
Preparation of ITO transparent conductive film by sol-gel method
LI Zhi-hua(李芝华)1, REN Dong-yan(任冬燕)2
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Department of Materials Engineering, Mian Yang Vocational and Technical College, Mianyang 621000, China
Received 6 March 2006; accepted 9 August 2006
Abstract: The ITO transparent conductive films were prepared on substrate of quartz glass by sol-gel method. The raw materials were nitrate indium, acetylacetone and the dopant of anhydrous chloride (SnCl4). The process from gel to crystalline film and the microstructure of the films were investigated by DTA-TG, XRD and SEM. The influence of preparation processes on the electricity performance of the films was also studied by four-probe apparatus. The results show that the crystallization process of ITO xerogel completes when the heat treatment temperature reaches 600 ℃. The ITO films possesses on vesicular structures accumulated by spherical particles, and both heat treatment temperature and cooling rate have important effects on the resistivity of ITO films.
Key words: ITO film; sol-gel process; resistivity; transparency
1 Introduction
Indium tin oxide(ITO) films are widely used as transparent conductive layers in a large variety of applications such as thin film transistor(TFT)[1,2], liquid crystal displayers(LCD)[3,4], smart mirrors for the windows, solar cells, electroluminescent devices[5,6], sensors and organic light emitting diodes(OLED)[7]. Several procedures have been developed for the preparation of ITO films, e.g. magnetron sputtering [8-10], activated reactive evaporation[11,12], chemical vapor deposition[13,14], and sol-gel method[15,16]. Recently, the sol-gel method attracts much attention for the advantage of low cost, controllable technique and the formation of large area films. The effects of rapid thermal annealing procedure for densification of sol-gel ITO thin films were well researched by CHICA et al[17]. The morphology, the optical and electrical properties of the ITO films fabricated by sol-gel procedure have been fully studied by STOICA et al[18].
The direct influence on electrical and optical performances of ITO transparent conductive films by doping concentration of Sn, heat-treat temperature and annealing velocity is usually studied at present, while the deep mechanism of influence on one aspect of ITO film’s performance by those factors is studied insufficiently. In this paper, the smaller resistivity and higher film density have been obtained by choosing proper sintering temperature, annealing velocity and withdraw technique. The structural, morphological, and especially electrical properties of ITO films were synthetically investigated by DTA-TG, XRD, SEM and four-probe apparatus. The aim of this paper is to elucidate the crystallization process of ITO films and the consequences of those factors such as heat-treat temperature, annealing velocity, withdraw technique and micro-morphology on electrical properties.
2 Experimental
In(NO3)3·5H2O crystals were prepared by dissolving the metallic indium into nitrate acid at first. Anhydrous indium nitrate (In(NO3)3) was mixed with acetylacetone and diethylenetriamine(DTA) according to the appropriate ratio, and then the mixture was heated at 60-65 ℃ for 3 h by stirring to make a solution and sol. Meanwhile, chloride (SnCl4) was dissolved in ethanol. The two mixtures above were mixed by stirring for 5-10 min. Then the ITO sol with various ratios could be obtained. In the present experiment, cleaned and dried quartz glass with the size of 30 mm × 20 mm × 3 mm was selected as substrate. The ITO film was obtained by the withdraw technique.
The phase transformation processes were investigated by PTC-1 type thermal analyzer. The phase and crystallization of the ITO films treated at different temperatures were analyzed by X-ray diffraction (SIMENS D500X). The resistivities of the films were measured by WS-1 four-probe apparatus. And the microstructures and morphology were studied by a KYKY-Amray2800 scanning electron microscope.
3 Results and discussion
3.1 Effect of treatment temperature on crystallization
Fig.1 shows the DTA/TG curves of the xerogel film. It can be seen from the TG curve that the mass of the xerogel film decreases obviously with the treatment temperature increasing to 530 ℃. At the same time, it can also be seen from the DTA analysis that an exothermic peak exists at 335.37 ℃ for the decomposing of organic framework in the gel, and an exothermic peak locates at 483.05 ℃ for the oxidation of carbon. An exothermic peak at 578.21 ℃ can also be found, but the mass does not change at this temperature. It is proposed that the exothermic peak results from the transformation of In2O3 to polycrystalline cubic bixbyite structure. The mass of the ITO xerogel almost keeps a constant at the temperature higher than 530 ℃.
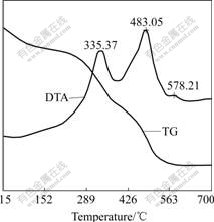
Fig.1 DTA/TG analysis of xerogel ITO powder
Fig.2 shows the XRD pattern after the xerogel powder is heated at 600 ℃ for 1 h. The xerogel powder has sharp structural XRD peaks after heated at 600 ℃, indicating that the crystallized grains become obviously coarse, and the crystalline structure is formed. By comparing the XRD results with the JDPDs data base, the heated powder has the single phase with the structure of bixbyite phase as that of In2O3, indicating that the dopant of SnO2 is dissolved into In2O3 and a homogenous solution forms after the xerogel is heated at 600 ℃.
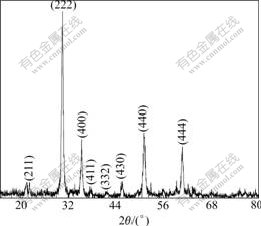
Fig.2 XRD pattern of xerogel ITO powder heated at 600 ℃ for 1 h
3.2 Effects of treatment temperature on conductivity
Fig.3 shows the resistivity of the ITO films heated at the temperatures from 200 ℃ to 800 ℃. It can be seen that the conductivity of the ITO film increases with the treatment temperature up to 600 ℃. There are two reasons for the results: one is that the higher treatment temperatures enhance the crystallization of ITO films, resulting in the increase of the ionic-transference rate; the other is that the formation possibility of the oxygen-ionic vacancies increases with the temperature, resulting in the increase of the conductivity.
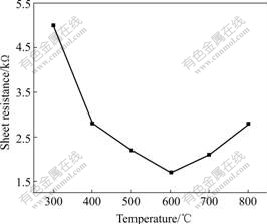
Fig.3 Effects of treatment temperature on resistivity of ITO film
However, the conductivity decreases with the increasing temperature when the treatment temperature is higher than 600 ℃. Because SnO2 may be reduced as SnO, i.e. the valence of the Sn ion transforms from +4 to +2 at higher temperatures, and then the ionic conductivity decreases. On the other hand, the high temperatures make In2O3 decompose into InO, and also reduce the conductivity.
3.3 Effect of cooling rate on conductivity
Fig.4 shows the effect of various cooling rates on the conductivity of ITO films. Curve 1 in Fig.4 indicates the resistivities of the ITO films which are withdrawn for 5 times and then slowly heated to 600 ℃ and cooled in furnace. Curve 2 indicates the resistivities of the ITO films which are withdrawn for 5 times and then slowly heated to 600 ℃ and cooled in air. It can be seen that the resistivities of the films cooled in air are about half lower than that cooled in furnace.
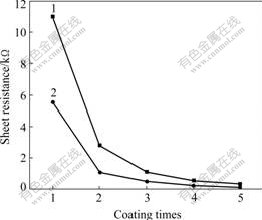
Fig.4 Effects of various cooling rates on resistivity of ITO films
This may be related to the influence of cooling rates on the distribution of the oxygen vacancies in the films, because the oxygen vacancies may be formed when the oxygen atoms run out from the films at high treatment temperatures. The concentration of the anion vacancies increases and the electron concentration increases, resulting in the conductivity of the film increased.
3.4 Effects of withdraw technique on ITO-film conductivity
Fig.5 shows the influence of the withdraw times on the resistivity of the ITO films on quartz-glass substrate at the withdrawing rate of 8 cm/min. It can be seen that the resistivity of the films decreases with the increasing withdraw times. The relationship between the resistivity of the film and the withdraw times is not linear. The decreased resistivity results predominantly from the first coating, because the first layer is formed by the sol coated on substrate. However, the followed coating is formed on the first one and the sol has better wetting ability on the coating than that on the substrate, resulting in that the followed coating has much higher density and lower resistivity.
Table 1 presents the effect of withdraw rate on the resistivity of ITO films formed on general glass substrate after once withdrawing. Analysed with Fig.5, the withdraw rates between 3 cm/min and 21 cm/min almost don’t affect the conductivity of the films, and the resistivity changes for the film thickness for the various withdraw rates. One possible reason for that can be described as following: when the viscosity of sol is small enough, the influence of the withdraw rates on the film thickness is small, and the film thickness depends predominantly on the wetting ability between the sol and substrate.
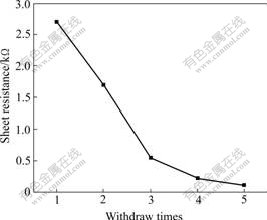
Fig.5 Effects of withdraw times on resistivity of ITO film
Table 1 Effects of withdraw rates on resistivity

3.5 Effects of microstructure on conductivity
Figs.6 and 7 display the surface morphologies of ITO films on quartz which are heated at 600 ℃ for 1 h by slow cooling and quick cooling, respectively. The ITO film by sol-gel technology is porous and consists of spheroidic particles. The spherical particle has the less size than 100 nm. The pores homogenously distribute among the particles. The pores dispersed inside the film may affect the conductivity. In order to increase the conductivity of the ITO film prepared by sol-gel method,it is necessary to increase the film density by some useful treatment to improve the microstructure of the film.
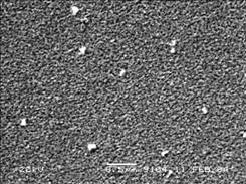
Fig.6 Surface morphology of ITO films under low cooling rate

Fig.7 Surface morphology of ITO films under high cooling rate
For the same substrate, the size of particle and pores can be decreased obviously and the density can be improved by quick cooling, as shown in Fig.7. At the same time, it can also be found that the conductivity can also be improved as shown in Fig.4. Other treatment technologies can also improve the ITO-film micro- structure, e.g., the higher treatment temperature and longer treatment time are helpful for the diffusion, resulting in higher density and conductivity. However, the decomposition of In2O3 might take place during ITO film treated at higher treatment temperatures and with longer treatment time, and affect the conductivity.
4 Conclusions
1) The withdrawn ITO xerogel films crystallize during heat-treatment and the crystallization process finishes when the treatment temperature raises to 600 ℃.
2) The resistivities of ITO films decrease with the increase of the treatment temperature and the resistivity becomes minimum at the treatment temperature of 600 ℃. The quick cooling makes the resistivity obviously decrease.
3) The conductivity increases with the withdrawing times. The withdrawing rate does not obviously affect the conductivity of the ITO films when the withdrawing rates are between 3 cm/min and 21 cm/min.
4) The SEM investigation of the ITO films reveals that the ITO films prepared by sol-gel technique consist of spherical particles with pores. The density affects intensively the film’s conductivity. The density and conductivity of the ITO films can be increased obviously by quick cooling.
References
[1] PYO S W, KIM Y M, KIM J H, SHIM J H, JUNG L Y, KIM Y K. An organic electrophosphorescent device driven by all-organic thin-film transistor using photoacryl as a gate insulator [J]. Current Applied Physics, 2002, 2(5): 417-419.
[2] IL R J, JIN C Y, KEUN W I, RYU J I, CHOI Y J, WOO I K, LIM B C, JANG J. High performance a-Si TFT with ITO/n+ ohmic layer using a Ni-silicide [J]. Journal of Non-Crystalline Solids, Part B, 2000, 266/269: 1310-1314.
[3] SON K S, CHOI D L, LEE H N, LEE W G. The interfacial reaction between ITO and silicon nitride deposited by PECVD in fringe field switching device [J]. Current Applied Physics, 2002, 2(3): 229-232.
[4] AMARAL A, CARVALHO C N, BROGUEIRA P, LAVAREDA G, MELO L V, GODINHO M H. ITO properties on anisotropic flexible transparent cellulosic substrates under different stress conditions [J]. Materials Science and Engineering B, 2005, 118(1-3): 183-186.
[5] ISHIBASHI K, WATABE K, SAKURAI T, OKADA O, HOSOKAWA N. Large area deposition of ITO films by cluster type sputtering system [J]. Journal of Non-Crystalline Solids, 1997, 218: 354-359.
[6] ALAM M J, CAMERON D C. Investigation of annealing effects on sol-gel deposited indium tin oxide thin films in different atmospheres [J]. Thin Solid Films, 2002, 420/421: 76-82.
[7] HATTON R A, DAY S R, CHESTERS M A, WILLIS M R. Organic electroluminescent devices: enhanced carrier injection using an organosilane self assembled monolayer (SAM) derivatized ITO electrode [J]. Thin Solid Films, 2001, 394(1-2): 291-296.
[8] MA H, CHO J S, PARK C H. A study of indium tin oxide thin film deposited at low temperature using facing target sputtering system [J]. Surface and Coatings Technology, 2002, 153(2-3): 131-137.
[9] KIM D, KIM S J. AFM observation of ITO thin films deposited on polycarbonate substrates by sputter type negative metal ion source [J]]. Surface and Coatings Technology, 2003, 176(1): 23-29.
[10] ZHANG K R, ZHU F R, HUAN C, WEE A. Indium tin oxide films prepared by radio frequency magnetron sputtering method at a low processing temperature [J]. Thin Solid Films, 2000, 376(1-2): 255-263.
[11] TADATSUGU M, SATOSHI I, TOSHIHIRO M. High rate deposition of transparent conducting oxide thin films by vacuum arc plasma evaporation [J]. Thin Solid Films, 2002, 416(1-2): 92-96.
[12] MA J, ZHANG D H, ZHAO J Q, TAN C Y, YANG T L, MA H L. Preparation and characterization of ITO films deposited on polyimide by reactive evaporation at low temperature [J]. Applied Surface Science, 1999, 151(3-4): 239-243.
[13] CHANDRASEKHAR R, CHOY K L. Innovative and cost-effective synthesis of indium tin oxide films [J]. Thin Solid Films, 2001, 398/399: 59-64.
[14] EDERTH J, HESZLER P, HULTAKER A, NIKLASSON G A, GRANQVIST C G. Indium tin oxide films made from nanoparticles: models for the optical and electrical properties [J]. Thin Solid Films, 2003, 445(2): 199-206.
[15] JIAO Z, WU M H, GU J Z. The gas sensing characteristics of ITO thin film prepared by sol-gel method [J]. Sensors and Actuators B: Chemical, 2003, 94(2): 216-221.
[16] STOICA T F, GARTNER M, LOSURDO M, TEODORESCU V, BLANCHIN M, STOICA T, ZAHARESCU M. Spectroellipsometric study of the sol–gel nanocrystalline ITO multilayer films [J]. Thin Solid Films, 2004, 455/456: 509-512.
[17] GHICA C, CANUT B, BLANCHIN M G, ROGER J A, OUESLTI M, BESSA?S B. Rapid thermal annealing procedure for densification of sol-gel indium tin oxide thin films [J]. Crystal Engineering, 2002, 5(3-4): 187-193.
[18] STOICA T F, GARTNER M, LOSURDO M, ZAHARESCU M. Morphology, structure and optical properties of sol–gel ITO thin films [J]. Materials Science and Engineering B, 2203, 101(1-3): 222-226.
(Edited by LI Xiang-qun)
Corresponding author: LI Zhi-hua; Tel: +86-731-8830838; E-mail: ligfz@mail.csu.edu.cn