Trans. Nonferrous Met. Soc. China 31(2021) 3506-3529
Review of gold leaching in thiosulfate-based solutions
FengXIE1, Jun-nanCHEN1, JianWANG2, WeiWANG1
1. School of Metallurgy, Northeastern University, Shenyang 110004, China;
2. Faculty of Materials, Metallurgy and Chemistry, Jiangxi University of Science and Technology, Ganzhou 341000, China
Received 3September 2021; accepted 8November 2021
Abstract:
umerous non-cyanide leaching lixiviants have been developed, among which thiosulfate is considered the most promising alternative to cyanide due to its non-toxicity, low price, high leaching rate and excellent characteristics in dealing with carbonaceous and copper-bearing gold ores. The traditional copper-ammonia-thiosulfate system has been studied extensively.However, with many years of process development, there are still some problems and challenges with this gold leaching system. A series of studies using nickel-, cobalt- and ferric-based catalyst to substitute copper have been conducted with the purpose of reducing the consumption of thiosulfate. A variety of non-ammonia thiosulfate leaching systems including oxygen-thiosulfate, copper-thiosulfate, copper-EDA-thiosulfate, ferric-EDTA-thiosulfate, and ferric-oxalate-thiosulfate leaching systems have been also developed to eliminate the potential side-effect of ammonia. In this review, the basic theory and process development of some main gold leaching systems based on thiosulfate solutions were systematically summarized to illustrate the research status on thiosulfate leaching process. The potential effects of various additives such as organic ligands containing amino, carboxyl or hydroxy functional groups on gold thiosulfate leaching were described in detail. The potential opportunity and challenge for promoting the industrial development of thiosulfate-based gold leaching systems were also discussed.
Key words:
thiosulfate; gold; leaching; additives;
1 Introduction
Cyanide has been used as the predominant lixiviant for extracting gold from ores and concentrates over one hundred years [1,2]. However, cyanidation process is facing with doubts and challenges even from the day of its birth due to the high toxicity of cyanide which may seriously threaten the local environment and life safety [3-6]. With increasing concerns and awareness on the public security and environment protection, many researchers have devoted to developing novel green lixiviants for gold extraction to take the place of cyanidation [7-9]. More than 20 kinds of non-cyanide leaching lixiviants including thiourea, thiosulfate,halogen, thiocyanate, and polysulfide, etc. have been widely investigated in past few years[10,11]. And a key factor affecting ultimate commercial success is the stability of the lixiviant and the gold complex in solutions [10]. Thiourea labelled with a potential carcinogen is considered with a gloomy application prospect [12-14]. Halide leaching system is comparatively less attractive because of the strongcausticity of the lixiviant[15]. The limited availability of thiocyanate to the temperature and solution pH is a significant restriction in gold leaching and if thiocyanate has to be detoxified by oxidation to cyanate and sulfate, it would further increase the operating costs [16]. And a relatively high polysulfide concentration is usually required for high gold extraction [17].
Among these lixiviants, thiosulfate is considered as the most promising alternative to cyanide due to the relatively low cost, non-toxicity, fast leaching kinetics, and potentially excellent performance in dealing with carbonaceous and copper-bearing gold ores [7,18-20]. The earliest documented literature of gold leaching in thiosulfate solutions can be traced back to the published paper of WHITE in 1905, which recorded the slow dissolution behavior of gold in sodium thiosulfate solution [21]. The first technique for gold thiosulfate leaching appeared in the reported Von Petra process in 1880, in which thiosulfate solution was used for extracting gold and silver from ores after chlorination roasting [22]. The von Petra process had been also applied to silver-rich sulfide ores in South America for many years prior to World War II [23]. BEREZOWSKY et al [24] published a patent to extract precious metals (Au, and Ag) from copper-bearing sulfide gold ores in ammonium-thiosulfate solution. The use of ammonium-thiosulfate solutions to leach gold and silver from the oxidizing leaching residue of copper sulfide concentrates was reported by BEREZOWSKY and SEFTON in 1979 [25]. The authors claimed that the leaching rate of gold was higher than that by conventional cyanidation and halogen leaching method. Between 1981 and 1983, KERLEY [26,27] published two patents on using copper-ammonium-thiosulfate solutions to extract precious metals (Au, and Ag) from refractory gold ores, especially those manganese-bearing gold ores. It was believed that ammonia acted as a role of accelerating the dissolution of precious metals and stabilizing thiosulfate and the solution pH value. Unfortunately, the gold plant based on KERLEY’s patents failed to operate successfully in Mexico later. TERARAKELYAN et al [28] from Soviet Union reported that the presence of copper ions (Cu2+) can accelerate the dissolution of gold in thiosulfate solutions [28]. In 1994, Newmont Gold Corporation published a patent on the use of copper-ammonia-thiosulfate solution for heap leaching of pre-robbing carbonaceous gold ores [29]. Later, Newmont successfully established and operated a plant for direct heap leaching of low-grade carbonaceous sulfide gold ores using copper-ammonia-thiosulfate solutions in Carlin, Nevada, USA. A new technique combining the bio-oxidation pretreatment with heap leaching in thiosulfate solutions was developed later for treating gold ores associated with polymetallic minerals. In 2003, the plant processed 1.24 million tons of gold ores and produced 55790 ounces of gold in total [30].
In recent years, copper-ammonia-thiosulfate leaching system gets much more attention. Numerous works have been done on thiosulfate leaching system concerning thermodynamics, leaching kinetics, speciation distribution as well as the stability of thiosulfate and various gold recovery options [31-35]. However, the traditional copper-ammonia-thiosulfate system failed to be applied commercially in large scale due to the high consumption of thiosulfate and the sensitivity of ammonia to the leaching conditions [36,37]. In addition, usually a high concentration of ammonia is required to reduce the consumption or decomposition of thiosulfate due to the instability of thiosulfate in the leaching solution [38,39]. This will not only increase the operation cost, but also inevitably cause environmental pollution with the emission of ammonia-nitrogen wastewater and tailings. Thus, some non-ammonia thiosulfate-based leaching systems for gold extraction were developed [9,40,41]. Besides, some researchers conducted a series of studies using nickel-, cobalt- and ferric-based catalyst to substitute copper with the purpose to reduce the consumption of thiosulfate due to their weak reactivity to thiosulfate. The effects of various additives and different minerals on the thiosulfate leaching systems have also been investigated [42-45]. Some novel thiosulfate leaching systems for gold extraction from ores have thus been formed [46-51]. Based on the main formula of each leaching solutions, some representative thiosulfate-based leaching systems reported in literatures (in the absence of additives) are summarized in Table 1. The ammonia leaching system mainly consistsof copper-ammonia, nickel-ammonia, and cobalt-ammonia thiosulfate leaching systems. The non-ammonia leaching system includes oxygen-thiosulfate, copper-thiosulfate, copper-EDA-thiosulfate, ferric-EDTA-thiosulfate, ferric-oxalate-thiosulfate leaching system, etc.
In this review, the basic theory and process development of some main gold leaching systems based on thiosulfate solutions are summarized to illustrate the research status on thiosulfate leaching process. The potential effects of various ions, organic additives, and minerals on gold thiosulfate leaching are described in details. The existing problems and potential solutions for thiosulfate-based leaching systems are also discussed.
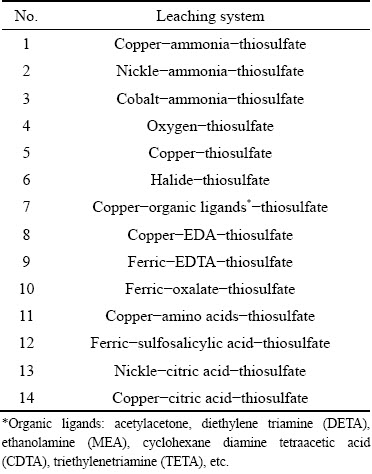
Table 1 Some representative thiosulfate-based leaching systems
2 Copper-ammonia-thiosulfatesystem
2.1 Basic theory
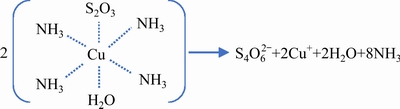
Thiosulfate ion (S2O32-), one of the oxygen-containing anion groups of sulfur, is a structural analogy of sulfate ions (SO42-) with one oxygen atom replaced by a sulfur atom. Figure 1 illustrates the structure of thiosulfate ion, which consistsof a non-regular tetrahedral S—SO3 structure with a central S and a peripheral S. The atomic size of S is larger than that of O, forming a weaker π bond. Besides, the S—S bond is longer than that of S—O bond [52]. The chemical properties of thiosulfate ions such as reducibility, strong complexing capacity, and the ability to form sulfides are predominated by the peripheral sulfide-like S atoms[53]. Thus, thiosulfate ions have a strong capacity of complexing with metal ions and generate strong σ bonds with metal ions while the peripheral S atoms serve as ligands [38]. Besides, thiosulfate ions can complex with many metal ions such as gold, silver, platinum, copper, iron, nickel, and cobalt [38].
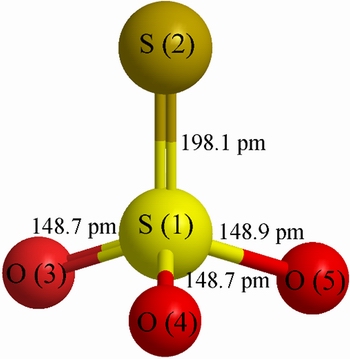
Fig. 1 Structure diagram of thiosulfate ion [52]
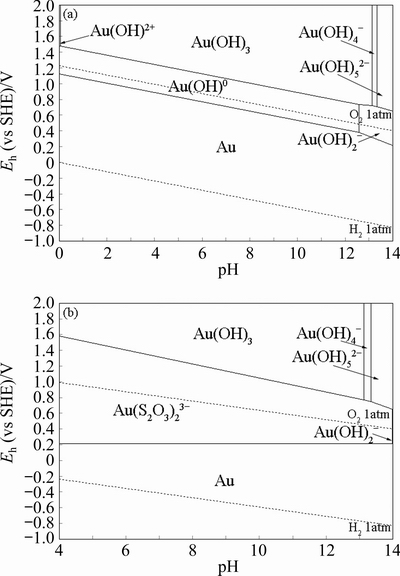
Fig. 2 Eh-pH diagrams for gold-water system (a) and gold-thiosulfate-water system(b) (298 K, 1 atm, [Au]T=0.001 mol/L, [S2O32-]T=0.1 mol/L) [57]
In simple thiosulfate solutions (such as sodium thiosulfate), Au(I) can react with thiosulfate ions to form different gold-thiosulfate complexes:
Au++S2O32-=Au(S2O3)-,β=1.0?1010.40 (1)
Au++2S2O32-=Au(S2O3)23-,β=1.0?1024.00 (2)
The Au(S2O3)23- complex is much more stable and usually the dominating species in thiosulfate solutions [54,55]. Extended X-ray analysis shows that Au(S2O3)23- complex ions are composed of coordination of one Au atom with two S atoms (S—Au—S) [52]. The dissociation energy analysis indicates that the Au—S bond belongs to a strong metal—S bond in aqueous solutions [56]. Figure 2 illustrates theEh-pH diagram of gold-water and gold-thiosulfate-water system. The gold leaching in alkaline oxygen-thiosulfate solution is demonstrated as
4Au+8S2O32-+O2+2H2O=4Au(S2O3)23-+4OH- (3)
Thermodynamically, the presence of thiosulfate ions can greatly reduce the oxidation potential of gold in aqueous solution, and the Au(S2O3)23-complex can stably exist in the stable region of water, which further demonstrates the feasibility of gold leaching with thiosulfate solutions [7]. CHANDRA and JEFFREY [58] considered that the dissolution behavior of gold in thiosulfate solution can be described as an electrochemical process. SENANAYAKE [59] carried out a detailed analysis on the anodic oxidation kinetics of gold in thiosulfate solution,indicating that the anodic oxidation of gold is conducted by the adsorption of MS2O3- ion pairs (M+ represents Na+, K+ and NH4+). The major reaction processes are shown as follows:
Adsorption: Au+MS2O3-=Au(S2O3)M-(ads) (4)
Anodic oxidation:
Au(S2O3)M-(ads)=Au(S2O3)M0(ads/aq)+e (5)
Desorption:
Au(S2O3)M0(ads/aq)+MS2O3-=M2Au(S2O3)2-(aq) (6)
The dissolution of gold in thiosulfate solutions exhibited an extremely slow kinetics due to the limitation of dissolved oxygen (see the leaching reaction shown in Eq. (3)). Thus, copper ions are introduced in the leaching solution to act as an oxidant. The experimental results indicated that Cu2+ can accelerate the dissolution rate of gold in thiosulfate solutions even at ambient temperature (25 °C) [46]. The electrochemical studies suggested that increasing the concentration of copper ions can promote the dissolution of gold in anodic area in thiosulfate solutions. When the potential maintains at 0.3 V, the dissolution rate of gold is much faster [60]. SENANAYAKE [61] considered the leaching mechanism of gold in copper-thiosulfate solution,as given in Eqs. (7)-(9):
Au+2S2O32-+Cu(OH)20=Au(S2O3)23-+Cu(OH)2- (7)
Au+S2O32-+Cu(OH)2(S2O3)2-=Au(S2O3)23-+Cu(OH)2- (8)
2Cu(OH)2-+0.5O2+H2O=2Cu(OH)20+2OH- (9)
Gold is catalytically oxidized to form Au(S2O3)23- in the solution by copper-bearing species (Cu(OH)20, and Cu(OH)2(S2O3)2-)and the dissolved oxygen. ZHANG and NICOL[60] believed that gold leaching in copper-thiosulfate solution was carried out through the intermediate product of [(S2O3)3Cu·O2]5- (Eqs. (10)-(12)):
Cu(S2O3)35-+O2=[(S2O3)3Cu·O2]5- (10)
[(S2O3)3Cu·O2]5-+4Au+8S2O32-+2H2O=4Au(S2O3)23-+Cu(S2O3)35-+4OH- (11)
[(S2O3)3Cu·O2]5-+4S2O32-+2H2O=2S4O62-+Cu(S2O3)35-+4OH- (12)
However, though the dissolution of gold in thiosulfate solutions is promoted with the presence of copper, the consumption of thiosulfate is also increased due to the catalytic effect of copper ions on thiosulfate decomposition [62,63]. To effectively stabilize the thiosulfate solutions, ammonia is thus introduced as a copper ligand in thiosulfate leaching system. Subsequently, a great number of studies to analyze the leaching mechanism in copper-ammonia-thiosulfate solutions have been reported[19,64,65]. It is believed that Au(I) can form complexes with both NH3 and S2O32- to form Au(NH3)2+ and Au(S2O3)- or Au(S2O3)23- in copper-ammonia-thiosulfate solutions. However, AYLMOREand MUIR [7] highlighted that gold mainly exists in the form of Au(S2O3)23- complex in copper-ammonia-thiosulfate leaching system. For copper ions (Cu+/Cu2+), they may form various complexes with ammonia, thiosulfate and hydroxyl in the copper-ammonia-thiosulfate solutions, including monovalent copper-ammonia complexes (Cu(NH3)+ and Cu(NH3)2+), divalent copper-ammonia complexes (Cu(NH3)2+, Cu(NH3)22+, Cu(NH3)32+, Cu(NH3)42+ and Cu(NH3)52+), monovalent copper-thiosulfate complexes (Cu(S2O3)-, Cu(S2O3)23- and Cu(S2O3)35-) and divalent copper hydroxide complexes (Cu(OH)+, Cu(OH)20, Cu(OH)3-and Cu(OH)42-), etc[19]. According to Eh-pH diagrams of copper-ammonia-thiosulfate-water system (Fig. 3) and the species distribution diagram of copper-complexes (Fig. 4), the Cu(S2O3)35- complex and Cu(NH3)42+ complex are the dominating phase of monovalent and divalent copper ions, respectively, in copper-ammonia-thiosulfate solutions under the specified conditions [57].
Therefore, the leaching mechanism of gold in copper-ammonia-thiosulfate solution can be described as
Au+5S2O32-+Cu(NH3)42+=Au(S2O3)23-+Cu(S2O3)35-+4NH3 (13)
2Cu(S2O3) 35-+8NH3+0.5O2+H2O=2Cu(NH3)42++2OH-+6S2O32- (14)
The mechanism of electrochemical catalytic leaching of gold in copper-ammonia-thiosulfate solution shown in Fig. 5 is generally accepted by researchers [7]. SENANAYAKE [19] presented an explicit dissolution behavior of gold in copper-ammonia-thiosulfate solutions and the reactions are shown as follows:
Adsorption:
Au+S2O32-+Cu(NH3)p(S2O3)0=Au(S2O3)2Cu(NH3)p2- (15)
Au+2S2O32-+Cu(NH3)m2+=Au(S2O3)2Cu(NH3)p2-+(m-p)NH3 (16)
Au+Cu(NH3)p(S2O3)22-=Au(S2O3)2Cu(NH3)p2- (17)
Redox reaction:
Au(S2O3)2Cu(NH3)p2-=Au(S2O3)23-+Cu(NH3)p+ (18)
Generally, in the copper-ammonia-thiosulfate leaching system, ammonia plays a role of stabilizing copper ions by forming Cu(NH3)42+ complex while copper ions serve as the oxidizing agent. In the cathodic area, Cu(NH3)42+ is reduced into Cu(S2O3)35- and newly-generated Cu(S2O3)35- complex can be oxidized back into Cu(NH3)42+ in the presence of dissolved oxygen. In the anodic area (hypothetical), Au+ ions react with S2O32- to form
Au(S2O3)23- complex. It is noted that the electrochemical potential of oxidizing gold to form Au(S2O3)23- ions in the presence of copper ions (0.10 mol/L) is only about 0 V (vs SHE) in ammonia-thiosulfate solutions [66].
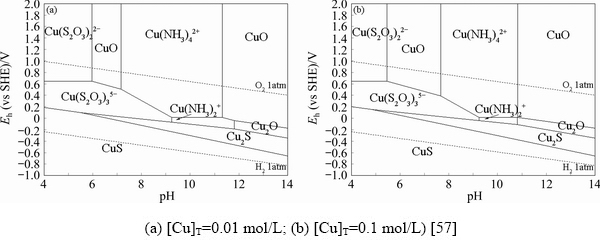
Fig. 3 Eh-pH diagrams for copper-ammonia-thiosulfate-water system (298 K, 1 atm,[NH3]T=1.0 mol/L, [S2O32-]T=0.1 mol/L)
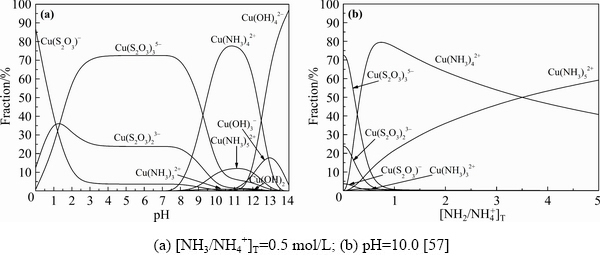
Fig. 4 Species distribution diagrams of copper complexes under different pH and different ammonia concentrations (298 K, 1 atm, [Cu]T=0.01 mol/L, [S2O32-]T=0.1 mol/L)
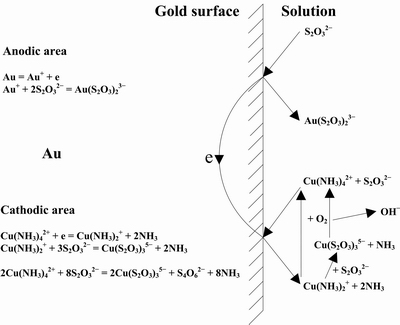
Fig. 5 Electrochemical catalytic mechanism of gold leaching in copper-ammonia-thiosulfate system [7]
According to the leaching mechanism described above, the species including copper, ammonia, thiosulfate, and oxygen all play critical roles in the leaching system. Some researchers claimed that, apart from the role as an oxidant, the copper ions in thiosulfate leaching system can also,
(1)Compete with gold at the adsorption sites on the surface of associated minerals, thus reducing the loss of gold caused by the adsorption on the mineral surface [67].
(2) Maintain a high oxidizing potential near the mineral surface in order to prevent gold from precipitating on the mineral surface and thus to reduce gold loss [67].
(3) Significantly increase the leaching rate of gold. However, excessive addition of copper ions will form the sulfide, oxide, and other passivating substances on the gold surface [40].
(4) Enhance the mass transfer of lixiviant to gold surface and improve the leaching kinetics of gold. Besides, Cu(I) may weaken or remove the passivation layer on gold surface [68], and the addition of Cu(II) can also eliminate part of the formed passivation layer [69].
For ammonia, it is noted that,
(1) Ammonia can hinder the dissolution of gangue minerals such as iron oxides, silicates and carbonates [7,70].
(2) Ammonia can hinder the formation of passivation film on gold surface or remove the passivation film, thus accelerating the dissolution rate of gold [69].
(3) As shown in Eqs. (19)and(20), ammonia can directly complex with gold to generate the Au(NH3)2+ complex, and then the ligand of NH3 is substituted by S2O32-. Thus, the more stable Au(S2O3)23- complex is formed. Besides, ammonia can prevent the adsorption of sulfur-containing substances on the surface of gold and accelerate the gold dissolution process [38,71]:
4Au+8NH3+2H2O+O2=4Au(NH3)2++4OH- (19)
Au(NH3)2++2S2O32-=Au(S2O3)23-+2NH3 (20)
(4) Ammonia can accelerate the dissolution of gold in thiosulfate solution by forming the intermediate Au(S2O3)(NH3)2-(ads) product in the absence of copper ions (Eqs. (21)-(23)) [20]:
Adsorption:
Au(s)+S2O32-+NH3=Au(S2O3)(NH3)2-(ads) (21)
Oxidization:
Au(S2O3)(NH3)2-(ads)=Au(S2O3)(NH3)-(ads/aq)+e (22)
Desorption:
Au(S2O3)(NH3)-(ads/aq)+S2O32-=Au(S2O3)23-(aq)+NH3 (23)
(5) Ammonia and thiosulfate serve as ligands to directly leach gold [72]:
4Au+4S2O32-+4NH3+2H2O+O2=4Au(NH3)(S2O3)-+4OH- (24)
(6) Ammonia serves as the stabilizer to complex copper ions and reduce the consumption of thiosulfate by lowering the reactivity of copper ions with thiosulfate [73,74].
Controlling thiosulfate and oxygen concentration is also critical to the goldleaching process in copper-ammonia-thiosulfate solutions [73,75]. A suitable thiosulfate concentration can accelerate the initial leaching rate of gold while too low concentration of thiosulfate will lead to the precipitation of gold or copper, which is not beneficial to the leaching process [61]. The dissolved oxygen can re-oxidize the Cu(I) complex to the Cu(II) complex, thus regenerating the oxidizingagent in the thiosulfate leaching system [75,76]. The gold leaching rate in thiosulfate solutions is actually limited by the reduction of oxygen due to the oxidation potential of gold is higher than the reduction potential of oxygen [68]. However, excessive oxygen will result in higher consumption of thiosulfate and may cause passivation of gold, leading to a slow gold leaching kinetics [75,77]. Oxygen can also accelerate the dissolution of sulfide minerals and promote the release of encapsuled gold in thiosulfate solutions for gold-bearing sulfide ores [77].
2.2 Effect of ions, additives, and minerals
2.2.1 Cations (Potassium, calcium, sodium, ammonium, cesium and thallium)
It was reported the dissolution rate of gold in different thiosulfate solutions follows the order K+>NH4+ >Na+ and follows the order Ca2+>NH4+>Na+ when ammonia is present [58]. ZHANG et al [68] reported that the dissolution rate of gold in calcium thiosulfate solution (0.1 mol/L) or copper (2 mmol/L)-calcium thiosulfate (0.1 mol/L) solution was faster than that in same concentration of sodium thiosulfate and copper-sodium thiosulfate solutions [68]. However, SITANDO et al [40] believed that the dissolution rate of gold in calcium thiosulfate solution (0.1 mol/L) was faster than that of sodium thiosulfate solution (0.1 mol/L) in the absence of copper ions, while in the presence of 2 mmol/L copper ions, the dissolution rate of gold in both thiosulfate solutions was similar. SENANAYAKE [78] proposed that calcium ions can promote the dissolution of gold to form Au(S2O3)23- complex by stabilizing the intermediate product of Au2(S2O3)2Ca. Based on the oxidation mechanism of gold in thiosulfate solution proposed by SENANAYAKE [59], the cations such as Na+, K+ and NH4+ can also stabilize the intermediate oxidation products of gold. The addition of cesium can also accelerate the dissolution rate of gold in ammonium thiosulfate solution [58]. BEK and SHEVTSOVA [79] conducted an electrochemical study and reported that the addition of TlNO3 (5×10-6-1×10-4 mol/L) could enhance the exchange current density and electron transfer coefficient, thus accelerating the anodic dissolution of gold in sodium thiosulfate solution. In addition, the results confirmed that thallium ions can catalyze the dissolution of gold by adsorbing on the surface of gold and forming a catalytic activation layer [79]. MELASHVILI et al [80] summarized that the addition of thallium (Tl2SO4) can improve the exchange current density of the gold electrode oxidation reaction in magnesium thiosulfate solution. The catalytic effect of thallium on gold oxidation reaction can be realized by the diffusion of Tl+ to the surface of gold electrode and transferring charges to convert to Tl3+. However, GUDKOV et al [81] argued that Tl+ can catalyze the conversion of Au(OH)3 to Au(S2O3)23- owning to its strong reducibility, high stability and specific adsorption site on the surface of gold. The main catalytic reaction is shown in Eqs. (25)-(27):
2Au(OH)3+2Tl++4S2O32-=2Au(S2O3)23-+2Tl3++6OH- (25)
2Tl3++2S2-+2OH-+O2=2Tl++S2O32-+H2O (26)
2Au(OH)3+3S2O32-+2S2-+O2=2Au(S2O3)23-+4OH-+H2O (27)
2.2.2 Anions
(1)Carbonate
SENANAYAKE [61] found that the presence of carbonate ions would cause a significant decrease of gold leaching rate during thiosulfate leaching process. The dominant form of copper ions will convert to Cu(CO3)22- complex when adding carbonate ions in alkaline copper-thiosulfate solutions, which may weaken the reaction of Eq. (28), thus reducing the decomposition of thiosulfate to tetrathionate (S4O62-) [61]. However, the addition of carbonate ions can also promote the reaction of Eqs. (29) and (30), and finally increase the consumption of thiosulfate and promote the accumulation of trithionate (S3O62-) and sulfate in solution. Meanwhile, gold may precipitate due to insufficient thiosulfate ions [61]. Some studies suggested that carbonate ions (CO32-) will react with calcium ions (Ca2+) and then form the insoluble calcium carbonate precipitation coated on the gold surface during the leaching process, leading to a significant decline of the gold leaching rate [82]:
2Cu(S2O3)22-=2Cu(S2O3)-+S4O62- (28)
S2O32-+O2=S2O52- (29)
2S2O52-=S3O62-+SO42- (30)
(2)Sulfite
It has been confirmed that the addition of sulfite ions (SO32-) can minimize the oxidative consumption of thiosulfate ions to the greatest extent during the leaching process, and significantly reduce the consumption and decomposition of thiosulfate [37,83]. The presence of sulfite ions can also hinder the formation of copper-bearing sulfides (CuS, and Cu2S), and prevent sulfur or sulfide from precipitating on the gold surface, thus improving the stability of leaching solution and increasing the gold leaching rate [38,84-86]. GUDKOV et al [81] suggested that the addition of sodium sulfite could stabilize the leaching solution and accelerate the dissolution of gold and silver. However, sulfite ions will also cause a rapid reduction of copper ions which will dramatically decrease with an excessive increase of sulfite in the leaching solutions, as given in Eqs. (31) and (32), leading to a sharp decline of the gold leaching rate [87]. BREUER and JEFFREY [88] reported that the presence of SO42- can hinder the complexation of SO32- on the axial ligand site of copper ions, thus lowering the reactivity of Cu2+ with SO32- and eventually decreasing the reduction of copper ions by sulfite ions.
2Cu2++SO32-+2OH-=2Cu++SO42-+H2O (31)
2Cu2++2SO32-=2Cu++S2O62- (32)
In addition, in the absence of oxygen, the addition of sulfite ions can not only effectively reduce the influence of trithionate and tetrathionate on the adsorption and recovery of Au(S2O3)23- complex by anion exchange resin, but also slowly generate thiosulfate ions during the leaching process, thus reducing the consumption of thiosulfate, as given in Eqs.(33)-(36) [38,89]. The addition of sulfite ions into the eluent can reduce the affinity of resin to Au(S2O3)23- complex by forming the Au(S2O3)(SO3)3- complex, which is beneficial for the elution process of gold [90]. However, the presence of sulfite ions can also react with the Au(S2O3)23- complex, as given in Eqs. (37)-(39) when concentration of thiosulfate in the leaching solution is extremely low, resulting in the decline of gold content in the leaching solution [61].
S4O62-+SO32-=S3O62-+S2O32- (33)
S3O62-+SO32-=S2O62-+S2O32- (34)
S2O62-+2OH-=SO42-+SO32-+H2O (35)
S4O62-+SO32-+2OH-=SO42-+2S2O32-+H2O (36)
2Au(S2O3)23-+SO32-=2Au+3S2O32-+S3O62- (37)
2Au(S2O3)23-+2OH-=Au2S+3S2O32-+SO42-+H2O (38)
2Au(S2O3)23-+SO32-+2OH-=2Au+4S2O32-+SO42-+H2O (39)
(3)Sulfate
JEFFREY et al [75] suggested that thiosulfate ion firstly complexes with copper-ammonia complex at the axial ligand site, and then reacts with copper ions to generate tetrathionate, as given in Eqs. (40)and(41) [75]. The presence of SO42- can competitively adsorb with S2O32- on the cupric axial ligand site, and replace S2O32- at the axial ligand site before Cu2+ reacts with S2O32-, thus lowering the reactivity of Cu2+ with S2O32-, and decreasing the reduction of copper ions and the oxidation consumption of thiosulfate [88].
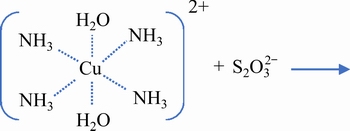
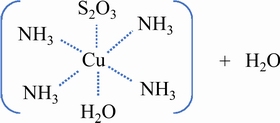 (40)
(40)
 (41)
(41)
(4)Trithionate/tetrathionate
Trithionate/tetrathionate is the decomposition product of thiosulfate from its reduction or oxidation, as given in Eqs. (42)-(46). BREUER and JEFFREY [88] suggested that the addition of a trace amount of trithionate/tetrathionate(10 mmol/L) can significantly accelerate the reduction of copper ions in the copper-ammonia-thiosulfate solution. And trithionate/tetrathionate ions can competitively adsorb with Au(S2O3)23- complex on the resin in the recycling process of gold using anion exchange resin or during the resin elution process. Thus, some Au(S2O3)23- remains in the leaching solution and cannot be effectively recovered [91,92]. In addition, polythionate ions such as trithionate/tetrathionate can be adsorbed on the gold surface during the leaching process, passivating the gold surface and hindering the dissolution of gold [93].
2Cu2++2S2O32-=S4O62-+2Cu+ (42)
2S4O62-+3OH-=S3O62-+2.5S2O32-+1.5H2O (43)
2S2O32-+0.5O2+H2O=S4O62-+2OH- (44)
3S2O32-+2O2+H2O=2S3O62-+2OH- (45)
S2O32-+2O2+2OH-=2SO42-+H2O (46)
(5)Pyrosulfite
Pyrosulfite (S2O52-) is the intermediate product of oxidative decomposition of thiosulfate in thiosulfate solutions [94]. CHU et al [95] found that the gold dissolution rate decreases with the increase of pyrosulfite (0-25 mmol/L), and the presence of pyrosulfite (10 mmol/L) can accelerate the reduction of copper ions in copper-ammonia-thiosulfate solution. It was reported that the gold leaching rate can be almost negligible when the concentration of pyrosulfite was 25 mmol/L [75].
(6)Phosphate and chloride
The addition of phosphate and chloride can significantly weaken the redox reaction between copper ions and thiosulfate in copper-ammonia-thiosulfate solution [88]. It has been proven that the presence of phosphate can eliminate the adverse effect of arsenopyrite during the leaching process in copper-ammonia-thiosulfate solution, thus reducing the consumption of thiosulfate and promoting the dissolution of gold [96]. As given in Eqs. (47) and (48), chloride ions can promote the dissolution rate of gold in ammonia-thiosulfate solutions [78,97]. The leaching rate of gold increased from 39.3% to 98.0% with the addition of 1.0 mol/L sodium chloride from a gold-bearing oxidized ore. Furthermore, in the process of leaching calcined gold concentrates with copper-ammonia-thiosulfate solution, the addition of chloride ion (0-0.6 mol/L) can slightly increase the gold leaching rate and reduce the consumption of thiosulfate [47].
2Au+4Cl-+0.5O2+H2O=2AuCl2-+2OH- (47)
AuCl2-+2S2O32-=Au(S2O3)23-+2Cl- (48)
2.2.3Organic additives
(1)Humic acid
XU et al [47] found that the addition of humic acid (HA) in copper-ammonia-thiosulfate solution could effectively improve the gold leaching rate and reduce the consumption of thiosulfate, better than the effect of chloride, sulfite, carboxymethyl cellulose, carboxymethyl starch, etc in the process of leaching roasted residue from a refractory gold ore. The catalytic mechanism of HA is probably because:HA can weaken the redox reaction between copper ions and thiosulfate by complexing at the cupric axial ligand site;HA can eliminate the affinity of associated minerals such as sulfide minerals and iron-bearing minerals to thiosulfate, thus weakening the catalytic effect of associated minerals on the decomposition of thiosulfate;HA can prevent the passivation substance from precipitating on the surface of gold or gold-bearing materials owning to the electrostatic repulsion by non-selective adsorption on the mineral surface;HA can release part of the encapsuled gold from gold ores [47,48,98].
Moreover, the addition of HA can also improve the stability of in-situ regenerated thiosulfate ions during the oxygen pressure-thiosulfate leaching process [98].
(2)Amino acid
Amino acids have the capability of complexing with heavy metals such as copper and play a role of stabilizing thiosulfate during the gold leaching process in thiosulfate solutions [99]. FENG and DEVENTER [100] summarized the effect of four common amino acids (L-valine, glycine, DL-α-alanine and L-histidine) during the process of leaching gold-bearing pyrite concentrates in copper-ammonia-thiosulfate solution [100]. The results indicated that amino acids have a stronger complexing capacity with copper ions than the effect of ammonia and the copper-amino acid complexes are more stable in the leaching solution. Thus, amino acids can reduce the consumption of thiosulfate by weakening the reactivity of copper-amino acid complexes to thiosulfate ions. In addition, the presence of amino acids can greatly improve the gold leaching rate, while the consumption of thiosulfate gradually decreases with increasing concentration of amino acids. Among four common amino acids, L-histidine had the best performance during the gold leaching process in thiosulfate solutions [100].
(3)EDTA, NTA and TEA
FENG and DEVENTER [101] reported that the addition of a small amount of EDTA (ethylenediaminetetraacetic acid) in the copper-ammonia-thiosulfate leaching system can reduce the redox potential of Cu(II)/Cu(I), thus decreasing the potential of the mixed solution and the consumption of thiosulfate [101]. The addition of EDTA can promote the leaching rate of gold by inhibiting the formation of passivation layer of sulfur or copper sulfide on the gold surface, reducing the interference of heavy metal ions and improving the stability of copper and thiosulfate in the leaching solution. Besides, the complexing capacity of EDTA with copper ions is stronger than that of ammonia. Thus, an excessive addition of EDTA will accelerate Cu(NH3)42+ complex to convert to copper-EDTA complexes, resulting in a lower gold leaching rate [101,102]. Furthermore, the addition of EDTA/NTA (nitrilotriacetic acid) proved the ability of improving the gold leaching rate and reducing the consumption of thiosulfate in leaching a copper-bearing sulfide gold ore with copper-ammonia-thiosulfate solution [85]. ZHAO et al [103] introduced TEA (triethanolamine) as an additive during the gold leaching process in copper-ammonia-thiosulfate solution. The results indicated that the addition of a small amount of TEA could improve the gold dissolution rate and reduce the consumption of thiosulfate. Furthermore, the presence of TEA can increase the redox potential of Cu(II)/Cu(I) and the potential of mixed solution. When the molar ratio of Cu/TEA was 1:1, the dissolution rate of gold can be increased by 50% approximately and the consumption of thiosulfate can be decreased by about 10% compared with that in the absence of TEA [103].
(4)Carboxymethyl cellulose
Carboxymethyl cellulose (CMC) contains a great number of carboxymethyl and carboxyl groups, which can complex with copper ions at the axial sites. Thus, CMC plays a role of stabilizing thiosulfate ions by reducing the reactivity between copper ions and thiosulfate ions [104]. The addition of CMC can eliminate the adverse effect of arsenopyrite during the gold leaching process in copper-ammonia-thiosulfate solution, reducing the consumption of thiosulfate and promoting the dissolution of gold at the same time [96]. In the process of leaching sulfide ores with copper-ammonia-thiosulfate solution, the addition of CMC can improve the leaching rate of gold and silver by stabilizing thiosulfate, dispersing particles of associated minerals and reducing the passivation substance in the lixiviant[104]. In the process of leaching a refractory calcined gold concentrate, XU et al [47] also reported that the presence of CMC/CMS (carboxymethyl starch) in copper-ammonia-thiosulfate solution can improve the gold leaching rate and reduce the consumption of thiosulfate, and the effect increases with an increase of concentration of the additives (0-30 mg/L).
2.2.4 Minerals (Sulfide minerals and hematite)
Sulfide minerals such as arsenopyrite can accelerate the decomposition of thiosulfate and hinder the dissolution of gold in thiosulfate leaching process [48,96]. The effect of sulfide minerals on the decomposition of thiosulfate follows the order: pyrite > arsenopyrite > chalcopyrite > galena > sphalerite. The order of the dissolution rate of gold in the presence of different sulfide minerals is as follows: sphalerite > arsenopyrite > pyrite ≈ galena > chalcopyrite [48]. However, the electro- chemical studies indicated that the presence of sulfide minerals can improve the leaching rate of gold in thiosulfate solution, and the reduction efficiency of oxygen turns higher due to the lower overpotential on the surface of sulfide minerals. Therefore, the electrons lost from gold during the leaching process are more easily gained by oxygen atom by transferring to the surface of sulfide minerals. Minerals such as pyrite, chalcopyrite, chalcocite and pyrrhotite all have a similar effect [40,68]. The presence of hematite can significantly reduce the dissolution of gold in copper-ammonia-thiosulfate solution, and the gold leaching rate gradually decreases with an increase of hematite content (0-12 g/L) [105]. It was reported that in the process of leaching sulfide ores containing 4.3 g/t gold with copper-ammonia-thiosulfate solution, the presence of hematite can reduce the gold leaching rate while the rate decreases with increasing the concentration of hematite (0-16 g/L) [105]. The study also shows that the addition of natural surfactants (Gempolym M47) in thiosulfate leaching process could weaken the adverse effect of hematite by hindering hematite from precipitating on the gold surface and preventing the dispersion of pulp. Meanwhile, the stability of thiosulfate can be enhanced by weakening the reactivity between copper ions and thiosulfate [105].
3 Nickel/cobalt-ammonia-thiosulfate systems
3.1 Nickel-ammonia-thiosulfate
In order to solve the problem of high consumption of thiosulfate caused by the catalytic oxidation by copper ions, ARIMA et al [106] carried out a study using nickel-based catalysis instead of copper-based catalyst in ammonium thiosulfate solutions. The leaching mechanism of nickel-ammonia-thiosulfate solution is shown in Fig. 6, and the overall reaction can be expressed as
2Au+Ni3O4+18NH3+4H2O+4S2O32-=2Au(S2O3)23-+3Ni(NH)62++8OH- (49)
3Ni(NH)62++6OH-+1/2O2=Ni3O4+18NH3+3H2O (50)
A gold extraction rate of 95% can be obtained after 24 h leaching in nickel-ammonia-thiosulfate system from a 100 wt.%75 μm of silicate gold ore containing 16 g/t Au. The consumption of ammonium thiosulfate was merely 1.2 kg/t which is less than the consumption of 1.5 kg/t sodium cyanide in a standard cyanidation process. The optimal leaching conditions were confirmed to be1×10-4 mol/L NiSO4, 0.05 mol/L (NH4)2S2O3, 0.5 mol/L NH3·H2O and pH value of 9.5. Compared to the traditional copper-based catalysis system with the consumption of thiosulfate of 3-21 kg/t (10-4-10-3 mol/L Cu2+) for silicate gold ores, the nickel-based catalysis can significantly reduce the consumption of thiosulfate to 1-5 kg/t (1×10-4-5×10-3 mol/LNi2+). Moreover, the consumption of thiosulfate catalyzed by nickel (1×10-4 mol/L) can be reduced by 1-5 kg/t compared to copper-based catalysis for sulfide gold minerals containing copper (0-0.3 wt.%) and iron (4.3-28.2 wt.%) [106]. In addition, ARIMA et al [107] demonstrated the feasibility of usingstrong basic anion exchange resin for the adsorption and recovery of gold from nickel-ammonia-thiosulfate leachate. The results show that the adsorption capacity of 1 g/L Dow 21K strong basic anion exchange resin for gold in nickel-ammonia-thiosulfate leaching solution is 95 mg/g. The elution efficiency order of different eluents for gold-bearing resin is OH- Cl-<<>3-<<>-<-<<>4-. Besides, the resins containing 5.0 mg/g gold can be completely desorbed by 2.5 mol/L ClO4-solution[107].
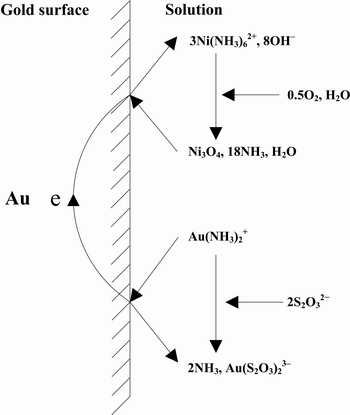
Fig. 6 Schematic diagram of gold leaching in nickel-ammonia-thiosulfate system [106]
XU et al [108] proposed a multi-stage leaching process to extract gold and silver from the complex polymetallic sulfide ores containing high concentrations of iron, arsenic and antimony using nickel-ammonia-thiosulfate solutions. The technique mainly consistsof three steps: Oxygen pressure-sulfuric acid leaching, silicofluoric acid leaching, and nickel-ammonia-thiosulfate leaching. Test results show that 98.2% Cu, 95.8% Zn, 95.7% Pb, 91.6% Fe, 71.1% As and 48.8% Sb can be leached from the sulfide ores through these leaching processes. And the leaching rates of gold and silver were achieved at 94.7% and 96.8%, respectively,in the nickel-ammonia-thiosulfate leaching solutions [108]. The competitive adsorption behavior between nickel and gold almost does not occur when the anion exchange resin is used to recover the nickel-ammonia-thiosulfate leaching solutions due to the extremely low affinity of the anion exchange resin to nickel-ammonia complexes [109]. XU et al [109] further proposed the process of nickel-ammonia-thiosulfate leaching combined with anion exchange resin (IRA-400) recovery to extract gold from calcined gold concentrates. Compared with the copper-ammonia catalysis system, the consumption of thiosulfate can be reduced from 43.6 kg/t to 19.5 kg/t in nickel-ammonia catalysis system, and further reduced to 13.5 kg/t after five times circulation of leaching solutions. In addition, the results indicated that the leaching of gold with nickel-ammonia-thiosulfate solutions was controlled by the diffusion process, and the catalytic mechanism of gold leaching is shown in Fig. 7. Ammonia can catalyze the anodic dissolution of gold to form Au(NH3)2+ and then the ligand of NH3 is substituted by S2O32-. Ni3O4 catalyzes the cathodic reduction of oxygen.
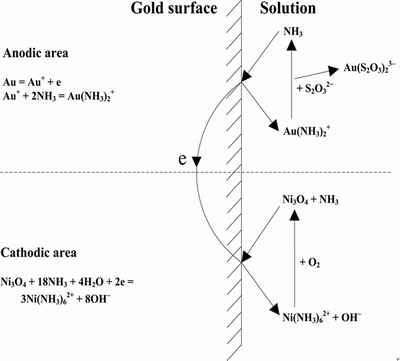
Fig. 7 Mechanism of gold leaching in thiosulfate solutions with nickel-ammonia catalysis [109]
3.2 Cobalt-ammonia-thiosulfate
LIU et al [110] conducted a thermodynamic study on the cobalt-ammonia-thiosulfate system and considered the gold leaching mechanism in cobalt-ammonia-thiosulfate solution can be described in Fig. 8. In the anodic area, ammonia ions first move to the gold surface and form complexes with gold to generate an intermediate product of Au(S2O3)(NH3)- complex. Then, NH3 is substituted by S2O32-, and thus the more stable Au(S2O3)23- complex is formed. In the cathodic area, Co(NH3)63+ is reduced to Co(NH3)x2+ (x=1-5), and the newly-generated Co(NH3)x2+ complex can be oxidized back to Co(NH3)63+ complex with the oxidization catalytic effect of dissolved oxygen. The detailed process is illustrated in the following reactions given in Eqs. (51)-(56). And the overall reaction is shown in Eq. (3) as the combination of anodic reactions and cathodic reactions [110].
Au+S2O32-+NH3=Au(S2O3)(NH3)2-(ads) (51)
Au(S2O3)(NH3)2-(ads)= Au(S2O3)(NH3)-+e (52)
Au(S2O3)(NH3)-+S2O32-=Au(S2O3)23-+NH3 (53)
Au+S2O32-+NH3+Co(NH3)63+=Au(S2O3)(NH3)-+Co(NH3)x2++(6-x)(NH3) (x=1-6) (54)
Au+2S2O32-+Co(NH3)63+=Au(S2O3)23-+Co(NH3)x2++(6-x)(NH3) (x=1-6) (55)
Co(NH3)x2++(6-x)NH3+1/4O2+1/2H2O=Co(NH3)63++OH- (x=1-6) (56)
Due to the lower reduction potential of Co3+/Co2+ than that of Cu2+/Cu+, and a more stable octahedral structure of Co(NH3)63+ complex, the consumption of thiosulfate in cobalt-ammonia-thiosulfate system is lower than that in copper-ammonia-thiosulfate system [110]. It was reported that the leaching process was controlled by the diffusion of solid product layer in the process of leaching calcined gold concentrates with cobalt-ammonia-thiosulfate solutions [46]. Compared with the copper-ammonia catalysis system, the cobalt-ammonia-thiosulfate leaching system has several advantages such as the lower consumption of thiosulfate and higher catalytic activity in gold leaching, and the gold-bearing resin can be eluted just by one-step elution owing to the weak affinity of strong basic anion exchange resin to cobalt-ammonia complexes [46,50,111].
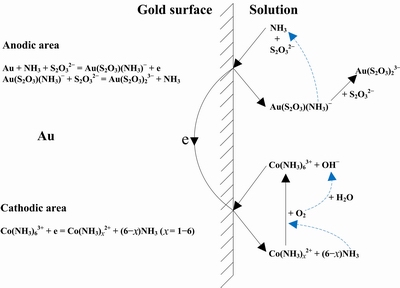
Fig. 8 Mechanism of gold leaching in thiosulfate solutions with cobalt-ammonia catalysis [110]
4 Non-ammonia-based leaching systems
4.1 Copper-citrate-thiosulfate system
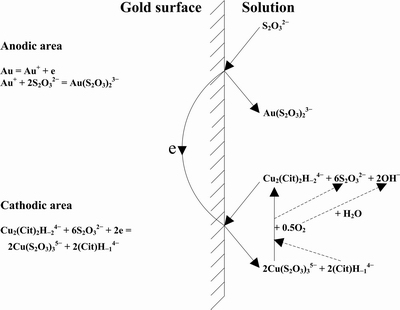
Fig. 9 Mechanism for gold leaching in copper-citrate-thiosulfate solutions [9]
The stabilizers of citric acid and citrate in copper-thiosulfate leaching system were introduced in Refs. [9,62,112,113]. The Eh-pH diagram and species distribution of the copper-citrate-thiosulfate leaching system were established by thermodynamic calculation. The gold leaching mechanism was determined based on the experimentalanalysis (Fig. 9). In the anodic area, aurous ions (Au+) react with thiosulfate ions to form Au(S2O3)23- complex and stably exist in the solution. In the meantime, 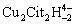 complex acquires electrons at the cathodic area of gold surface and is reduced to the Cu(S2O3) 35- complex (Eqs. (57)and (58)). The dissolved oxygen in the solution acts as the oxidant to re-oxidize Cu(S2O3)35-to
complex acquires electrons at the cathodic area of gold surface and is reduced to the Cu(S2O3) 35- complex (Eqs. (57)and (58)). The dissolved oxygen in the solution acts as the oxidant to re-oxidize Cu(S2O3)35-to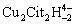 complex for further gold leaching. The gold in copper-citrate-thiosulfate system is catalyzed by the redox couple of
complex for further gold leaching. The gold in copper-citrate-thiosulfate system is catalyzed by the redox couple of 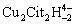 /Cu(S2O3)35-[9].
/Cu(S2O3)35-[9].
2Au+10S2O32-+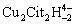 =2Au(S2O3)23-+2Cu(S2O3)35-+
=2Au(S2O3)23-+2Cu(S2O3)35-+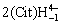 (57)
(57)
2Cu(S2O3)35-+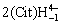 +0.5O2+H2O=
+0.5O2+H2O=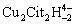 +6S2O32-+2OH- (58)
+6S2O32-+2OH- (58)
The experimental results show that the gold extraction from a free milling ore in this leaching system can achieve at 96.1% after 9 h leaching which is much faster than that under typical cyanidation conditions. Increasing temperature(30-90 °C) and thiosulfate concentration (0.01-0.20 mol/L) can increase the gold extraction rate. A modest increase of copper and citrate concentration can facilitate gold dissolution, but excessive addition of copper and citrate may cause a slight decrease of the gold leaching rate. Based on kinetics analysis, the leaching process from the free milling gold ore may be controlled by the product layer diffusion, giving an activation energy of 34.48 kJ/mol[62].
4.2 Copper-EDA-thiosulfate system
4.2.1 Basic theory
It has been reported that organic compounds containing amino functional groups including polyamines, amino acids, and ethanol amines, etc can form complexes with copper ions [90,114]. Among above amino organic ligands, ethylene- diamine (EDA), diethylenediamine (DETA), triethylenediamine (TETA), ethanolamine (MEA) and glycine can effectively complex with copper ions [114,115]. In 2011, Metal Asia International Ltd. publicly published a patent on using copper-EDA-thiosulfate leaching and ion exchange resin to extract gold from gold-bearing ores [116]. Later, a research team from Kunming University of Science and Technology in China conducted a series of studies on the copper-EDA-thiosulfate leaching system [117]. For a limonite-bearing gold ore, as high as 94.3% of gold extraction was achieved and the consumption of thiosulfate merely achieved at 1.12 kg/t under the conditions of 0.1 mol/L Na2S2O3, 0.06 mol/L EDA, 0.005 mol/L CuSO4, and 1.5 kg/t CTAB (cetyltrimethyl ammonium bromide). It was believed that the gold leaching mechanism in copper-ethylenediamine-thiosulfate solution can be described as Eqs. (59)-(63) [117,118]:
Cu2++2en=Cu(en)22+ (59)
Au+Cu(en)22+=Au++Cu(en)2+ (60)
3Au++Cu(en)2+=Cu++2Au+Au(en)23+ (61)
Au(en)23++2S2O32-+2e=Au(S2O3)23-+2en (62)
Cu(en)22++Au+4S2O32-=Cu(S2O3)23-+Au(S2O3)23-+2en (63)
When copper powder instead of CuSO4 was used to form copper-ethylenediamine-thiosulfate leaching solution, a high gold recovery of 95.38% was achieved under the experimental conditions of 0.2 mol/L thiosulfate, pH 11, EDA/Cu2+ molar ratio of 6:1, and Cu/Au+ mass ratio of 150:1, and 40 °C [119]. The reduction of Au(S2O3)23-complex on the surface of copper powder follows the first-order kinetics model with an apparent activation energy of 39.82 kJ/mol. The processes of roasting pretreatment and copper-ethylenediamine-thiosulfate leaching were combined to extract gold from a refractory gold ores containing high arsenic (1.67 wt.%) and high sulfur (9.51 wt.%) contents [120]. The gold extraction reached 80.3% in the copper-ethylenediamine-thiosulfate leaching system, compared with 63.0% for the traditional copper-ammonia-thiosulfate leaching system. And the thiosulfate consumption decreased from 11.3 to 4.14 kg/t [120].
4.2.2 Effect of additives
The addition of Co2+(8-32 mg/L) and Ni2+(5-15 mg/L) in copper-EDA-thiosulfate leaching system can not only improve the redox potential of the mixed solution, but also reduce the formation of passivation film of sulfur on the gold surface, and finally accelerate the dissolution rate of gold [121]. An optimal dissolution rate of gold can be achieved under the experimental conditions that the concentrations of Co2+ and Ni2+are 16 mg/L and 10 mg/L, respectively. Noteworthily, the addition of lead (PbNO3) can promote the gold dissolution rate in the process of leaching sulfide gold ores with thiosulfate solutions [114]. However, the presence of lead ions can also significantly increase the consumption of thiosulfate, and the emission of lead-bearing wastewater or tailings will seriously pollute the ecological environment.
Moreover, CTAB (cetyltrimethyl ammonium bromide) can readily dissolve to water and hydrolyze to CTA+ and Br-, as given in Eq. (64), while CTA+ can adsorb the negatively charged clay in the pulp. In the process of leaching limonite-bearing gold ores with copper-EDA-thiosulfate solution, the addition of CTAB could flocculate the clay and fine particles, thus eliminating the adsorption of clay on the gold surface and reducing the absorption of fine particles on the reagent surface, eventually improving the gold leaching rate and decreasing the consumption of thiosulfate [117]. Furthermore, CTAB also plays a role of stabilizing the Au(S2O3)23- complex by forming [CTA+]3- [Au(S2O3)23-]·nH2O and [CTA+][AuBr2-]·nH2O ion pairs, as given in Eqs. (65)and(66) [117]:
CTAB=CTA++Br- (64)
Au(S2O3)23-+3CTA++nH2O=[CTA+]3[Au(S2O3)23-]·nH2O (65)
[CTA+]3[Au(S2O3)23-]·nH2O+2Br-=[CTA+][AuBr2-]·nH2O+2CTA++2S2O32- (66)
4.3 Ferric-EDTA/oxalate-thiosulfate system
CHANDRA and JEFFREY [51] proposed ferric-oxalate-thiosulfate leaching system and conducted a series of studies on gold leaching. The results indicated that the dissolution of gold can keep stable above 24 h when the molar ratio of oxalic acid to ferric is 3:1. HEATH et al [122] demonstrated that ferric-EDTA and ferric-oxalate complexes are effective oxidizing agents for gold leaching in thiosulfate solution no matter in anaerobic or aerobic conditions. It is suggestedthat this process can be used for such as in-situ or underground leaching where it is extremely difficult to control oxygen concentration which makes the traditional copper-ammonia-thiosulfate solution unsuitable for gold leaching [122]. The ferric-EDTA/oxalate-thiosulfate leaching systems still have the capacity to extract gold form gold ores even after seven days leaching in the absence of oxygen.
It was found that thiourea can be used as a catalyst for this gold leaching process. Compared with copper-ammonia-thiosulfate leaching system, the ferric-oxalate-thiosulfate system in the presence of thiourea has the advantages of faster gold leaching kinetics and lower thiosulfate consumption [51]. Ferric-EDTA and ferric-oxalate complexes have a low reaction activity with thiosulfate and do not react with the additive of thiourea. However, the presence of sulfide minerals such as pyrite and pyrrhotite can catalyze the decomposition of thiosulfate and rapidly reduce ferric complexes to ferrous complexes, resulting in a significant decline of gold leaching rate [123]. Therefore, the ferric-EDTA/oxalate-thiosulfate system may have a promising application prospect for gold leaching in-situ after overcoming the influence of sulfide minerals [122,123]. HEATH et al [122] also found that solution pH plays an important role during gold leaching process in ferric-EDTA/oxalate-thiosulfate systems. When the pH value is less than 5, the stability of thiosulfate becomes worse. While the pH value is too high, the oxidizing capacity of ferric-EDTA complexes turns weaker and ferric ions readily generate ferric oxalate precipitates. Therefore, a suitable pH range of 6-7 for ferric-EDTA/oxalate-thiosulfate leaching system needs to be maintained [122].
Noteworthily, it has been demonstrated that the addition of thiourea can increase the exchange current density of the gold electrode oxidation reaction in magnesium thiosulfate solution [80]. CHANDRA and JEFFREY [57] suggested that the addition of thiourea can promote the half reaction of gold oxidation in thiosulfate solutions. ZELINSKY and NOVGORODTSEVA[124] highlighted that the presence of thiourea can promote the oxidation of gold. A small amount of thiourea can greatly increase the oxidation rate of thiosulfate and the dissolution rate of gold, and gradually remove the passivation substance on gold surface. In the gold leaching process with ferric-EDTA/oxalate-thiosulfate solution, the presence of thiourea plays a role of catalyzing the dissolution of gold [51,122,125]. Besides, the addition of thiourea can also improve the gold extraction rate and the initial gold leaching rate form gold-bearing sulfide ores in thiosulfate solution [114].
4.4 Pressurized oxygen-thiosulfate system
In order to overcome the problem caused by the usage of ammonia and reduce the high consumption of thiosulfate caused by the oxidation of copper ions, JI et al [82] conducted a series of studies on directly thiosulfate leaching of pre-robbing carbonaceous gold ores in a closed leaching reactor with slightly increased pressure (68.95-689.5 kPa) and temperature (60-80 °C). The results indicated that the gold extraction can exceed 80% after 6h leaching. Compared with the traditional copper-ammonia-thiosulfate leaching system, the oxygen pressure-thiosulfate leaching process has some advantages such as eco-friendly, easy to control, and lower consumption of thiosulfate [82]. ZHANG and NICOL [126] reported that the dissolution rate of gold in alkaline oxygen-thiosulfate solution was significantly slower than that in copper-ammonia-thiosulfate solution, and the oxidation decomposition product of thiosulfate would form a passivation film of sulfur on the gold surface, hindering the dissolution rate of gold. It was considered that the leaching rate of gold in oxygen-thiosulfate solution is 1×10-7 mol·m-2·s-1, two orders of magnitude lower than that in a typical cyanidation process. The leaching rate of gold in oxygen-thiosulfate solution can be increased by one order of magnitude by elevating the temperature and dissolved oxygen and reducing the concentration of copper. The addition of sulfide minerals can increase the leaching rate of gold in oxygen-thiosulfate solution to the same order of magnitude as a typical cyanide process [40]. Some research also reported that the addition of thallium ions can accelerate the dissolution of gold in oxygen-thiosulfate solution by adsorption effect [79].
In recent years, relevant researches on the pressurized oxygen-thiosulfate leaching process were carried out. XU et al [98] suggested that thiosulfate can be generated in situ by adding sulfur in the oxygen pressure-thiosulfate leaching process, and meanwhile, sulfur can be generated by the oxidation pretreatment of gold-bearing sulfide ores. They proposed to combine the oxidation pretreatment with pressurized oxygen-thiosulfate leaching process to deal with sulfide gold ores. YANG et al [127] proved that increasing the temperature and pressure is beneficial to gold leaching in thiosulfate solutions by thermodynamic calculation. In addition, A-21S Tulsion strong basic anion exchange resin was used to recover gold from the pregnant solution after oxygen pressure-thiosulfate leaching process. More than 98% gold in the leaching solution can be recovered with 1 g/L dosage of the resin, and the eluent consistingof sodium chloride and sodium sulfite can completely desorb the gold-bearing resins [127]. It is worth noting that increasing temperature and pressure is beneficial to the dissolution of gold in thiosulfate solution, and meanwhile, the consumption of thiosulfate is also increased. The leaching process operated at elevated pressure will cause a significant increase of the operating cost, especially when the gold content in ore is dramatically low. Therefore, the pressure oxygen-thiosulfate leaching process is more suitable to deal with high grade gold-bearing materials.
In addition, ammonium alcohol polyvinyl phosphate (AAPP) contains a great number of negatively charged phosphoric acid groups and hydroxyl groups, which can non-selectively adsorb on the surface of gold and passivation substance, resulting in the phenomenon of electrostatic repulsion between gold and passivation substance, thus preventing the formation of passivation substance on the gold surface, and ultimately improving the gold leaching rate [127]. Similarly, AAPP can reduce the affinity of mineral surface to thiosulfate ions, thus weakening the catalytic oxidation effect of minerals to thiosulfate [127]. In the oxygen pressure-thiosulfate leaching process for oxidized gold ore, the addition of AAPP can inhibit the formation and accumulation of passivation substance such as FeOOH, Fe2O3, Al2O3, S0on the gold surface. And the leaching rate of gold can significantly increase to 90% while the consumption of sodium thiosulfate gradually decreases with an increase concentration of AAPP (0-4 g/L) [127].
4.5 Copper-thiosulfate system
Although the consumption of thiosulfate may be higher when there is no ammonia in copper thiosulfate solutions, interestingly, some studies proved that the copper thiosulfate leaching system works well in some cases. The leaching rates of gold from different types of gold ores in copper-thiosulfate solution were compared. The results indicated that the gold leaching rate from oxidized ores was slower than that of pyrite, while the rate from copper-bearing gold ores was much faster [68]. As discussed in previous sections, the leaching mechanism of gold in copper-thiosulfate solution is well illustrated in literatures (Eqs. (7)-(9)) [61].
Gold is catalytically oxidized to form Au(S2O3)23- in the solution by copper-being species (Cu(OH)20, Cu(OH)2(S2O3)2-)and the dissolved oxygen. ZHANG and NICOL [60] believed that gold leaching in copper-thiosulfate solution was carried out through the intermediate product of [(S2O3)3Cu·O2]5- (shown in Eqs. (10)-(12)) [60].
Researchers at Barrick Gold Corporation (2013) conducted a study on using copper-calcium thiosulfate solutions for gold leaching from the oxygen-pressure-leaching residue of carbonaceous gold ores. The results indicated the optimum leaching solution pH was 8.0 in copper-calcium thiosulfate solution and at higher pH, the presence of sulfate (SO42-) will generate gypsum (CaSO4) precipitated on the gold surface, resulting in a decline of gold extraction [92]. Moreover, the addition of resins can reduce the loss of gold by decreasing the absorption on the surface of associated minerals and increase the gold recovery significantly [92]. In 2014, Barrick Gold Corporation adopted the process of grinding-pressurized oxidation-thiosulfate leaching(copper-calcium thiosulfate)-resin adsorption to deal with refractory carbonaceous sulfide gold ores [128]. Figure 10 illustrates a simplified flow diagram of the process reported by Barrick Gold Corporation [92,128]. In the resin elution stage, ammonium thiosulfate was used to remove the impurities such as copper-thiosulfate complexes; then the mixed solution consistingof S3O62- and SO32- was used to elute the gold-bearing resin. A flow rate of 2 BV was controlled to get a higher concentration of gold-bearing solution during the elution process, and then the eluent can be directly applied to the electrowinning process of gold [90].
5 Problems and challenge
In recent years, thiosulfate leaching system, especially the traditional copper-ammonia-thiosulfate system has been studied extensively.Many researchers considered thiosulfate is the most promising alternative to cyanidation. However, with many years of process development, there are still some problems and challenges that the thiosulfate-based leaching systems must face to. These include (but not limited) the high consumption of thiosulfate, the passivation of gold surface, the potential hazard of ammonia, and more critically that, the gold leaching kinetics in thiosulfate solutions at ambient temperature is dramatically slow though the gold extraction rate is also readily affected by other operating conditions such as pH and dissolved oxygen, and the type of gold ores. Some critical factors that potentially hinder the practice of thiosulfate-based gold leaching system are discussed.

Fig.10 Flow diagram of thiosulfate leaching gold process from Barrick Gold Corporation [128]
5.1 High consumption of thiosulfate
Thiosulfate (S2O32-) is prone to be decomposed by oxidation reaction and the catalytic effect of associated minerals during the leaching process. In addition, a high concentration of copper ions can greatly consume thiosulfate ions by a series of redox reactions. Elevating temperature can improve the poor leaching kinetics in thiosulfate system under ambient conditions, which may increase the consumption of thiosulfate at the same time. Thus, the high consumption of thiosulfate becomes one of the major barriers restricting the industrial application and development of thiosulfate system. Based on the published literatures, regenerating thiosulfate in-situ and improving the stability of thiosulfate during the leaching process may efficiently resolve the problem of high thiosulfate consumption. Other methods and techniques to reduce the consumption of thiosulfate include:
(1) Reduce the content of dissolved oxygen in the pulp appropriately by blowing N2 [85];
(2) Increase the pulp density appropriately to lower the consumption of thiosulfate [85];
(3) Use nickel-, cobalt- or ferric-based catalysis instead of copper catalysis [51,85,110];
(4) Add inorganic additives such as Cl-, SO42- and PO43- to weaken the reactivity between thiosulfate and copper ions to reduce the consumption of thiosulfate [88];
(5) Add sulfite (SO32-) to increase the stability of thiosulfate ions and further reduce the consumption of thiosulfate [18];
(6) Reduce the usage or concentration of reagents (CuSO4 or thiosulfate) to reduce the consumption of thiosulfate [85,129,130];
(7) Add organic reagents (EDTA, NTA, HA, etc) to improve the stability of thiosulfate and reduce the consumption of thiosulfate [47,85,101];
(8) Use sulfur, SO2 and ammonia to regenerate ammonium thiosulfate in the solution to reduce the consumption of thiosulfate [130];
2NH3+SO2+S+H2O=(NH4)2S2O3 (67)
(9) Use sulfur to regenerate thiosulfate in-situ during oxygen pressure leaching process to reduce the consumption of thiosulfate, as given in Eqs. (68)-(70) [98]:
5S+6OH-+3O2=2S2O32-+SO32-+3H2O (68)
SO32-+S=S2O32- (69)
2S+2OH-+O2=S2O32-+H2O (70)
5.2 Passivation of gold surface
In the process of leaching gold or gold ores with thiosulfate solutions, the gold surface is prone to form passivation film, which seriously impedes the dissolution of gold. Table 2 lists the reported composition of the passivation film in thiosulfate solutions under different leaching conditions. They mainly consist of the copper-bearing sulfides (CuS2, CuS, and CuS2) and the decomposition products of thiosulfate such as sulfur, polysulfides (S3O62-, and S4O62-) and polysulfides (Sx2-, Sn>4, and 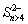 ). In addition, the dissolved products of associated minerals are also important parts of the passivation substance on gold surface. It has been proved that the addition of Cu(I) [68], Cu(II) [69], ammonia [69,93,126,131], EDTA [101], thiourea[124], humic acid [48,98], ammonium alcohol polyvinyl phosphate [127] caneffectively hinder the formation of passivation layer and weaken or eliminate the passivation film during the gold leaching process in thiosulfate solutions. And the usage of additives is one of the major measures to remove the passivation on gold surface and improve the leaching kinetics.
). In addition, the dissolved products of associated minerals are also important parts of the passivation substance on gold surface. It has been proved that the addition of Cu(I) [68], Cu(II) [69], ammonia [69,93,126,131], EDTA [101], thiourea[124], humic acid [48,98], ammonium alcohol polyvinyl phosphate [127] caneffectively hinder the formation of passivation layer and weaken or eliminate the passivation film during the gold leaching process in thiosulfate solutions. And the usage of additives is one of the major measures to remove the passivation on gold surface and improve the leaching kinetics.
5.3 Potential problems with ammonia
In the traditional copper-ammonia-thiosulfate leaching system, the potential problems and risks caused by the presence of ammonia have seriously restricted and hindered the development and commercial application of thiosulfate gold leaching system. Ammonia is difficult to be disposed, transported and stored because it readily volatilizes into the air and causes damage to the human body, and pollutes the ecological environment. Some developed countries (USA, Germany) and China have issued standards and specific occupational exposure limits of ammonia in the workplace(Table 3) [132-134]. In China, ammonia exposure concentration should be lower than20 mg/m3 in 8 h working day (40 h working week), while in Germany and America, the exposure limits of ammonia concentration are 14 mg/m3 and 25 mg/m3, respectively. In China, a 15 min exposure concentration limit of 30 mg/m3 is required, slightly lower than that in USA of 35 mg/m3 of the concentration limit.
Table 2 Composition of passivation layer on gold or gold ore surface in thiosulfate solutions

Table 3 Threshold limiting value of ammonia gas at workplace
The addition of ammonia in the leaching system also produces a large amount of wastewater and tailings containing ammonia or ammonium. The water hazard class regulation (WGK) issued by Germany has clearly stipulated that ammonium ion (NH4+) is a low-hazard substance to water (WGK=1), while ammonia (NH3) is a hazardous substance to water (WGK=2) [135]. Direct discharge of the unpurified wastewater containing ammonia or ammonium to the environment will not only threaten the survival of aquatic animals, but also seriously pollute the water resources. Natural bacteria exhibit a slow oxidation rate to ammonia in natural environment, making it extremely difficult to be decomposed. Besides, nitrate is the final metabolic product of ammonia, which can accelerate the growth of aquatic algae, causing eutrophication and further contaminating groundwater resources. It is worth noting that countries all over the world are tightening the regulations on the purification and discharge of industrial ammonia-nitrogen wastewater, and the emission has been strictly supervised and controlled. Therefore, the operation cost and economic feasibility by the purification treatment of ammonia-containing wastewater and tailings becomes one of the most important barriers to the large-scale application of copper-ammonia-thiosulfate leaching system.
6 Summary
Thiosulfate is considered as the most promising alternative reagent to cyanide, especially, in dealing with copper-bearing or carbonaceous gold ores when using cyanide, or cyanide consumption is high. However, the thiosulfate-based gold leaching processes are still far away from large scale application because the overall gold recovery is generally lower and reagent consumption is higher compared to cyanidation. The use of the copper-ammonia-thiosulfate leaching system and its modifications has been studied extensively, both in theory and process development. Basically, copper ions sever as the oxidants while ammonia acts as the stabilizer to complex with copper ions and prevents the copper ions from precipitating in alkaline solution. However, the use of ammonia not only increases the complexity of thiosulfate leaching systems, but also intensifies the sensitivity due to the instability of ammonia. In addition, the presence of ammonia in thiosulfate leaching system will produce a large amount of wastewater and tailings containing ammonia or ammonium, which may cause great damages to the surrounding environment.Nickel and cobalt ions can also be used as the oxidants for gold in ammonia-thiosulfate solutions. However, the benefits of using these precious metals for gold leaching and the potential operating cost must be balanced.
A variety of non-ammonia-based thiosulfate leaching systems have been developed in recent years. Some of them become attractive ways for dealing with refractory gold ores. Studies confirm that organic ligands containing amino, carboxyl or hydroxy functional groups can efficiently reduce the consumption of thiosulfate and slightly increase the gold leaching rate compared with those in the presence of ammonia. Furthermore, the addition of EDTA, oxalate, citrate, HA, CMC/CMS, AAPP and other additives all exhibited similar effect during the gold leaching process in thiosulfate solutions. However, the high consumption of thiosulfate is still a critical issue associated with these leaching systems.
Another critical factor that may potentially affect the application of thiosulfate-based gold leaching systems is the recovery of gold from thiosulfate leach solutions. Though there are already some studies on this issue such as using anion-exchange resins, there still lacks solid evidence that proves resins are more effective in thiosulfate solutions than carbon in cyanide-leach solutions and may overcome passivation to improve gold recovery (with or without additives). In other word, the gold leaching and recovery processes developed for thiosulfate-based solutions must exhibit an equivalent low operating costs to match that of cyanidation-carbon adsorption process. However, the threat of increasing public concerns over cyanide management and the tightening regulations on disposal of cyanide tailings issued by local governments worldwide provide economic incentive for existing cyanidation plants to switch from cyanide to thiosulfate. The thiosulfate-based gold leaching system may be realistically used in practice if an overall leaching-recovery process with a reasonable gold recovery and well reagent recycling or destruction and impurity control can be effectively developed.
Acknowledgments
Theauthorsaregratefulforthefinancialsupportsfrom the Fundamental Research Funds for Central Universities of China(No. N182502044)
References
[1] BOTZ M M, MUDDER T I, AKCIL A U. Cyanide treatment: Physical, chemical and biological processes [M]//Developments in Mineral Processing.Amsterdam: Elsevier, 2005: 672-702.
[2] KUYUCAK N, AKCIL A. Cyanide and removal options from effluents in gold mining and metallurgical processes [J]. Minerals Engineering, 2013, 50/51: 13-29.
[3] DONATO D B, NICHOLS O, POSSINGHAM H, MOORE M, RICCI P F, NOLLER B N. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife [J]. Environment International, 2007, 33(7): 974-984.
[4] JOHNSON C A. The fate of cyanide in leach wastes at gold mines: An environmental perspective [J]. Applied Geochemistry, 2015, 57: 194-205.
[5] MANAR R, BONNARD M, RAST C, VEBER A M, VASSEUR P. Ecotoxicity of cyanide complexes in industrially contaminated soils [J]. Journal of Hazardous Materials, 2011, 197: 369-377.
[6] ADAMS M, LLOYD V. Cyanide recovery by tailings washing and pond stripping [J]. Minerals Engineering, 2008, 21(6): 501-508.
[7] AYLMORE M G, MUIR D M. Thiosulfate leaching of gold—A review [J]. Minerals Engineering, 2001, 14(2): 135-174.
[8] AYLMORE M G. Alternative lixiviants to cyanide for leaching gold ores [M]//Developments in Mineral Processing.Amsterdam: Elsevier, 2005: 501-539.
[9] WANG J, XIE F, WANG W, BAI Y L, FU Y, DREISINGER D. Eco-friendly leaching of gold from a carbonaceous gold concentrate in copper-citrate-thiosulfate solutions [J]. Hydrometallurgy, 2020: 191: 105204.
[10] ADAMS M D. Advances in gold ore processing [M]. Amsterdam: Elsevier, 2005: 501-560.
[11] MUNIVE G T, ENCINAS M A, SALAZAR CAMPOY M M, ?LVAREZ V E, VAZQUEZ V M, CHOQUE D C. Leaching gold and silver with an alternative system: Glycine and thiosulfate from mineral tailings [J]. JOM, 2020, 72(2): 918-924.
[12] ZHENG Su, Wang Yun-yan, CHAI Li-yuan. Research status and prospect of gold leaching in alkaline thiourea solution [J]. Minerals Engineering, 2006, 19(13): 1301-1306.
[13] ?RG?L S, ATALAY?. Reaction chemistry of gold leaching in thiourea solution for a Turkish gold ore [J]. Hydrometallurgy, 2002, 67(1/2/3): 71-77.
[14] PARKER G K, HOPE G A. Spectroelectrochemical investigations of gold leaching in thiourea media [J]. Minerals Engineering, 2008, 21(6): 489-500.
[15] BAGHALHA M. Leaching of an oxide gold ore with chloride/hypochlorite solutions [J]. International Journal of Mineral Processing, 2007, 82(4): 178-186.
[16] LI J S, SAFARZADEH MS, MOATS M S, MILLER J D, LEVIER K M, DIETRICH M, WAN R Y. Thiocyanate hydrometallurgy for the recovery of gold. Part I: Chemical and thermodynamic considerations [J]. Hydrometallurgy, 2012, 113/114: 1-9.
[17] EARLET Drummond. Polysulfides as an alternative green gold leaching technology [C]//SME Annual Meeting and Exhibit and CMA’s 111th National Western Mining Conference. UAS: Mining Engineering, 2009:652-658.
[18] ZHANG X M, SENANAYAKE G. A review of ammoniacal thiosulfate leaching of gold: An update useful for further research in non-cyanide gold lixiviants [J]. Mineral Processing and Extractive Metallurgy Review, 2016, 37(6): 385-411.
[19] SENANAYAKE G. Analysis of reaction kinetics, speciation and mechanism of gold leaching and thiosulfate oxidation by ammoniacalcopper(II) solutions [J]. Hydrometallurgy, 2004, 75(1-4): 55-75.
[20] SENANAYAKE G. Catalytic role of ammonia in the anodic oxidation of gold in copper-free thiosulfate solutions [J]. Hydrometallurgy, 2005, 77(3/4): 287-293.
[21] WHITE H A. The solubility of gold in thiosulphates and thiocyanates [J]. South African Journal of Science, 1905, 1(1).
[22] LIDDELL D M. Handbook of non-ferrous metallurgy [M]. New York: McGraw-Hill, 1945: 357.
[23] FLETT D S, WILSON J C, DERRY R. Chemical study of thiosulphate leaching of silver sulfide [J]. Transactions of the Institution of Mining and Metallurgy, Section C: Mineral Processing and Extractive Metallurgy, 1983, 92: 216-223.
[24] BEREZOWSKY G S, SEFTON V B, GORMELY L S. Recovery of precious metals from metal sulphides: US4070182 [P]. 1978.
[25] BEREZOWSKY R M G S, SEFTON V B. Recovery of gold and silver from oxidation leach residues by ammoniacalthiosulphate leaching [C]//108th AIME Annual Meeting. New Orleans,1979: 17-34.
[26] KERLEY B J Jr. Recovery of precious metals from difficult ores: US4369061 [P]. 1983-01-18.
[27] KERLEY B J Jr. Recovery of precious metals from difficult ores: US4269622 [P]. 1981.
[28] TERARAKELYAN K A, BAGDASARYAN K A, OGANYAN A G, MKRTCHYAN R T, BABAYAN G G. On technological expediency of sodium thiosulphate usage for gold extraction from raw material [J]. Izv VUZ TsvetnMetall, 1984(5): 72-76.
[29] WAN R Y, LEVIER MK, CLAYTON R B. Hydrometallurgical process for the recovery of precious metal values from precious metal ores with thiosulfate lixiviant: US5354359 [P]. 1994-10-11.
[30] WAN R Y, LEVIER K M. Solution chemistry factors for gold thiosulfate heap leaching [J]. International Journal of Mineral Processing, 2003, 72(1/2/3/4): 311-322.
[31] LIU Xiao-liang, JIANG Tao, XU Bin, ZHANG Yan, LI Qian, YANG Yong-bin, HE Ying-he. Thiosulphate leaching of gold in the Cu-NH3-S2O32--H2O system: An updated thermodynamic analysis using predominance area and species distribution diagrams [J]. Minerals Engineering, 2020: 151: 106336.
[32] DONG Zhong-lin, JIANG Tao, XU Bin, YANG Yong-bin, LI Qian. An eco-friendly and efficient process of low potential thiosulfate leaching-resin adsorption recovery for extracting gold from a roasted gold concentrate [J]. Journal of Cleaner Production, 2019, 229: 387-398.
[33] GROSSE A C, DICINOSKI G W, SHAW M J, HADDAD P R. Leaching and recovery of an oxide gold concentrate using ammoniacal thiosulfate solutions [J]. Hydrometallurgy, 2003, 69(1/2/3):1-21.
[34] ESCOBAR-LEDESMA F R, ARAG?N-TOBAR C F, ESPINOZA-MONTERO P J, de la TORRE-CHAUVIN E. Increased recovery of gold thiosulfate alkaline solutions by adding thiol groups in the porous structure of activated carbon [J]. Molecules, 2020, 25(12): 2902.
[35] MAHANDRA H,FARAJI F,GHAHREMAN A. Novel extraction process for gold recovery from thiosulfate solution using phosphonium ionic liquids [J]. ACS Sustainable Chemistry & Engineering, 2021, 9(24): 8179-8185.
[36] FLEMING C A, MCMULLEN J, THOMAS K G, WELLS J A. Recent advances in the development of an alternative to the cyanidation process: Thiosulfate leaching and resin in pulp [J]. Mining Metallurgy & Exploration, 2003, 20(1): 1-9.
[37] MARCHBANK A R, THOMAS K G, DREISINGER D, FLEMING C. Gold recovery from refractory carbonaceous ores by pressure oxidation and thiosulfate leaching: US5536297 [P]. 1996-07-16.
[38] GROSSE A C, DICINOSKI G W, SHAW M J, HADDAD P R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors: A review [J]. Hydrometallurgy, 2003, 69(1/2/3): 1-21.
[39] LAMPINEN M, LAARI A, TURUNEN I. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption [J]. Hydrometallurgy, 2015, 151:1-9.
[40] SITANDO O, DAI X, SENANAYAKE G. Gold dissolution in non-ammoniacalthiosulphate solutions: Comparison of fundamentals and leaching studies [C]//World Gold Conference 2015. Johannesburg: The Southern African Institute of Mining and Metallurgy,2015.
[41] SITANDO O, SENANAYAKE G, DAI X, NIKOLOSKI A N, BREUER P. A review of factors affecting gold leaching in non-ammoniacal thiosulfate solutions including degradation and in-situ generation of thiosulfate [J]. Hydrometallurgy, 2018, 178: 151-175.
[42] JIANG Y, ZI F, CHEN Y. Method for directly recovering gold in thiosulfate system: CN112375919-A [P]. 2021. (in Chinese)
[43] YANG H, ZHAO H, TONG L. A method of using triethanolamine as the leaching of gold using thiosulfate of additive: CN109680163-A [P]. 2019. (in Chinese)
[44] ZI F,CHEN Y,CHEN S. Method for recovering gold in thiosulfate system: CN111004922-A [P]. 2019. (in Chinese)
[45] ZI F,JIANG Y,CHEN Y. Method for directly recovering gold in thiosulfate system by using active carbon: CN112267030-A [P]. 2020. (in Chinese)
[46] XU B, LI K, LI Q, YANG Y B, LIU X L, JIANG T. Kinetic studies of gold leaching from a gold concentrate calcine by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution [J]. Separation and Purification Technology, 2019, 213: 368-377.
[47] XU B, YANG Y B, JIANG T, LI Q, ZHANG X, WANG D. Improved thiosulfate leaching of a refractory gold concentrate calcine with additives [J]. Hydrometallurgy, 2015, 152: 214-222.
[48] XU B, YANG Y B, LI Q, JIANG T, ZHANG X, LI G H. Effect of common associated sulfide minerals on thiosulfate leaching of gold and the role of humic acid additive [J]. Hydrometallurgy, 2017, 171: 44-52.
[49] XU B, KONG W H, LI Q, YANG Y B, JIANG T, LIU X L. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution [J]. Metals, 2017, 7(6): 222.
[50] ZHANG Y, XU B, ZHENG Y Q, LI Q, YANG Y B, LIU X L, JIANG T, LYU X J. Hexaamminecobalt(III) catalyzed thiosulfate leaching of gold from a concentrate calcine and gold recovery from its pregnant leach solution via resin adsorption [J]. Minerals Engineering, 2021, 171: 107079.
[51] CHANDRA I, JEFFREY M I. A fundamental study of ferric oxalate for dissolving gold in thiosulfate solutions [J]. Hydrometallurgy, 2005, 77(3/4): 191-201.
[52] TRINAPAKUL M, KRITAYAKORNUPONG C, TONGRAAR A, VCHIRAWONGKWIN V. Active site of the solvated thiosulfate ion characterized by hydration structures and dynamics [J]. Dalton Transactions, 2013, 42(30): 10807-10817.
[53] BRENT HISKEY J, ATLURI V P. Dissolution chemistry of gold and silver in different lixiviants [J]. Mineral Processing and Extractive Metallurgy Review, 1988, 4(1/2): 95-134.
[54] WEBSTER J G. The solubility of gold and silver in the system gold-silver-sulfur-oxygen-water at 25 °C and 1atm [J]. GeochimicaetCosmochimicaActa, 1986, 50(9): 1837-1845.
[55] SENANAYAKE G. Gold leaching in non-cyanide lixiviant systems: critical issues on fundamentals and applications [J]. Minerals Engineering, 2004, 17(6): 785-801.
[56] BRYCE R A, CHARNOCK J M, PATTRICK R A D, LENNIE A R. EXAFS and density functional study of gold(I) thiosulfate complex in aqueous solution [J]. The Journal of Physical Chemistry A, 2003, 107(14): 2516-2523.
[57] WANG Jian. Applied fundamental research on copper-citrate-thiosulfate leaching gold system [D]. Shenyang, Northeastern University, 2020. (in Chinese)
[58] CHANDRA I, JEFFREY M I. An electrochemical study of the effect of additives and electrolyte on the dissolution of gold in thiosulfate solutions [J]. Hydrometallurgy, 2004, 73(3/4): 305-312.
[59] SENANAYAKE G. Kinetic model for anodic oxidation of gold in thiosulfate media based on the adsorption of MS2O3- ion-pair [J]. Hydrometallurgy, 2005, 76(3/4): 233-238.
[60] ZHANG S C, NICOL M J. An electrochemical study of the dissolution of gold in thiosulfate solutions. Part II. Effect of copper [J]. Journal of Applied Electrochemistry, 2005, 35(3): 339-345.
[61] SENANAYAKE G. Role of copper(II), carbonate and sulphite in gold leaching and thiosulphate degradation by oxygenated alkaline non-ammoniacal solutions [J]. Minerals Engineering, 2005, 18(4): 409-426.
[62] WANG J, XIE F, WANG W, BAI Y L, FU Y, CHANG Y F. Leaching of gold from a free milling gold ore in copper-citrate-thiosulfate solutions at elevated temperatures [J]. Minerals Engineering, 2020, 155: 106476.
[63] BREUER P L, JEFFREY M I. Thiosulfate leaching kinetics of gold in the presence of copper and ammonia [J]. Minerals Engineering, 2000, 13(10/11): 1071-1081.
[64] BYERLEY J J, FOUDA S A, REMPEL G L. Kinetics and mechanism of the oxidation of thiosulphate ions by copper(II) ions in aqueous ammonia solution [J]. Journal of the Chemical Society, Dalton Transactions, 1973(8): 889-893.
[65] AYLMORE M G, MUIR D M. Thermodynamic analysis of gold leaching by ammoniacal thiosulfate using Eh/pH and speciation diagrams [J]. Mining, Metallurgy & Exploration, 2001, 18(4): 221-227.
[66] AYLMORE M G. Thiosulfate as an alternative lixiviant to cyanide for gold ores [M]//Gold Ore Processing. Amsterdam: Elsevier, 2016: 485-523. (Book)
[67] AYLMORE M G, MUIR D M, STAUNTON W P. Effect of minerals on the stability of gold in copper ammoniacal thiosulfate solutions—The role of copper, silver and polythionates [J]. Hydrometallurgy, 2014, 143: 12-22.
[68] ZHANG H, DAI X, BREUER P. Factors affecting gold leaching in thiosulfate-O2 solutions [C]//Proceedings of the ALTA 2013 Gold Sessions. Perth, 2013: 30-31.
[69] SMITH S R, ZHOU C Q, BARON J Y, CHOI Y, LIPKOWSKI J. Elucidating the interfacial interactions of copper and ammonia with the sulfur passive layer during thiosulfate mediated gold leaching [J]. ElectrochimicaActa, 2016, 210: 925-934.
[70] ABBRUZZESE C, FORNARI P, MASSIDDA R, VEGLI? F, UBALDINI S. Thiosulphate leaching for gold hydrometallurgy [J]. Hydrometallurgy, 1995, 39(1/2/3): 265-276.
[71] CHEN J Y, DENG T, ZHU G,ZHAO J. Leaching and recovery of cold in thiosulfate based system—A research summary at ICM [J]. Transactions of the Indian Institute of Metals, 1996, 49(6): 841-849.
[72] SENANAYAKE G. The role of ligands and oxidants in thiosulfate leaching of gold [J]. Gold Bulletin, 2005, 38(4): 170-179.
[73] SITANDO O. Gold leaching in thiosulfate-oxygen solutions [D]. Perth, Australia: Murdoch University, 2017.
[74] WAN R. Importance of solution chemistry for thiosulphate leaching of gold [C]//Proceedings of the World Gold 97. Singapore, 1997: 159-162.
[75] JEFFREY M I, BREUER P L, CHU C K. The importance of controlling oxygen addition during the thiosulfate leaching of gold ores [J]. International Journal of Mineral Processing, 2003, 72(1/2/3/4): 323-330.
[76] SENANAYAKE G, MUIR D M. Chloride processing of metal sulphides: Review of fundamentals and applications [C]//Proceedings of the National Academy of Sciences of the United States of America. 2003: 517-531.
[77] FENG D, van DEVENTER J S J. The role of oxygen in thiosulphate leaching of gold [J]. Hydrometallurgy, 2007, 85(2/3/4): 193-202.
[78] SENANAYAKE G. Gold leaching by copper(II) in ammoniacalthiosulphate solutions in the presence of additives. Part I: A review of the effect of hard–soft and Lewis acid-base properties and interactions of ions [J]. Hydrometallurgy, 2012, 115/116: 1-20.
[79] BEK R Y, SHEVTSOVA O N. The effect of thallium ions on the gold dissolution rate in thiosulfate electrolytes [J]. Russian Journal of Electrochemistry, 2012, 48(11): 1046-1051.
[80] MELASHVILI M, DREISINGER D, CHOI Y. Cyclic voltammetry responses of gold electrodes in thiosulphate electrolyte [J]. Minerals Engineering, 2016, 92: 134-140.
[81] GUDKOV A S, ZHUCHKOV I A, MINEEV GG. Mechanism and kinetics of sulfite-thiosulfate dissolution of gold [J]. Russian Journal of Non-Ferrous Metals, 2010, 51(5): 393-397.
[82] JI J, FLEMING C, WEST-SELLS P G. A novel thiosulfate system for leaching gold without the use of copper and ammonia [C]//Hydrometallurgy 2003: 5th International Symposium Honoring Professor Ian M. Ritchie.USA: The Minerals, Metals & Materials Society, 2003: 227-244.
[83] HEMMATI M. A study of thiosulfate leaching of gold from carbonaceous ore and the quantitative determination of thiosulfate in the leached solution [J]. Mining and Metallurgical Engineering, 1987: 29-31.
[84] ZIPPERIAN D, RAGHAVAN S, WILSON J P. Gold and silver extraction by ammoniacal thiosulfate leaching from a rhyolite ore [J]. Hydrometallurgy, 1988, 19(3): 361-375.
[85] XIAC, YENW T, DESCHENES G. Improvement of thiosulfate stability in gold leaching [J]. Mining, Metallurgy & Exploration, 2003, 20(2): 68-72.
[86] BAGDASARYAN K A, EPISKOPOSYAN M L, TER-ARAKELYAN K A, BABAYAN G G. The kinetics of the dissolution of gold and silver in sodium thiosulphate solutions [J]. Izv VUZ Tsvetn Metallurgy, 1983, 5: 64-68.
[87] BREUER P L, JEFFREY M I. An electrochemical study of gold leaching in thiosulfate solutions containing copper and ammonia [J]. Hydrometallurgy, 2002, 65(2/3): 145-157.
[88] BREUER P L, JEFFREY M I. The reduction of copper(II) and the oxidation of thiosulfate and oxysulfur anions in gold leaching solutions [J]. Hydrometallurgy, 2003, 70(1/2/3): 163-173.
[89] WEST-SELLS P G, JI J, HACKL R P. A process for counteracting the detrimental effect of tetrathionate on resin gold adsorption from thiosulfate leachates [C]//Proceedings of the TMS Fall Extraction and Processing Conference. USA: The Minerals, Metals & Materials Society, 2003: 245-256.
[90] JEFFREY M I, HEWITT D M, DAI X, BRUNT S D. Ion exchange adsorption and elution for recovering gold thiosulfate from leach solutions [J]. Hydrometallurgy, 2010, 100(3/4): 136-143.
[91] NICOL M J, O'MALLEY G. Recovering gold from thiosulfate leach pulps via ionexchange [J]. JOM, 2002, 54(10): 44-46.
[92] CHOI Y, BARON J, WANG Q. Thiosulfate processing–from lab curiosity to commercial application [C]//Proceedings of the World Gold. Brisbane, 2013: 45-50.
[93] JEFFREY M I, WATLING K, HOPE G A, WOOODS R. Identification of surface species that inhibit and passivate thiosulfate leaching of gold [J]. Minerals Engineering, 2008, 21(6): 443-452.
[94] BYERLEY J J, FOUDA S A, REMPEL G L. ChemInform abstract: Activation of copper(II) ammine complexes by molecular oxygen for the oxidation of thiosulphate ions [J]. ChemischerInformationsdienst, 1975, 6(40).
[95] CHU C K, BREUER P L, JEFFREY M I. The impact of thiosulfate oxidation products on the oxidation of gold in ammonia thiosulfate solutions [J]. Minerals Engineering, 2003, 16(3): 265-271.
[96] YANG Y B, ZHANG X, XU B, LI Q, JIANG T, WANG Y X. Effect of arsenopyrite on thiosulfate leaching of gold [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(10): 3454-3460.
[97] LI Ru-xiong, KUANG Sheng-lu. Leaching gold with thiosulphate solution containing added sodium chioride and sodium dodecyl sulphonate [J]. Engineering Chemistry & Metallurgy, 1998, 19(1): 77-82. (in Chinese)
[98] XU B, LI K, ZHONG Q, YANG Y B, JIANG T. Study on the oxygen pressure alkaline leaching of gold with generated thiosulfate from sulfur oxidation [J]. Hydrometallurgy, 2018, 177: 178-186.
[99] MICHEL D, FRENEY J. Electrochemical investigation of the thiosulphate gold leaching [C]//CD Proceedings of CIM Gold Symposium, Annual General Meeting. Montreal: The Canadian Institute of Mining, Metallurgy and Petroleum, 1998.
[100] FENG D, van DEVENTER J S J. The role of amino acids in the thiosulphate leaching of gold [J]. Minerals Engineering, 2011, 24(9): 1022-1024.
[101] FENG D, van DEVENTER J S J. Thiosulphate leaching of gold in the presence of ethylenediaminetetraacetic acid (EDTA) [J]. Minerals Engineering, 2010, 23(2): 143-150.
[102] SMITH R, MARTELL A, MOTEKAITIS R. Critically selected constants of metal complexes database, Version 5.0 software [M]. National Institute of Standards and Technology, 1998.
[103] ZHAO H F, YANG H Y, CHEN X, CHEN G B, TONG L L, JIN Z N. Effect of triethanolamine as a new and efficient additive on thiosulfate-copper-ammonia system leaching of gold [J]. JOM, 2020: 72(2): 946-952.
[104] FENG D, van DEVENTER J S. Thiosulphate leaching of gold in the presence of carboxymethyl cellulose (CMC) [J]. Minerals Engineering, 2011, 24(2): 115-121.
[105] FENG D, van DEVENTER J S J. Effect of hematite on thiosulphate leaching of gold [J]. International Journal of Mineral Processing, 2007, 82(3): 138-147.
[106] ARIMA H, FUJITA T, YEN W T. Using nickel as a catalyst in ammonium thiosulfate leaching for gold extraction [J]. Materials Transactions, 2004, 45(2): 516-526.
[107] ARIMA H, FUJITA T, YEN W T. Gold recovery from nickel catalyzed ammonium thiosulfate solution by strongly basic anion exchange resin [J]. Materials Transactions, 2003, 44(10): 2099-2107.
[108] XU B, YANG Y B, LI Q, JIANG T, LI G H. Stage leaching of a complex polymetallic sulfide concentrate: Focus on the extraction of Ag and Au [J]. Hydrometallurgy, 2016, 159: 87-94.
[109] XU B, LI K, DONG Z L, YANG Y B, LI Q, JIANG T. Eco-friendly and economical gold extraction by nickel catalyzed ammoniacal thiosulfate leaching-resin adsorption recovery [J]. Journal of Cleaner Production, 2019, 233: 1475-1485.
[110] LIU X L, XU B, YANG Y B, LI Q, JIANG T, HE Y H. Thermodynamic analysis of ammoniacalthiosulphate leaching of gold catalysed by Co(III)/Co(II) using Eh-pH and speciation diagrams [J]. Hydrometallurgy, 2018, 178: 240-249.
[111] DONG Zhong-lin, JIANG Tao, XU Bin, ZHANG Bang-sheng, LIU Gui-qing, LI Qian, YANG Yong-bin. A systematic and comparative study of copper, nickel and cobalt-ammonia catalyzed thiosulfate processes for eco-friendly and efficient gold extraction from an oxide gold concentrate [J]. Separation and Purification Technology, 2021: 272: 118929.
[112] WANG Jian, XIE Feng, PAN Yue, WANG Wei. Leaching of gold with copper-citrate-thiosulfate solutions [J]. Mineral Processing and Extractive Metallurgy Review, 2021: 1-10.
[113] XIE Feng, WANG Jian, FU Yan, WANG Wei, LU Dian-kun CHANG Yong-feng. A kind of composite sulfur hydrochlorate catalytic citric acid-leaching of gold using thiosulfate technique: CN110205499(A), CN110205499(B) [P]. 2019. (in Chinese)
[114] CHEN Xia. Process of leaching precious metals: US9150942 B2 [P]. 2015.
[115] WANG Jian, PAN Yue. Copper-catalyzed glycine-thiosulfate composite gold leaching process: CN112961991A [P]. 2021-06-15. (In Chinese)
[116] CHEN Xia, YEN W T. Process for extracting gold from gold-bearing ore: US7985277[P]. 2011.
[117] YU Hong, ZI Fu-ting, HU Xian-zhi. The copper–ethanediamine–thiosulphate leaching of gold ore containing limonite with cetyltrimethyl ammonium bromide as the synergist [J]. Hydrometallurgy, 2014, 150: 178-183.
[118] CHEN Xia, YEN W T. Effect of lead ion and minerals on thiosulfate-gold leaching [C]//Hydrometallurgy 2008: 6th International of Symposium. Littleton, 2008: 760-768. (Proceedings or a chapter in an edited book)
[119] GUO L X, HU X Z, ZI F T. Substitution recovery of gold from copper-ethylenediamine-thiosulfate leaching solution with copper power [J]. Russian Journal of Non-Ferrous Metals, 2018, 59(3): 261-268.
[120] WANG Q, HU X Z, ZI F T, YANG P, CHEN Y L, CHEN S L. Environmentally friendly extraction of gold from refractory concentrate using a copper–ethylenediamine–thiosulfate solution [J]. Journal of Cleaner Production, 2019, 214: 860-872.
[121] NIE Y H, CHI H, ZI F T, HU X Z, YU H, HE S Q. The effect of cobalt and nickel ions on gold dissolution in a thiosulfate-ethylenediamine (en)-Cu2+ system [J]. Minerals Engineering, 2015, 83: 205-210.
[122] HEATH J A, JEFFREY M I, ZHANG H G, RUMBALL J A. Anaerobic thiosulfate leaching: Development of in situ gold leaching systems [J]. Minerals Engineering, 2008, 21(6): 424-433.
[123] ZHANG H, JEFFREY M. A study of pyrite catalysed oxidation of thiosulfate [C]//Hydrometallurgy 2008: Proceedings of the 6th International Symposium. Phoenix, 2008: 769-778.
[124] ZELINSKY A G, NOVGORODTSEVA O N. Effect of thiourea on the rate of anodic processes at gold and graphite electrodes in thiosulfate solutions [J]. ElectrochimicaActa, 2013, 109: 482-488.
[125] ZHANG H G, NICOL M J, STAUNTON W P. An electrochemical study of an alternative process for the leaching of gold in thiosulfate solutions [C]//Treatment of Gold Ores. Quebec: The Canadian Institute of Mining, Metallurgy and Petroleum, 2005: 243-257.
[126] ZHANG S C, NICOL M J. An electrochemical study of the dissolution of gold in thiosulfate solutions Part I: Alkaline solutions [J]. Journal of applied electrochemistry, 2003, 33(9): 767-775.
[127] YANG Y B, GAO W, XU B, LI Q, JIANG T. Study on oxygen pressure thiosulfate leaching of gold without the catalysis of copper and ammonia [J]. Hydrometallurgy, 2019, 187: 71-80.
[128] BARON J Y, CHOI Y, JEFFREY M. Double-refractory carbonaceous sulfidic gold ores. Gold Ore Processing [M]//Gold Ore Processing. Amsterdam: Elsevier. 2016: 909-918.
[129] LANGHANS J W Jr, LEI K P V Jr, CARNAHAN T G Jr. Copper-catalyzed thiosulfate leaching of low-grade gold ores [J]. Hydrometallurgy, 1992, 29(1/2/3): 191-203.
[130] AYLMORE M G. Treatment of a refractory gold—copper sulfide concentrate by copper ammoniacal thiosulfate leaching [J]. Minerals Engineering, 2001, 14(6): 615-637.
[131] WATLING K, HOPE G A, JEFFREY M I, WOODS R. Surface products on gold leached in ammoniacalcopper(II) thiosulfate solution [J]. ECS Transactions, 2006, 2(3): 121-132.
[132] ACGIH. 2015 TLVs? and BEIs?: Threshold limit values for chemical substances and physical agents and biological exposure indices [C]//American Conference of Governmental Industrial Hygienists. Cincinnati, 2015: 35.
[133] HARTWIG A. List of MAK and BAT values 2017: Permanent senate commission for the investigation of health hazards of chemical compounds in the work area. Report 53 [M]. Bonn: Deutsche Forschungsgemeinschaft, 2017: 19-166.
[134] LAN Wen-xing, WANG Wei, OU Jian-hua, HUANG Qiu-xia. Practical application study of occupational exposure limits for hazardous agents in workplace.Part 1: chemical hazardous agents (GBZ 2.1—2007) [J]. Technology & Development of Chemical Industry, 2014, 43(3): 58-62. (in Chinese)
[135] GOS S, RUBO A. The relevance of alternative lixiviants with regard to technical aspects, work safety and environmental safety [EB/OL]. 2001.
硫代硫酸盐提金技术综述
谢锋1,陈俊南1,王剑2,王伟1
1. 东北大学 冶金学院,沈阳 110004;
2. 江西理工大学 材料冶金化学学部,赣州 341000
摘 要:目前文献已报导研发多种非氰浸金试剂。因无毒、价格低廉、浸出率高,并且在处理含碳、含铜金矿石方面具有优异的性能,硫代硫酸盐被认为是最有发展潜力的非氰提金试剂。传统的铜-氨-硫代硫酸盐浸金体系得到广泛研究,但经过多年的工艺改进与发展,该浸出体系仍面临一些关键难题。为了降低硫代硫酸盐的消耗,研究者采用镍基、钴基和铁基催化剂替代铜,并进行一系列的工艺开发研究。针对无氨体系,研发包括氧-硫代硫酸盐、铜-硫代硫酸盐、铜-乙二胺-硫代硫酸盐、铁-乙二胺-硫代硫酸盐、铁-草酸-硫代硫酸盐等多种非氨-硫代硫酸盐浸金体系。作者系统总结目前以硫代硫酸盐为基础的浸金体系的基本理论和工艺发展现状,重点阐述无氨硫代硫酸盐浸金工艺的技术研究现状,并针对性详细介绍含氨基、羧基或羟基官能团的有机配体添加剂对硫代硫酸盐浸金的潜在影响。探讨加快硫代硫酸盐体系工业应用所面临的机遇和挑战。
关键词:硫代硫酸盐;金;浸出;添加剂
(Edited by Bing YANG)
Corresponding author:FengXIE, Tel: +86-24-83672298, E-mail: xief@smm.neu.edu.cn;
1003-6326/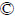 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
Abstract:Numerous non-cyanide leaching lixiviants have been developed, among which thiosulfate is considered the most promising alternative to cyanide due to its non-toxicity, low price, high leaching rate and excellent characteristics in dealing with carbonaceous and copper-bearing gold ores. The traditional copper-ammonia-thiosulfate system has been studied extensively.However, with many years of process development, there are still some problems and challenges with this gold leaching system. A series of studies using nickel-, cobalt- and ferric-based catalyst to substitute copper have been conducted with the purpose of reducing the consumption of thiosulfate. A variety of non-ammonia thiosulfate leaching systems including oxygen-thiosulfate, copper-thiosulfate, copper-EDA-thiosulfate, ferric-EDTA-thiosulfate, and ferric-oxalate-thiosulfate leaching systems have been also developed to eliminate the potential side-effect of ammonia. In this review, the basic theory and process development of some main gold leaching systems based on thiosulfate solutions were systematically summarized to illustrate the research status on thiosulfate leaching process. The potential effects of various additives such as organic ligands containing amino, carboxyl or hydroxy functional groups on gold thiosulfate leaching were described in detail. The potential opportunity and challenge for promoting the industrial development of thiosulfate-based gold leaching systems were also discussed.








 (40)
(40) (41)
(41)



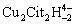 complex acquires electrons at the cathodic area of gold surface and is reduced to the Cu(S2O3) 35- complex (Eqs. (57)and (58)). The dissolved oxygen in the solution acts as the oxidant to re-oxidize Cu(S2O3)35-to
complex acquires electrons at the cathodic area of gold surface and is reduced to the Cu(S2O3) 35- complex (Eqs. (57)and (58)). The dissolved oxygen in the solution acts as the oxidant to re-oxidize Cu(S2O3)35-to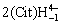 (57)
(57)
 ). In addition, the dissolved products of associated minerals are also important parts of the passivation substance on gold surface. It has been proved that the addition of Cu(I) [68], Cu(II) [69], ammonia [69,93,126,131], EDTA [101], thiourea[124], humic acid [48,98], ammonium alcohol polyvinyl phosphate [127] caneffectively hinder the formation of passivation layer and weaken or eliminate the passivation film during the gold leaching process in thiosulfate solutions. And the usage of additives is one of the major measures to remove the passivation on gold surface and improve the leaching kinetics.
). In addition, the dissolved products of associated minerals are also important parts of the passivation substance on gold surface. It has been proved that the addition of Cu(I) [68], Cu(II) [69], ammonia [69,93,126,131], EDTA [101], thiourea[124], humic acid [48,98], ammonium alcohol polyvinyl phosphate [127] caneffectively hinder the formation of passivation layer and weaken or eliminate the passivation film during the gold leaching process in thiosulfate solutions. And the usage of additives is one of the major measures to remove the passivation on gold surface and improve the leaching kinetics.

 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press