
Synthesis and emission analysis of novel rare earth complex Eu(TTA)3(2NH2-Phen)
L? Yu-guang(吕玉光)1,2, LI Gang(李 刚)1, SHI Chun-hui(石春卉)3, YU Lian-sheng(于连生)1,
LUAN Fang(栾 芳)1, ZHANG Fu-jun(张福俊)4
1. College of Pharmacy, Jiamusi University, Jiamusi 154007, China;
2. Provincial Key Laboratory of Biomaterials, College of Materials Science and Engineering,
Jiamusi University, Jiamusi 154007, China;
3. First Affiliated Hospital, Jiamusi University, Jiamusi 154002, China;
4. Key Laboratory of Luminescence and Optical Information, Beijing Jiaotong University, Beijing 100044, China
Received 13 July 2009; accepted 18 March 2010
Abstract: The rare earth ternary complex of Eu3+ with thenoyltrifluoroacetone, and 4, 7-2NH2 phenanthroline was synthesized and well characterized by UV, fluorescent, IR spectrometry and X-ray diffractometry (XRD) as well as elemental analysis. The results show that the complex of Eu(III) emits strong red luminescence when excited by UV light, and Eu(TTA)3(2NH2-Phen) has the higher sensitized luminescent efficiency and longer lifetime than Eu(TTA)3(Phen). In device of ITO/PVK/Eu(TTA)3(2NH2-Phen)/Al, the spectra of Eu(TTA)3(2NH2-Phen) with different ratios for spin-cast film were monitored. The main emitting peak at 614 nm can be attributed to the transition of 5D0→7F2 of Eu3+ and this process results in the enhancement of red emission from electroluminescence device. The effect and mechanism of the ligands on the luminescence properties of europium complex were discussed. The results show that the luminescence intensity of the title complexes greatly increases in comparison with that of their corresponding complexes, revealing that the second ligands form very good synergistic effect with the first ligands. The title complexes possess excellent thermal stability properties, and are hopefully developed into fine PL and EL red materials.
Key words: synthesis; ternary complex; luminescence; electroluminescence
1 Introduction
In recent years, rare earth complex fluorescence materials have generated much research interest in many fields such as materials science, chemistry, biological technology and information technology. Luminescent phosphors have attracted more attention in the development of different luminescent display systems[1-4]. Many rare earth complexes have been developed as the emitters in organic photo-luminescence and electroluminescence devices[5-9]. As some lanthanide ions, e.g. Eu3+ and Tb3+, possess good luminescence characteristics (high color purity) based on the transitions between the 4f energy levels, many compounds activated with Eu3+ and Tb3+ have been studied for practical application as laser materials. Many research groups have extensively studied various Eu(III) complexes for the purpose of achieving desirable luminescent properties[10-15]. However, the quantum efficiency of most of these lanthanide complexes is still low. This may be mostly due to inefficiency of the energy transfer, particularly, triplet–triplet transfer in these lanthanide complexes. One of the most important problems in this field is a selection of suitable ligands, which would provide high efficiency of emission of the metal ions
In this work, we have successfully synthesized complex Eu(TTA)3(2NH2-Phen) with remarkably sharp red emission bands. In PVK/Eu(TTA)3(2NH2-Phen) blend, the red emission of europium complex was enhanced and PVK emission was quenched. The results show that the complex of Eu (III) emits strong red luminescence when excited by UV light. The present study may be important and helpful for the development of red color rare earth display applications.
2 Experimental
2.1 Sample preparation
EuCL3?6H2O (1 mmol) and HTTA (3 mmol) were dissolved in 50 mL ethanol. pH value of the mixture was adjusted to 6-7 using triethylamine. Then, 4, 7-2NH2 phenanthroline in ethanol solution was added to the reaction mixture, and the molar ratio of 2NH2-Phen to Eu3+ ion was 1:1. The precipitate was filtered, washed with water and ethanol, dried at room temperature, and then stored in a silica-gel drier[16].
2.2 Measurements
Elemental analyses were performed on a Perkin- Elmer 240 C analytical instrument. X-ray diffraction (XRD) analysis was carried out by D/MAX2500VB2+/ PC(Cu Kα(λ=1.540 56 ?), V=40 kV, I=200.0 mA, λ=1.540 56 ?). Infrared spectra were recorded in the range of 4 000-400 cm-1 by a prostige-21IR spectro- photometer in KBr flake. UV-Vis spectra were performed on a UV-2501PCS double spectrophotometer. The excitation and emission spectra were recorded on a Shimadzu 5301 spectrofluorophotometer equipped with a 150 W xenon lamp as the excitation source. Spectra were recorded using monochromator with slit widths of 1.5 nm on both excitation and emission sides. Lifetimes were measured with a Spex 1934D phosphorimeter using a 450 W flash lamp as the excitation source (pulse width=3 ?s). The EL spectra were measured on a fluorolog-3 spectrophotometer (American SPEX Company). The luminance was measured by PR-650 spectra-scan spectrometer.
3 Results and discussion
3.1 IR spectrum analysis and XRD analysis
Infrared spectrum of Eu(TTA)3(2NH2-Phen) is shown in Fig1. Bands at 1 539, 2 438 and 730 cm-1 are corresponded to stretching vibration of —N=C, —N=O vibration, and rC—H vibration of 4,7-2NH2 phenanthroline, respectively. In addition, typical asymmetric vibration of the carbonyl group in HTTA is detected at about 1 605 and 1 549 cm-1. The peak at about 520 cm-1 reveals the presence of O→RE, which cannot be observed in the ligands. The elemental analysis data and the structure of the europium complex are shown in Table 1 and Fig.2. We conducted an investigation into samples by XRD. From the obtained grain size and complex XRD microstructural parameter, we found some patterns according to Scherrer formula: D=0.89λ/(βcosθ), for complex, grain size D=60.2 ?.
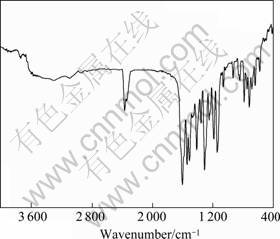
Fig.1 Infrared spectrum of complex Eu(TTA)3(2NH2-Phen)
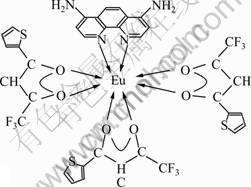
Fig.2 Structure of complex Eu(TTA)3(2NH2-Phen)
Table 1 Results of analytical data of complex (mass fraction, %)

3.2 UV absorption spectrum and fluorescence properties
UV spectrum of the complex Eu(TTA)3(2NH2-Phen) is shown in Fig.3. The complex exhibits absorption in the ultraviolet region with the maximal absorption of Eu(TTA)3(2NH2-Phen) at 275, 296 and 353 nm.
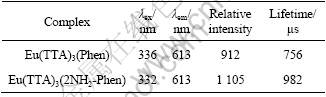
Fluorescence emission spectrum of the Eu(TTA)3(2NH2-Phen) complex is shown in Fig.4 (λex=332 nm). Five typical Eu3+ luminescence peaks appear at 583.0, 593.5, 614.0, 656.0 and 707.5 nm, which are due to 5D0→7F0, 5D0→7F1, 5D0→7F2, 5D0→7F3 and 5D0→7F4, respectively. As shown in Fig.4, the relative intensity of 5D0→7F2 is stronger than other luminescence emissions. Moreover, the emission peak positions of Eu(TTA)3(2NH2-Phen) indicate that there is a typical Eu3+ luminescence emission. In Table 2, the complex Eu(TTA)3(2NH2-Phen) also shows longer lifetime (about 982 ?s) than Eu(TTA)3(Phen) (about 756 ?s).
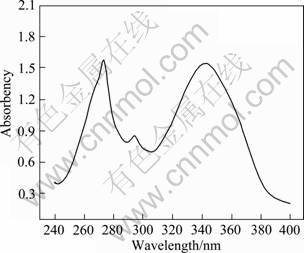
Fig.3 UV spectrum of complex Eu(TTA)3(2NH2-Phen)
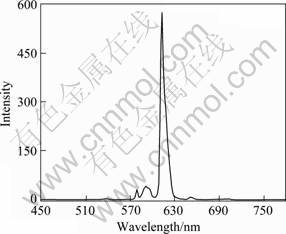
Fig.4 Typical emission spectrum of complex Eu(TTA)3(2NH2-Phen) excited at 332 nm
Table 2 Luminescence properties of rare earth complex
3.3 Electroluminescence
The device structure of the complex Eu(TTA)3(2NH2-Phen) was fabricated according to Refs.[17-18]. Poly(N-vinylcar-bazole) (PVK) was dissolved in 10 mg/mL chloroform. In order to improve the performance of Eu(TTA)3(2NH2-Phen) thin film, Eu(TTA)3(2NH2-Phen) was doped into PVK at mass ratio of 1:3. The PVK: Eu(TTA)3(2NH2-Phen) thin film was fabricated on the top of cleaned ITO coated glass substrate by spin-coating method. 2, 9-dimethyl-4, 7-diphenyl-1, 10-phenanthroline (BCP) and aluminum quinoline (Alq3) films were fabricated by thermal evaporation at a rate of about 0.3 ? /s under high vacuum of 266.644 μPa.
Fig.5 shows the electroluminescent spectrum of the complex Eu(TTA)3(2NH2-Phen) at a driving voltage of 13 V. The dependence of electroluminescence intensity on the driving voltage is obtained by using the time-base spectra. In the structural device, electro-luminescence intensity sharply increases when the driving voltage goes beyond 15 V. Furthermore, the enhancement of red emission in device of ITO/PVK/Eu(TTA)3(2NH2-Phen)/ Al is most likely due to the energy transfer enhancement from PVK and ligand to Eu3+. This process results in the enhancement of red emission from electro-luminescent device, which is made from metal complexes.
Fig.6 shows the power—voltage curves for the complex Eu(TTA)3(2NH2-Phen) structure devices at various driving voltages. The electron current in the Eu(TTA)3(2NH2-Phen) device sharply increases when the driving voltage goes beyond 12 V. It is found that the Eu(TTA)3(2NH2-Phen) structural device effectively improves the electro-luminescence intensity of lanthanide ions.
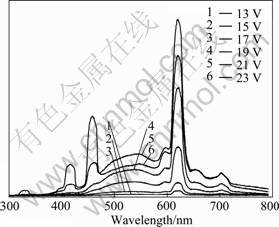
Fig.5 EL spectra of complex Eu(TTA) 3(2NH2-Phen) at various driving voltages
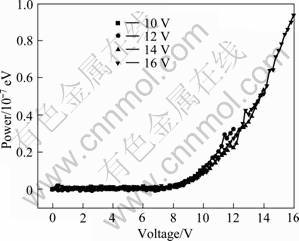
Fig.6 Power—voltage curves for complex Eu(TTA)3- (2NH2-Phen) at various driving voltages
4 Conclusions
1) By using a chemical coprecipitation method, the novel ternary complex Eu(TTA)3(2NH2-Phen) was successfully synthesized and characterized for photoluminescence and electro-luminescence properties.
2) Eu(TTA)3(2NH2-Phen) has higher sensitized luminescent efficiency and longer lifetime than Eu(TTA)3(Phen).
3) In PVK/Eu(TTA)3(2NH2-Phen) blend, the red emission of europium complex is enhanced and PVK emission is quenched. Results show that the complex of Eu (III) emits strong red luminescence when excited by UV light.
References
[1] KIDE J,HAYASE H, HONGAWA K. Bright red lightemitting organic electrolumine scent devices having a europium complex as an emitter [J]. Appl Phys Lett, 1994, 65: 2124-2128.
[2] WANG Qian-ming, YAN Bing, ZHANG Xin-hua. Photophysical properties of novel lanthanide complexes with long chain mono-eicosyl cis-butene dicarboxylate [J]. Photochemistry and Photobiology A: Chemistry, 2005, 174: 119-124.
[3] HASEGAWA M, ISHII A, KISHI S. Picosecond time-resolved luminescence of Pr (III) complexes: Intramolecular excitation energy transfer from ligand to Pr(III) [J]. Photochemistry and Photobiology A: Chemistry, 2006, 178: 220-224.
[4] HASEGAWA Y, KAWAI H, NAKAMURA K, YASUDA N, YANAGIDA S. Molecular design of luminescent Eu(III) complexes as lanthanide lasing material and their optical properties[J]. Journal of Alloys and Compounds, 2006, 408: 669-674.
[5] MURASE N, NAKAMOTO R, MATSUOKA J, TOMITA A. Role of Eu ions and defect centers in formation of high-temperature persistent spectral holes in glass [J]. Journal of Luminescence, 2004, 107: 256-260.
[6] ZHONG Guo-lun, WANG Yong-hong, WANG Cun-kuan, PU Bing- yin, FENG Yu, YANG Kong-zhang. Assemblies, characterization, and luminescent enhancement of organized molecular films based on rare earth complexes [J]. Journal of Luminescence, 2002, 99: 213-222.
[7] LV Yu-guang, ZHANG Jing-chang, CAO Wei-liang, ZHANG Fu-Jun, XU Zheng. Enhanced electroluminescence of Eu3+ by Tb3+in complexesTb1-xEux(TTA)3Dipy [J]. Journal of Luminescence, 2008, 128: 117-122.
[8] LIU Guo-cong, DUAN Xue-chen, LI Hai-bin, DONG Hui, ZHU Li-gang, LIANG Da-wen. Hydrothermal synthesis and luminescent properties of fishbone-like Eu3+-doped LaVO4 nanocrystals [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(1): 119-126. (in Chinese)
[9] HUANG Ming-chu, LIU Guo-cong, LI Hai-bin, YIN Zhi-min. Solvothermal synthesis and photoluminescence of CeO2?Eu3+ nanocrystals [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(2): 293-300. (in Chinese)
[10] GUAN Jun-bo, CHEN Biao, SUN You-yi, LIANG Hao. Effects of synergetic ligands on the thermal and radiative properties of Eu(TTA)3nL-doped poly(methyl metha -crylate) [J]. Journal of Non- Cryst Solids, 2005, 351: 849-855.
[11] QIAN Guo-dong, WANG Min-quan. In situ synthesis and photophysical properties of the Eu(TTA)3Dipy complex in vinyltriethoxysilane-derived gel glass [J]. Journal of Phys Chem Solids, 2002, 63: 1829-1834.
[12] LIU Yan, YE Chun-fang, QIAN Guo-dong, QIU Jian-rong. Enhanced lumen escence of Eu3+ by Gd3+ in ternary chelate doped in gel glasses via in situ technique [J]. Journal of Luminescence, 2006, 118: 158-164.
[13] NAKAMURA K, HASEGAWA Y, KAWAI H, YASUDA N, WADA Y, YANAGIDA S. High lasing oscillation efficiency of Eu (III) complexes having remarkably sharp emission band [J]. Journal of Alloys and Compounds, 2006, 408: 771-775.
[14] MANSEKI K, HASEGAWA Y, YUJI WADA Y, YANAGIDA S. Photophysical properties of tetranuclear Eu(III)complexes in polyphenylsilsesqioxane (PPSQ) [J]. Journal of Alloys and Compounds, 2006, 408: 805-808.
[15] GUO Dong-cai, WANG Xin, TAN Hui, WANG Li-ying, GOU Li-ning. Synthesis and properties of complexes of europium with phenanthroline derivatives and fatty acids [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(11): 2111-2117. (in Chinese)
[16] LV Yu-guang, ZHANG Jing-chang, CAO Wei-liang, ZHANG Fu-jun, XU Zheng. Synthesis and characteristics of a novel rare earth complex of Eu(TTA)2(N-HPA)Phen [J]. Photochemistry and Photobiology A: Chemistry, 2007, 188: 155-160.
[17] LV Yu-guang, LI Qiu-ping, SHI Chun-hui, LIU Hai-ran, LIU Feng-hua. Study of the luminescence properties of a novel rare earth complex Tb(DPC)22H2O [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2009, 74: 26-29.
[18] LV Yu-guang, SONG Chun-xiang, ZHANG Yu, SHA Jing-quan, LIU Cui-juan, ZHANG Fu-jun. Synthesis and electroluminescent property of ternary complexes Eu(TTA)3M [J]. Journal of Alloys and Compounds, 2010, 492: 259-263.
(Edited by LI Xiang-qun)
Foundation item: Project(B201015) supported by the Natural Science Foundation of Heilongjiang Province, China; Project(11551482) supported by the Scientific and Technical Research Project of Education Department of Heilongjiang Province, China; Projects(L2010-124, L2010-144) supported by the Research Fund for Jiamusi University, China; Project(E08050204) supported by the Research Fund for the Provincial Key Laboratory of Biomaterials Jiamusi University, China; Project(2009-360) supported by Health Commission of Heilongjiang Province, China; Project supported by Key Laboratory of Luminescence and Optical Information, Beijing Jiaotong University, China
Corresponding author: L? Yu guang; Tel: +86-454-8679535; E-mail: yuguanglv@163.com
DOI: 10.1016/S1003-6326(10)60651-6