles more effective in promoting Ni nucleation than 500 nm particles. The number of active nucleation sites on the electrode surface increases with increasing overpotential, resulting in an earlier peak current of crystal nucleation. And the 40 nm particles are more beneficial to strengthening by grain size refinement of the nickel matrix.
2) The anionic surfactant SDS is more effective at dispersing 40 nm silicon carbide particles than the cationic surfactant CTAB. The surface coating with CTAB is duller and rougher. However, the choice of surfactant has less influence on the dispersion of 500 nm silicon carbide particles.
Acknowledgements
The authors would like to thank the University of Alabama for providing the experimental and analytical facilities. In addition, KAN Hong-min would like to express her gratitude for awarding a scholarship to purse her research in the United States of America (the University of Alabama) as a visiting scholar by China Scholarship Council (CSC).
Contributors
KAN Hong-min conducted the investigation, wrote the first draft of manuscript and edited the draft of manuscript. MENG Yuan-yuan conducted the literature review and investigation. Ramana G REDDY edited the draft of manuscript.
Conflict of interest
KAN Hong-min, MENG Yuan-yuan and Ramana G REDDY declare that they have no conflict of interest.
References
[1] CALDERON J A, HENAO J E, GOMEZ M A. Erosion-corrosion resistance of Ni composite coatings with embedded SiC nanoparticles [J]. Electrochimica Acta, 2014, 124: 190-198. DOI: 10.1016/j.electacta.2013.08.185.
[2] SUN Chu-feng, LIU Xiao-qin, ZHOU Chun-yu, WANG Chao-nan, CAO Hong-wei. Preparation and wear properties of magnetic assisted pulse electrodeposited Ni-SiC nanocoatings [J]. Ceramics International, 2019, 45(1): 1348-1355. DOI: 10.1016/j.ceramint.2018.07.242.
[3] ALIZADEH M, TEYMURI A. Structure, indentation and corrosion characterizations of high-silicon Ni-Si nano-composite coatings prepared by modified electrodeposition process [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(3): 608-616. DOI: 10.1016/S1003-6326(19)64970-8.
[4] ZHOU Hai-fei, DU Nan, ZHU Li-wei, SHANG Jian-ku, QIAN Zhou-hai, SHEN Xiao-ming. Characteristics investigation of Ni-diamond composite electrodeposition [J]. Electrochimica Acta, 2015, 151: 157-167. DOI: 10.1016/ j.electacta.2014.10.122.
[5] LI Bao-song, ZHANG Wei-wei, HUAN Yu-xing, DONG Jia. Synthesis and characterization of Ni-B/Al2O3 nanocomposite coating by electrodeposition using trimethylamine borane as boron precursor [J]. Surface and Coatings Technology, 2018, 337: 186-197. DOI: 10.1016/j.surfcoat.2018.01.018.
[6] JIANG Wei, SHEN Li-da, QIU Ming-bo, WANG Xin, FAN Ming-zhi, TIAN Zong-jun. Preparation of Ni-SiC composite coatings by magnetic field-enhanced jet electrodeposition [J]. Journal of Alloys and Compounds, 2018, 762: 115-124. DOI: 10.1016/j.jallcom.2018.05.097.
[7] JIANG Yan-cheng, XU Yun-hua, WANG Mei, YAO Han-chao. Effects of pulse plating parameters on the microstructure and properties of high frequency pulse electrodeposited Ni-Co/ZrO2 nanocomposite coatings [J]. Journal of Materials Science: Materials in Electronics, 2017, 28(1): 610-616. DOI: 10.1007/s10854-016-5565-3.
[8] GYAWALI G, KIM H S, TRIPATHI K, KIM T H, LEE S W. Fabrication and characterization of electrodeposited Ni-SiC-h/BN composite coatings [J]. Journal of Materials Science & Technology, 2014, 30(8): 796-802. DOI: 10.1016/j.jmst.2014. 05.008.
[9] LARI BAGHAL S M, AMADEH A, HEYDARZADEH S M. Investigation of mechanical properties and operative deformation mechanism in nano-crystalline Ni-Co/SiC electrodeposits [J]. Materials Science and Engineering A, 2012, 542: 104-112. DOI: 10.1016/j.msea.2012.02.039.
[10] CORNI I, CHATER R J, BOCCACCINI A R, RYAN M P. Electro co-deposition of Ni-Al2O3 composite coatings [J]. Journal of Materials Science, 2012, 47(14): 5361-5373. DOI: 10.1007/s10853-012-6381-7.
[11] EROGLU D, VILINSKA A, SOMASUNDARAN P, WEST A C. Effect of a cationic polymer, polyethyleneimine, on Ni/SiC Co-deposition [J]. Journal of the Electrochemical Society, 2012, 160(2): D35-D40. DOI: 10.1149/2.041302jes.
[12] LEKKA M, LANZUTTI A, CASAGRANDE A, de LEITENBURG C, BONORA P L, FEDRIZZI L. Room and high temperature wear behaviour of Ni matrix micro- and nano-SiC composite electrodeposits [J]. Surface and Coatings Technology, 2012, 206(17): 3658-3665. DOI: 10.1016/ j.surfcoat.2012.03.016.
[13] EROGLU D, VILINSKA A, SOMASUNDARAN P, WEST A C. Use of dispersants to enhance incorporation rate of nano-particles into electrodeposited films [J]. Electrochimica Acta, 2013, 113: 628-634. DOI: 10.1016/j.electacta.2013.09. 113.
[14] CUI Wei, WANG Ke, WANG Kai-yu, WANG P. Effects of jet rate on microstructure, microhardness, and wear behavior of jet electrodeposited Ni-SiC composites [J]. Ceramics International, 2018, 44(6): 7214-7220. DOI: 10.1016/ j.ceramint.2018.01.169.
[15] JIANG Wei, SHEN Li-da, XU Ming-yang, WANG Zhan-wen, TIAN Zong-jun. Mechanical properties and corrosion resistance of Ni-Co-SiC composite coatings by magnetic field-induced jet electrodeposition [J]. Journal of Alloys and Compounds, 2019, 791: 847-855. DOI: 10.1016/j.jallcom. 2019.03.391.
[16] MA E. Materials science: Watching the nanograins roll [J]. Science, 2004, 305(5684): 623-624. DOI: 10.1126/ science.1101589.
[17] ZARGHAMI V, GHORBANI M. Alteration of corrosion and nanomechanical properties of pulse electrodeposited Ni/SiC nanocomposite coatings [J]. Journal of Alloys and Compounds, 2014, 598: 236-242. DOI: 10.1016/j.jallcom. 2014.01.220.
[18] UNAL E, KARAHAN I H. Effects of ultrasonic agitation prior to deposition and additives in the bath on electrodeposited Ni-B/hBN composite coatings [J]. Journal of Alloys and Compounds, 2018, 763: 329-341. DOI: 10.1016/ j.jallcom.2018.05.312.
[19] GHAZIOF S, GAO Wei. The effect of pulse electroplating on Zn-Ni alloy and Zn-Ni-Al2O3 composite coatings [J]. Journal of Alloys and Compounds, 2015, 622: 918-924. DOI: 10.1016/j.jallcom.2014.11.025.
[20] ZHOU Y, XIE F Q, WU X Q, ZHAO W D, CHEN X. A novel plating apparatus for electrodeposition of Ni-SiC composite coatings using circulating-solution co-deposition technique [J]. Journal of Alloys and Compounds, 2017, 699: 366-377. DOI: 10.1016/j.jallcom.2016.12.331.
[21] LI Bao-song, ZHANG Wei-wei, LI Dan-dan, HUAN Yu-xing, DONG Jia. Microstructural, surface and electrochemical properties of a novel Ni-B/Ni-W-BN duplex composite coating by co-electrodeposition [J]. Applied Surface Science, 2018, 458: 305-318. DOI: 10.1016/j.apsusc.2018.07.100.
[22] UNAL E, KARAHAN I H. Production and characterization of electrodeposited Ni-B/hBN composite coatings [J]. Surface and Coatings Technology, 2018, 333: 125-137. DOI: 10.1016/j.surfcoat.2017.11.016.
[23] HE Teng, HE Yi, LI Han, SU Zu-bo, FAN Yi, HE Ze. Fabrication of Ni-W-B4C composite coatings and evaluation of its micro-hardness and corrosion resistance properties [J]. Ceramics International, 2018, 44(8): 9188-9193. DOI: 10.1016/j.ceramint.2018.02.128.
[24] LI Bao-song, ZHANG Wei-wei. Microstructural, surface and electrochemical properties of pulse electrodeposited Ni-W/Si3N4 nanocomposite coating [J]. Ceramics International, 2018, 44(16): 19907-19918. DOI: 10.1016/ j.ceramint.2018. 07.254.
[25] ALLAHYARZADEH M H, ALIOFKHAZRAEI M, ROUHAGHDAM A R S, TORABINEJAD V. Electrodeposition of Ni-W-Al2O3 nanocomposite coating with functionally graded microstructure [J]. Journal of Alloys and Compounds, 2016, 666: 217-226. DOI: 10.1016/j.jallcom. 2016.01.031.
[26] LI Han, HE Yi, HE Teng, FAN Yi, YANG Qiang-bin, ZHAN Ying-qing. The influence of pulse plating parameters on microstructure and properties of Ni-W-Si3N4 nanocomposite coatings [J]. Ceramics International, 2016, 42(16): 18380-18392. DOI: 10.1016/j.ceramint.2016.08.171.
[27] GUGLIELMI N. Kinetics of the deposition of inert particles from electrolytic baths [J]. Journal of the Electrochemical Society, 1972, 119(8): 1009. DOI: 10.1149/1.2404383.
[28] FRANSAER J, CELIS J P, ROOS J R. Analysis of the electrolytic codeposition of non-Brownian particles with metals [J]. Journal of the Electrochemical Society, 1992, 139(2): 413-425. DOI: 10.1149/1.2069233.
[29] HWANG B J, HWANG C S. Mechanism of codeposition of silicon carbide with electrolytic cobalt [J]. Journal of the Electrochemical Society, 1993, 140(4): 979-984. DOI: 10.1149/1.2056239.
[30] EROGLU D, WEST A C. Mathematical modeling of Ni/SiC Co-deposition in the presence of a cationic dispersant [J]. Journal of the Electrochemical Society, 2013, 160(9): D354-D360. DOI: 10.1149/2.052309jes.
(Edited by ZHENG Yu-tong)
中文导读
碳化硅粒度及表面活性剂对Ni-SiC复合镀层中碳化硅含量及分布均匀性的影响
摘要:本文在电沉积镍的镀液中添加SiC颗粒于铜基体上制备Ni-SiC复合镀层,研究了表面活性剂种类和增强颗粒粒度对Ni-SiC复合镀层中碳化硅含量及分布均匀性的影响。SiC颗粒的粒度影响形核过电位,40 nm SiC颗粒比500 nm SiC颗粒提供更多成核活性点,且对于镍电结晶形核促进作用更明显。表面活性SDS对40 nm SiC颗粒的分散效果较CTAB更好,二者对500 nm SiC颗粒分散效果相似。CTAB从某种程度上增加了SiC颗粒的含量,但对于SiC颗粒的分散效果不佳,SiC颗粒仍有大量团聚,且通过阳离子表面活性剂CTAB所获镀层表面比较暗淡、粗糙。
关键词:Ni-SiC复合镀层;循环伏安;计时电流;表面活性剂SDS;表面活性剂CTAB
Foundation item: Project(20180550242) supported by the Liaoning Science and Technology Plan, China
Received date: 2020-07-21; Accepted date: 2021-01-20
Corresponding author: KAN Hong-min, PhD, Professor; Tel: +86-24-62268751; E-mail: kanhongmin2002@163.com; ORCID: https://orcid.org/0000-0002-9816-9554
Abstract: The electrodeposition of nickel-silicon carbide coatings on a copper electrode was done by mixing SiC particles in the nickel electrodeposition solution. The influence of surfactants and silicon carbide particle size on uniformity and quantity of silicon carbide particles in nickel-silicon carbide composite coatings was investigated. It was found that particle size affects the nucleation overpotential, with 40 nm silicon carbide nanoparticles more effective in promoting nickel nucleation than 500 nm particles due to an increase in active nucleation sites. In terms of surfactants, anionic surfactant sodium dodecyl sulfate (SDS) produced better dispersion of 40 nm silicon carbide particles than cationic surfactant cetyltrimethyl ammonium bromide (CTAB), but little difference was found between the two when 500 nm silicon carbide particles were used. Thus, although the suspension of silicon carbide particles can be improved and their co-deposition can be promoted with a cationic surfactant CTAB, it is less effective than an anionic surfactant SDS in terms of surface finish.


