硫化铜矿SAG(半自磨)磨矿废品浮选药剂的交互作用
来源期刊:中国有色金属学报(英文版)2021年第3期
论文作者:Ali AHMADI Mojtaba REZAEI Seyed Mohammad SADEGHIEH
文章页码:792 - 806
Key words:flotation; copper sulphide; reagent; interaction effect; reject ore
摘 要:浮选药剂在黏土矿中基础矿物的选矿中表现出复杂的行为。利用中心组合设计法研究浮选药剂对低品位高黏质硫化物矿中铜浮选效率的交互作用。初步结果表明,在石灰作pH调节剂的情况下,异丙基黄原酸钠(SIPX)和O-异丙基-N-乙基硫基氨基甲酸酯(IPETC)是最有效的捕收剂。在不同的pH值下,评估捕收剂(SIPX和IPETC)用量和起泡剂甲基异丁基甲醇(MIBC)用量对分离效率的影响。基于方差分析(ANOVA)结果,对于分离效率而言,捕收剂用量与pH值以及捕收剂用量与起泡剂用量之间的交互作用显著。在低捕收剂用量时,pH值从9提高到11可使IPETC的铜分离效率从81%提高到86%,SIPX的铜分离效率从77%提高到86%。方差分析结果表明,在pH值11、8.6 g/t SIPX、7 g/t IPETC和20 g/t MIBC的条件下,铜分离效率最高(88.7%)。最终,可得出结论,捕收剂SIPX和IPETC更适合于处理高黏质硫化矿。
Abstract: Flotation reagents have a complex behaviour in the beneficiation of base minerals in clayey ores. Interaction effects of reagents on the efficiency of copper flotation for a highly clayey low-grade sulphide ore were investigated using a central composite design. Preliminary results showed that sodium-isopropyl-xanthate (SIPX) and O-isopropyl-N-ethyl-thionocarbamate (IPETC) were found to be the most efficient collectors in the presence of lime as the pH regulator. The effects of dosage of collectors (SIPX and IPETC) and the dosage of methyl-isobutyl-carbonyl (MIBC) as frother on the separation efficiency were evaluated at different pH levels. Based on the analysis of variance (ANOVA), the interaction effects of the collector-pH and collector-frother were significant for the separation efficiency. At the low level of collector dosage, increasing pH from 9 to 11 enhanced copper separation efficiency from 81% to 86% for IPETC and from 77% to 86% for SIPX. Results of ANOVA showed that the maximum copper separation efficiency (88.7%) was obtained at the dosages of 8.6 g/t SIPX, 7 g/t IPETC and 20 g/t MIBC at pH 11. Finally, it was concluded that a mixture of SIPX and IPETC collectors was more suitable to treat highly clayey sulphide ores.
Trans. Nonferrous Met. Soc. China 31(2021) 792-806
Ali AHMADI, Mojtaba REZAEI, Seyed Mohammad SADEGHIEH
Department of Mining Engineering, Isfahan University of Technology, Isfahan 84156-83111, Iran
Received 25 March 2020; accepted 15 January 2021
Abstract: Flotation reagents have a complex behaviour in the beneficiation of base minerals in clayey ores. Interaction effects of reagents on the efficiency of copper flotation for a highly clayey low-grade sulphide ore were investigated using a central composite design. Preliminary results showed that sodium-isopropyl-xanthate (SIPX) and O-isopropyl-N-ethyl-thionocarbamate (IPETC) were found to be the most efficient collectors in the presence of lime as the pH regulator. The effects of dosage of collectors (SIPX and IPETC) and the dosage of methyl-isobutyl-carbonyl (MIBC) as frother on the separation efficiency were evaluated at different pH levels. Based on the analysis of variance (ANOVA), the interaction effects of the collector-pH and collector-frother were significant for the separation efficiency. At the low level of collector dosage, increasing pH from 9 to 11 enhanced copper separation efficiency from 81% to 86% for IPETC and from 77% to 86% for SIPX. Results of ANOVA showed that the maximum copper separation efficiency (88.7%) was obtained at the dosages of 8.6 g/t SIPX, 7 g/t IPETC and 20 g/t MIBC at pH 11. Finally, it was concluded that a mixture of SIPX and IPETC collectors was more suitable to treat highly clayey sulphide ores.
Key words: flotation; copper sulphide; reagent; interaction effect; reject ore
1 Introduction
Chalcopyrite as the most important copper sulphide mineral, is usually separated from gangue minerals such as pyrite by the froth flotation process [1,2]. Clayey contamination may significantly affect the beneficiation of copper sulphide resources. They present cation exchange interaction with many inorganic and organic ions in solution, affecting the performance of additives used to control the flotation systems [3]. Flotation is a complex and multivariate physicochemical separation method which might be influenced by various chemical, physical and operational factors.
Sulfhydryl collectors such as xanthate, dixanthogen, dithiophosphate and dithiocarbamate are considered as the effective collectors for treating copper sulphide and oxide ores [4,5]. The xanthate collectors such as potassium amyl xanthate (PAX) and sodium isopropyl xanthate (SIPX) are industrially used due to their low cost and wide application especially for copper sulphide [6]. Other thiol collectors such as thionocarbamates (TC), dithiophosphate (DTP) and dithiocarbamates (DTC) are applied to improving the separation efficiency and the frothing properties in the presence of xanthates [6].
Several studies carried out to evaluate various collectors on flotation efficiency have indicated that xanthates and thionocarbamates could significantly affect the copper recovery through the floatation process [7,8]. PEREZ-GARIBAY et al [9] reported that at high concentration of SIPX, different optimal bubble size distributions could be reached, enhancing copper recovery.
It has also been reported that using the mixture of collectors might have synergistic effects, leading to a reduction in reagents consumption, as a result of which the separation efficiency and recovery of coarse particles could be enhanced [10-13]. FINCH and SMITH [10] indicated that the surface tension of mixed surfactants in the flotation process was lower compared to the condition where each surfactant was used individually. HELBIG et al [14] investigated the synergistic effect between potassium n-butyl xanthate (PNBX) and a dithiocarbamate type collector for pyrite floatation. The results showed that using a mixture of these collectors could improve copper grade and recovery, increasing bubble loadings and improving froth characteristics. The mixture of thionocarbomate and mercapto benzothiazole (MBT) in the presence of SIPX presented the highest copper recovery.
The stability of bubbles is adjusted by using different type of frothers including surface-active and surface-inactive frothers [8,15]. The methyle- isobutyl-carbonyl (MIBC) is considered as one of the common aliphatic frothers with highly branched chains which indicates a high frothing ability and stability of the complexes [16]. Note that some collectors such as IPETC have a frothing capability [6].
Controlling pH is an essential key factor in separating valuable minerals in the presence of xanthates [11]. HUANG et al [12] stated that while increasing pH (above 10), butyl xanthate and butyl amine dithiophosphate could present a good hydrophobic characteristic in the copper flotation. The flotation rate and recovery of copper-bearing sulphide minerals were decreased by increasing pH [7,8]. Thionocarbomate and thiourea collectors separated chalcopyrite from pyrite better than xanthates at high pH values [13]. It was also stated that increasing pH from 8 to 13 decreased pyrite recovery in the presence of four different collectors namely, SIPX, sodium isobutyl xanthate (SIBX), sodium ethyl xanthate (NaEX) and potassium amyl xanthate (KAX), whereas chalcopyrite recovery was independent of pH level and xanthate collector type [17].
The synergetic interactions among common flotation reagents have been widely investigated over the past several decades [9,11,13,18-20]. PH and frother type are considered as the main key parameters, affecting collector adsorption and surface tension [21]. The mixture of amphipathic agents can affect surface pressure, molecular complex at the interface of monolayer film on the surface, surface potential, viscosity and rigidity [18,22].
The SAG mill reject of Sarcheshmeh copper concentrator consists of a low-grade ore with a high grindability index about 18-20 kW·h/t, obtained from the overflow of the mill discharge screen. Sometimes it is transferred to another copper concentrator such as Bavanat copper processing plant (Fars province, Iran) to produce a copper flotation concentrate. In addition to hardness, such an ore has a complex mineralogy, containing a higher level of clayey minerals. By increasing the mineralogical complexity of ores and decreasing grade and grain size of the valuable minerals, there is a continuous need for more selective and efficient reagents such as collectors, frothers and pH regulators. These reagents are applied to creating favourable conditions for the separation of the valuable minerals from gangues. Identifying the main variables, and considering interactions among various reagents are important in the optimization of a froth flotation system. Despite the complicated chemistry of various reagents applied in complex sulphide flotation such as highly clayey ores, a few researches have been evaluated the interaction effects among them. Hence, this work was conducted with the aim of improving the copper grade and recovery from a highly clayey low-grade copper sulphide ore obtained from Sarcheshmeh grinding circuit reject (Kerman, Iran). In this regard, the effects of three different collectors (including O-isopropyl-N-ethyl-thionocarbamate (IPETC), SIPX and PAX), pH (from 9 to 11) and frother dosage on the copper separation efficiency were investigated. The interaction among collectors, pH and frother were statistically evaluated using a central composite design.
2 Experimental
2.1 Materials
2.1.1 Ore
The samples used in this study were obtained from the rejected portion of the SAG Mill of Sarcheshmeh copper concentrator (Kerman Province, Iran). A representative sample was obtained from the overflow of the SAG mill discharge screen. It contained a low-grade ore with a particle size of >5 mm. In order to characterize the ore, the representative sample was subjected to different chemical and mineralogical analyses. Chemical analysis by X-ray fluorescence (XRF) (model: Varian 735) (Table 1) showed that the sample contained 62.17% SiO2, 12.88% Al2O3, 3.75% SO3 and 0.41% Cu (determined by inductively coupled plasma-optical emission spectrometry (ICP-OES) (model: Varian 735)). The contents of gold and silver, analyzed by the fire assay method (SGS’s FAI313) and ICP-MS, were 3.1×10-8 and 2.1×10-6, respectively. These precious metals were not considered economic and were not investigated in this study. X-ray diffraction analysis (XRD) (Philips model D6792) of the sample showed that the sample contained chalcopyrite as the main valuable mineral in association with quartz and some gangue clayey minerals such as chlorite, albite and muscovite (Table 2 and Fig. 1). In order to determine the liberation degree of chalcopyrite, the mineralogical investigations on polished section prepared from a mixture of sample and molding powder (with a mass ratio of 1:10) were conducted by grain counting method using an optical microscope (model: Olympus BX53F). The results showed that the size of the most of the chalcopyrite grains (84%) was below 90 μm.
2.1.2 Reagents
SIPX (90% purity in the form of pellet), IPETC (95% purity as liquid) and PAX (90% purity in the form of pellet) were used as flotation collectors. MIBC (99% purity as liquid) was used as the frother. The chemical structure of the used reagents in the flotation system is shown in Fig. 2 [22-24]. The pH of the pulp was adjusted using lime milk, sodium carbonate (Na2CO3) (99.9% purity) and sodium hydroxide (NaOH) (98% purity). In some tests, CMC (98% purity as a powder) and A3-3 (Na2SiO3:Na2S2O5:Al2(SO3)3) were used as depressants of pyrite and clayey minerals. Tap water was used throughout the experiments. All reagents used in batch flotation tests were of industrial grade.
2.2 Experimental and modelling procedures
The SAG mill reject sample was firstly crushed to below 2 mm by laboratory roll and gyradisc crushers. For each flotation test, 650 g was ground to 80% passing 74 μm in a laboratory stainless steel ball mill with 9 mm steel balls at a pulp density of 67% (w/v). The mill discharge was then transferred to a 2.5 L Denver flotation cell at the pulp density of 25% (w/v). After that, the desired amounts of reagents were added to the slurry while agitating at 1200 r/min and the slurry was conditioned for 5 min. During this conditioning step, collectors and the frother were added to the pulp. After 2 min, air was fed and the froth was collected for 8 min (32 min in one-factor experiments). The concentrates were collected after cumulative times of 0.5, 2, 4 and 8 min (and 16 and 32 min for one-factor experiments). The concentrates and tailings were filtered, dried, weighed, sampled and assayed for copper.
2.2.1 One-factor flotation experiments
The one-factor experiments were conducted to determine the type of collectors and pH regulators. Three types of collectors namely PAX, IPETC and SIPX, and three types of pH regulators including lime, Na2CO3 and NaOH with MIBC as frother were applied. pH was adjusted to be 11. These experiments were performed at the collector dosage of 10 and 5 g/t MIBC as frother. The froth was collected by hand scraping after starting the air flow injection.
2.2.2 Statistical design of flotation experiments
The effects of several parameters including the dosage of SIPX and IPETC collectors, the dosage of frother and pH level on the copper separation efficiency were investigated. The experimental range and levels of independent variables for the separation efficiency are given in Table 3.
Table 1 Chemical composition of representative sample ore using X-ray fluorescence (XRF) (wt.%)

Table 2 Mineralogical composition of representative sample ore using XRD (wt.%)

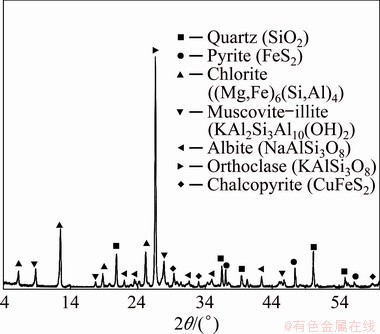
Fig. 1 XRD pattern of representative sample of copper ore
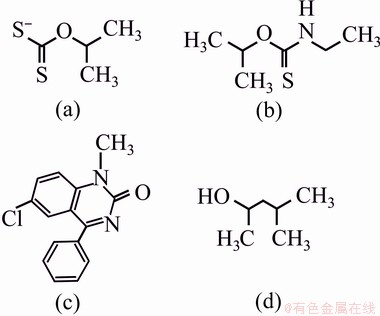
Fig. 2 Chemical structures of SIPX (a), IPETC (b), PAX (c) and MIBC (d)
Table 3 Level of variables in center composite design (CCD)
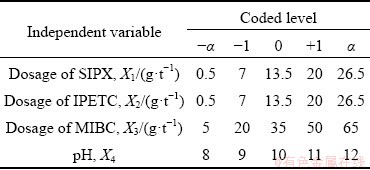
Response surface methodology (RSM) and the central composite design (CCD) were applied to investigating the interaction and optimizing the variables with a minimum number of experiments without the need for studying all possible combinations experimentally. The CCD method allows estimating the second degree polynomial relationships between the independent and dependent variables and gives information about interaction between them. To simplify the calculations and to uniform the comparison, variables were studied with their codified values [25]. Each independent factor was coded at five levels namely -α, -1, 0, +1 and α.
The independent parameters (Table 3) used for the optimization were coded in accordance with the following equation:
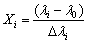 (1)
(1)
where Xi is the dimensionless coded value of each independent variable, λi is the corresponding actual value, λ0 is the value of λi at the center point, and △λi is the step change value [20,26]. In order to measure these values, five different levels were defined as follows:
1: maximum dosage of reagent or pH;
-1: minimum dosage of reagent or pH;
0: average of minimum and maximum dosages of reagent or pH;
-α: the difference between 0 and 1 subtracted from the minimum level;
α: the difference between 0 and 1 added to the maximum level.
The experimental results were analyzed using Design Expert (DX7), and a regression quadratic polynomial equation was proposed as follows:
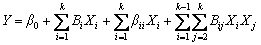 (2)
(2)
where Y is the predicted response, Xi and Xj are the input variables, β0 is the intercept term, βi is the linear effects, βii is the squared effect, and βij is the interaction term [27]. The validity of the equation was analyzed by using ANOVA (analysis-of- variance), and the fitting quality of the equation was judged from the coefficients of correlation and P-value [28].
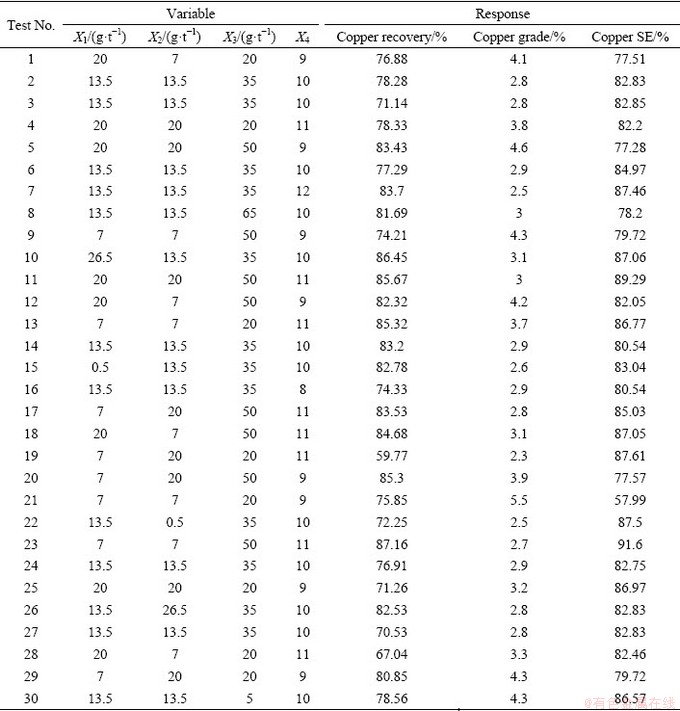
A set of 30 experimental runs, including duplicates, were designed using CCD (Table 4). The influence of the dosage of two collectors (SIPX and IPETC) and a frother (MIBC) at different levels of pulp pH were investigated in a batch mode. The experimental data were fitted on linear, interactive, quadratic and cubic models to predict the behaviour of the investigated parameters and the separation efficiency. The experimental conditions and their responses were presented in Table 4. A quadratic polynomial equation was determined to predict the behaviour of the investigated parameters and the separation efficiency. The coefficients of the model were estimated by multiple regression analysis. The fitting quality of the model was judged from their coefficients of correlation and regression.
Table 4 Experimental design of flotation tests and obtained results
Separation efficiency (SE) which is usually used to evaluate the flotation efficiency can be estimated for copper flotation according to Eq. (3). It is a combined index where both concentrate grade and metal recovery are considered:
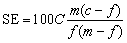 (3)
(3)
where C is the mass ratio of concentrate to feed, c and f are the grades of copper in the concentrate and the feed, respectively, and m is the metal content in the pure mineral.
In order to evaluate the efficiency of the one-factor flotation experiments and to identify the effective variables, a classical first-order flotation kinetic model was utilized which estimates the infinite copper recovery and kinetics rate by the following equation [14,29,30]:
R=R∞[1-exp(1-Kt)] (4)
where R is the copper recovery after time t, R∞ is the infinite copper recovery, K is the first order rate constant, and t is flotation time.
3 Results and discussion
3.1 One-factor experiments: Effect of collector type and pH regulator
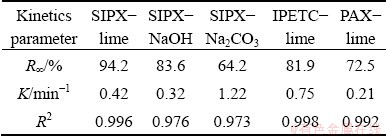
In order to investigate the influence of the type of collector and pH regulator on the copper flotation efficiency, some experiments were performed using three collectors namely PAX, SIPX and IPETC, and three pH regulators (pH value of 11) namely lime, Na2CO3 and NaOH. The diagrams of time-recovery and grade-recovery are plotted in Figs. 3 and 4, respectively. From Fig. 3(b) and 4(b), it could be concluded that maximum copper recovery occurs at minimum concentrate grade. As can be seen in Fig. 3 and Table 5, maximum copper recovery (94.2%) was obtained in the presence of lime as pH regulator and SIPX as collector where copper grade was 4.16%. However, in the presence of sodium carbonate, maximum flotation rate constant (1.22 min-1) and minimum recovery (64.2%), as well as maximum copper grade (4.77%), were achieved. Considering the related higher copper recovery and easier accessibility of lime as well as its lower price, lime was chosen as the more suitable pH regulator for the next experiments.
Figure 4 shows that copper recovery, after 35 min, in the presence of SIPX, IPETC and PAX reached 96%, 84% and 75%, respectively. The copper grade in the presence of SIPX, IPETC and PAX were also 4.2%, 6.0% and 3.6%, respectively. Among the collectors, PAX presented the lowest infinite recovery (72.5%) and kinetics rate (0.21 min-1). As can be seen in Table 5, IPETC in comparison with other collectors presented the highest kinetics rate (0.75 min-1), whereas SIPX demonstrated the highest copper recovery (94.2%).
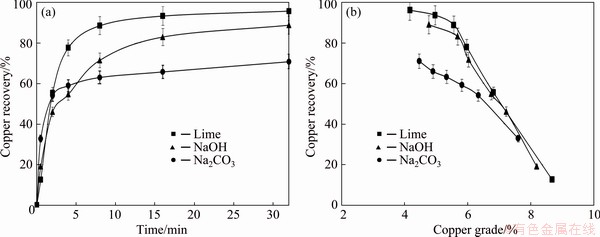
Fig. 3 Effect of pH regulator type on recovery-time (a) and recovery-grade (b) of concentrate in the presence of SIPX collector
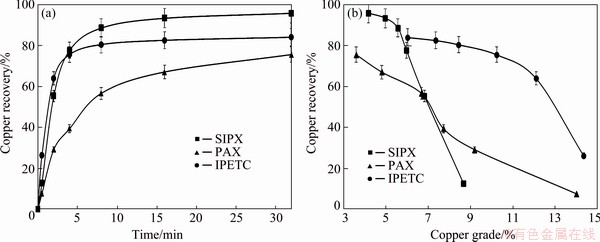
Fig. 4 Effect of collector type on recovery-time (a) and recovery-grade (b) of concentrate in the presence of lime as pH regulator
Table 5 Copper flotation kinetics variables of preliminary experiments using first-order kinetics model
Thus, the mixture of SIPX and IPETC as collectors and lime as pH regulator were utilized to evaluate the interaction influence of effective variables on the separation efficiency using a central composite design. It has also been reported that IPETC led to better results in water recovery and nickel grade compared to PAX [6]. Furthermore, the infinite copper recovery and kinetics rate of the experiments are given in Table 5. Xanthates and thionocarbamates are mainly considered as the anionic collectors [31]. The IPETC collector precipitates as copper-IPETC complex on the mineral surface and increases selectivity against pyrite according to the following equation [32]:
Cu+IPETC→Cu(IPETC′)+H++e (5)
where IPETC' is IPETC without hydrogen [32]. The adsorption of the anionic collector on the mineral surface is related to the anionic part of the collector, whereas the hydrophobic parts change the hydrophobicity [23]. The main chelating agents in the adsorption of xanthates and thionocarbamates on the copper sulphide minerals are xanthogen and thionocarbamate [6]. The ionic collectors are utilized to increase selective hydrophobicity, and nonionic collectors are developed to improve froth stability and interaction with the ionic reagents at the interface [33]. In the mixed collector systems, the stronger collector can be adsorbed on the fragile site of the mineral surface, which could make a great opportunity to adsorb the second collector on the appropriate sites [7,34].
3.2 Multi-factor experiments
A centeral composite design was used to evaluate the interaction effects among four chemical factors including SIPX dosage, IPETC dosage, MIBC dosage and pH level.
3.2.1 Model analysis
Response surface methodology (RSM) was used to optimize the investigated variables and determine the best-fitted regression model for the copper separation efficiency. The predicted model was given by the following equations:
SE=0.82+0.011X1-0.012X2+0.021X3+0.017X4-
0.0095X1X3-0.024X1X4-0.027 X2X3-
0.018X2X4+0.012 X1X2X3+0.014X1X2X4+
0.021X1X3X4+0.021X2X3X4+0.024X12X2+
0.039X12X32+0.028X12X42 (6)
Considering the separation efficiency, the results of the analysis of variance (ANOVA) are tabulated in Table 6. The Fisher variation ratio (F-value) is a statistical criterion of how well the variables describe the variation in the mean of data [35,36]. These results demonstrate that the model is significant with a confidence level of more than 99% at a calculated F-value of 16.59 with a very low probability (P-value <0.0001). It needs to be noted that the effect of the parameters and their interactions are significant with the significance level of 95% when their P-values are less than 0.05 [37]. The P-value for lack of fit is 0.196 which is not significant even with a confidence level of 90%.
Table 6 Significance of regression coefficients for separation efficiency
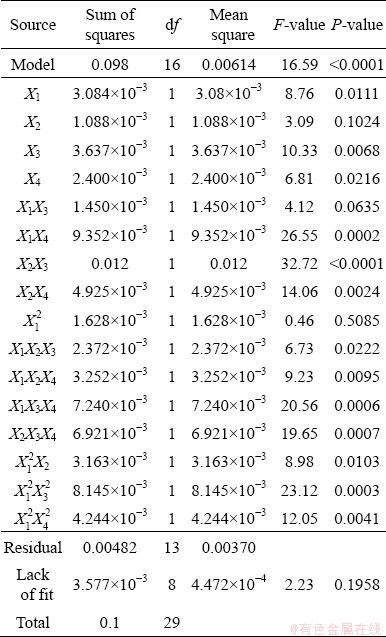
The accuracy and quality parameters of the fitted model are listed in Table 7. R2 and adjusted R2 were estimated to be 0.955 and 0.90 which emphasizes the precision of the fitted model. This coefficient indicates how much variability in the observed response values can be explained by the experimental factors and their interactions. The small values of R2 represent the less pertinence of the dependent variables in the model [38]. The adequate precision of the fitted model was 23.7 which needs to be more than 4 in the desirable models.
The plots of the fitted-equation were used to determine the individual effects and interactions of the investigated parameters on the copper separation efficiency. As can be seen in Eq. (5) and Table 6, the interaction effects of IPETC-SIPX, SIPX-MIBC and MIBC-pH were eliminated from the fitted model to reach better description due to their high P-values. Furthermore, Eq. (5) indicated that SIPX and IPETC have a significant interaction at the high values of SIPX (X12X2), whereas there is no significant interaction between IPETC and SIPX at the lower dosage of SIPX. The results demonstrated that the maximum copper recovery (87.2%) and separation efficiency (91.6%) as well as minimum copper grade (2.7%) were achieved at SIPX and IPETC dosages of 7 g/t, MIBC dosage of 50 g/t and pH of 11 (Run No. 23 in Table 4). Moreover, the minimum copper recovery (75.9%) and separation efficiency (58%), as well as the maximum copper grade (5.5%), were obtained at SIPX and IPETC dosages of 7 g/t, MIBC dosage of 20 g/t and pH value of 9 (Run No. 21). Among the main investigated parameters, MIBC dosage and pH level are significant with the confidence level of higher than 95%.
3.2.2 Interaction effect of collectors and frother
(1) Interaction of SIPX and MIBC
The results shown in Table 6 indicate a significant interaction between SIPX collector and MIBC frother with a confidence level of 90% (P-value of 0.063). The response surface plot for the copper separation efficiency (Fig. 5) was obtained by varying SIPX dosage from 7 to 21 g/t and MIBC dosage from 20 to 50 g/t at pH value of 10 and IPETC dosage of 13.5 g/t. The minimum separation efficiency (80%) was obtained at low dosages of SIPX and MIBC, which may be related to the production of larger bubbles and lower coating degree of particles by the collectors. At a low dosage of SIPX (below 9 g/t), increasing MIBC dosage from 20 to 50 g/t increased copper separation efficiency from 80% to 84%, which could be related to the generation of finer bubbles and higher gas hold-up. At the high dosage of SIPX, MIBC dosage had not a significant effect on the separation efficiency; however, at the low dosage of MIBC, increasing SIPX dosage from 7 to 19 g/t enhanced separation efficiency from 80% to 84%. Moreover, at the high amount of MIBC, the highest separation efficiency was obtained at a high and low dosage of SIPX. It can be concluded from Eq. (5) that this interaction has a negative influence on the copper separation efficiency. It has been reported [18] that molecular interaction between collector and frother has an important role in attaching air bubbles to the particles coated by collectors. The interaction effect between collector and frother was interpreted by linear and lamellar theories. The linear theory describes the particle attachment by penetration through the air-bubble film, while lamellar theory describes it without penetration [15]. The linear theory would be more appropriate due to high local concentration and hydrophilic properties of the surface [18]. The mutual adsorption of frother and collector on the particle surface indicated better results in the metal recovery, which could affect surface tension and increase collector role in frothing [16]. Generally, high dosage of frother produces highly mobile and relatively deep froth beds which affect the flotation performance [39]. Some researchers indicated that certain frothers have the ability to compensate for recovery deficiencies especially when xanthates are used [7,40]. WIESE and HARRIS [41] resulted that an increase in the frother dosage enhanced the recovery of valuable minerals. It was also reported that frother molecules accumulated at the mineral surface and facilitated the mineral-bubble attachment [14]. It has also been noted that collector addition decreased gas hold-up even at low dosage especially once the anionic frother was utilised, whereas increasing frother dosage enhanced it obviously [15]. It was reported that the air dispersion depended greatly on the frother, which caused the increase of gas hold-up and finer bubble generation [15].
Table 7 Accuracy parameters of fitted model using central composite design

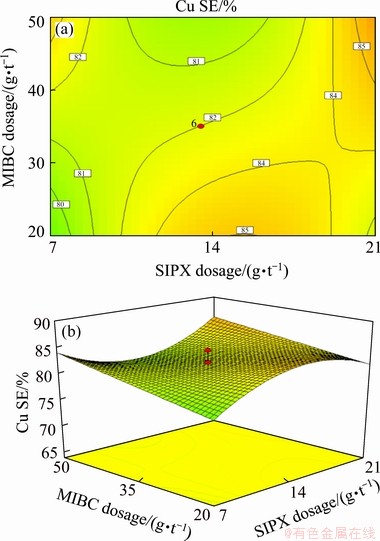
Fig. 5 Contour (a) and 3D (d) diagrams of interaction effect of MIBC and SIPX on copper separation efficiency at IPETC dosage of 13.5 g/t and pH of 10
(2) Interaction of IPETC and MIBC
The statistical analysis of ANOVA for copper separation efficiency (Table 6 and Eq. (5)) showed a negative interaction effect between IPETC collector and MIBC frother with a confidence level of 99% (P-value <0.0001). Figure 6 shows a response surface plot where the copper separation efficiency was affected by varying MIBC dosage from 20 to 50 g/t and IPETC dosage from 7 to 21 g/t. The highest copper separation efficiency (84%) occurred at the high dosage of IPETC, and the low dosage of MIBC, while the lowest copper separation efficiency (78%) was obtained at high dosages of IPETC and MIBC. It can be found that at the low dosage of IPETC (below 10 g/t), increasing MIBC dosage from 20 to 50 g/t had no significant influence on the copper separation efficiency. At the high dosage of IPETC (higher than 14 g/t), the separation efficiency was decreased from 84% to 78% by increasing MIBC from 20 to 50 g/t. This phenomenon could be resulted from enhancing the floatability of gangue minerals and decreasing selectivity at high dosage of MIBC which could decrease the grade of valuable minerals. It has also been reported [6] that thionocarbamates and dithiophosphate had a frothing ability which might affect the size and stability of air bubbles.
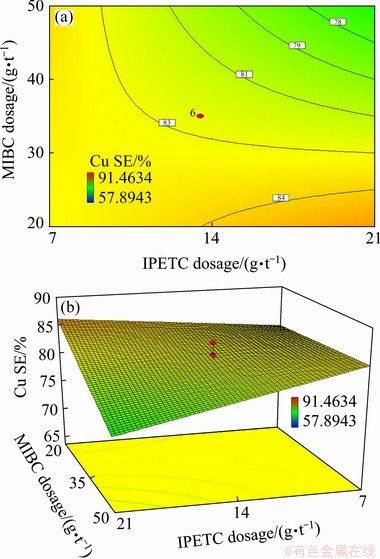
Fig. 6 Contour (a) and 3D (b) diagrams of interaction effect of MIBC and IPETC on copper separation efficiency at SIPX dosage of 13.5 g/t and pH of 10
(3) Interaction of SIPX-IPETC and MIBC
As can be found in Table 6, the interaction between the dosage of IPETC, SIPX and MIBC was significant with a confidence level of higher than 95% (P-value of 0.022). Figure 7 shows a block diagram which presents the influence of the dosage of the collectors (SIPX and IPETC) and the frother (MIBC) simultaneously on the copper separation efficiency. It can be seen that the maximum copper separation efficiency (87%) was reached at the high dosage of MIBC (50 g/t) and low dosage of collectors, whereas the minimum separation efficiency (74%) was gained at the low dosages of MIBC and collectors. It can also be noted that the interaction between collectors and frother has a positive effect on the separation efficiency (Eq. (5)).
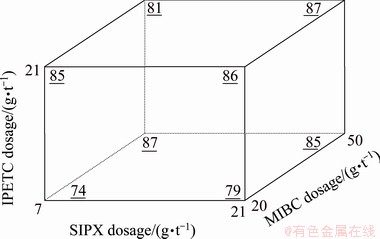
Fig. 7 Simultaneous mutual effect of IPETC, SIPX and MIBC on separation efficiency of copper (underlined values) at pH 10
3.2.3 Interaction effect of collectors and pH
(1) Interaction of SIPX and pH
The results of ANOVA analysis presented in Table 6 show that the dosage of the SIPX collector and the pH level had a significant negative effect on the copper separation efficiency with a confidence level of higher than 95% (P-value of 0.0002). Figure 8 represented the effect of SIPX dosage and pH on the separation efficiency. It can be seen that at the low pH levels (below 9), the efficiency was enhanced from 77% to 84% by increasing SIPX dosage from 7 to 21 g/t. However, at the high level of pH (above 10.5), the highest efficiency (86%) was achieved at both the lowest and the highest dosages of SIPX corresponding to about 7 and 21 g/L, respectively. At the low dosage of SIPX, separation efficiency was enhanced from 77% to 86% with increasing the pH level from 9 to 11. The interaction between collector dosage and pH has been previously investigated in several studies [11,26,42]. It was found that the collector hydrolysis was affected by alkaline pH which could increase the hydrophobicity of some minerals such as silicate [2]. The thionocarbamate collectors presented better copper selectivity against pyrite compared to the xanthates at alkaline pH values [43]. The dissolution and frothing ability of some frothers are improved at alkaline pH [16]. It has also been reported that the stability of foam bubbles was enhanced by increasing pH from 6 to 11, which could be affected by the hydration of frother and electrostatic charge on bubbles [16]. It was reported that pyrite flotation efficiency decreased with increasing pH from 5 to 12 due to the formation of hydrophilic metal hydroxides on the sulfide mineral surface [8,11]. GOKTEPE [17] also attributed the increasing effect of high pH values on the copper separation efficiency to the depression of pyrite mainly as a result of xanthate decomposition and metal-hydroxides formation on the mineral surface. It should be noted that the pulp pH affects the hydration of solid surfaces, excess of load density, the Zeta potential and the ionisation or solubility of surface agents [44]. The level of pH determines the proportion of xanthate and dixanthogen in copper sulphide flotation [45].
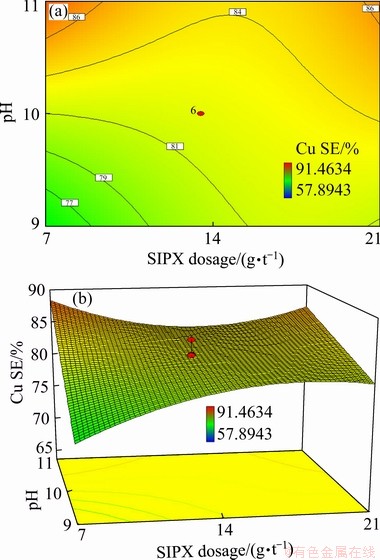
Fig. 8 Contour (a) and 3D (b) diagrams of interaction effect of SIPX and pH on copper separation efficiency in MIBC and IPETC dosages of 35 and 13.5 g/t, respectively
(2) Interaction of IPETC and pH
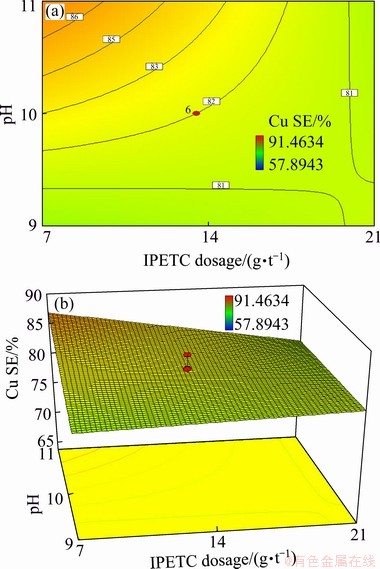
Fig. 9 Contour (a) and 3D (b) diagrams of interaction effect of IPETC and pH on separation efficiency in MIBC and SIPX dosage of 35 and 13.5 g/t, respectively
The dosage of IPETC collector and the pH level presented an obvious interaction on the copper separation efficiency with a confidence level of 99% (P-value of 0.0024) (Table 5 and Fig. 9). It can be seen that at the low level of IPETC dosage, increasing pH from 9 to 11 led to enhancing the copper separation efficiency from 81% to 86%. Varying pH at the high dosage of IPETC had no significant effect on the separation efficiency. The results indicated that at high levels of pH (above 10.5), the separation efficiency was decreased significantly from 86% to 81% by increasing IPETC from 7 to 21 g/t. However, at low levels of pH (below 9.5), increasing the IPETC dosage had no obvious effect on the copper separation efficiency. Thereby, high copper separation efficiency at a lower dosage of IPETC could be attributed to the selectivity improvement by decreasing pyrite floatability at alkaline pH. It was pointed out that at alkaline pH levels (above 8) low dosage of IPETC had no significant effect on the pyrite floatability, whereas chalcopyrite flotation indicated better results due to its natural floatability [46]. At higher pH values, chemical adsorption of IPETC on pyrite was replaced by its physical adsorption on the mineral surface, which caused the decrease of pyrite floatability [46]. It was also found that IPETC caused the lowest copper recovery and foam half-life in contrast to the other thionocarbamates [47]. Moreover, high dosage of IPETC could increase pyrite floatability as well as decrease foam half-life and height, which might reduce the copper separation efficiency [47].
(3) Interaction of SIPX-IPETC and pH
The results of ANOVA (Table 6) indicated a significant interaction between the collectors and pH with a confidence level of 99% (P-value of 0.0095). The block diagram shown in Fig. 10 is plotted to investigate the simultaneous effect of the dosage of collectors (SIPX and IPETC) and pH on the copper separation efficiency at MIBC dosage of 35 g/t. The results showed that the highest copper separation efficiency (90%) was achieved at a low dosage of collectors and the high level of pH (11), while the lowest copper separation efficiency (70%) was obtained at a low dosage of collectors and the low level of pH (9). As can be seen from Eq. (5), the interaction of these variables indicates a positive effect on copper separation efficiency. Among these variables, pH plays an important role in the separation efficiency. It needs to be noted that low levels of collector dosage and high level of pH can enhance the selectivity of the valuable copper- bearing minerals such as chalcopyrite and depress the gangue minerals such as pyrite.
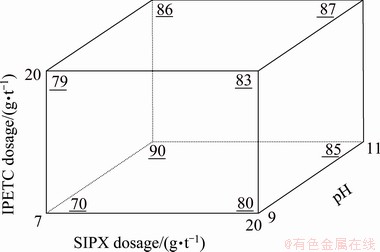
Fig. 10 Simultaneous mutual effect of IPETC, SIPX and pH on separation efficiency of copper (underlined values) at MIBC dosage of 35 g/t
3.2.4 Interaction of IPETC and SIPX
As can be seen in Table 6, IPETC and SIPX had no significant interaction, whereas these collectors indicated a significant interaction (X12X2) at high values of SIPX with a confidence level of 99% (P-value of 0.0103). The interaction effect of SIPX and IPETC dosage on the copper separation efficiency at pH value of 10 is shown in Fig. 11. The results indicate that high separation efficiency (85%) occurred at high dosage of SIPX and IPETC (20 g/t) and lower dosage of MIBC (35 g/t). It has also been reported that using a mixture of collectors could increase surface adsorption, which increases hydrophobicity and copper recovery [1]. The composition of the monolayers formed from the mixed collectors on the mineral surface depends on the collector dosage [33]. In the single collector system, the selectivity was generally decreased by increasing the collector dosage, while it was increased in the mixed collector systems without decreasing recovery due to increasing the hydrophobicity and alkyl chain interaction between the collectors [33,48,49]. It was evident that long hydrocarbon chains present in the collector structure increased the time of flotation compared to the collector with shorter hydrocarbon chain, which could positively affect the solubility of the collector [6]. The xanthogen and metal xanthate formation are considered as the main problem of utilisation of xanthate collectors, which caused in instability and low selectivity [13]. It was also reported that some additional reagents should be utilised to increase the selectivity of xanthates [43].
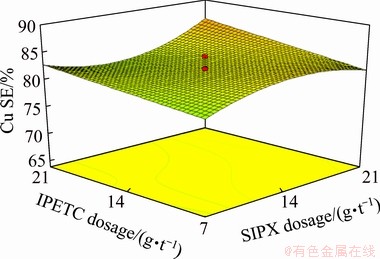
Fig. 11 Simultaneous mutual effect of IPETC and SIPX dosage on separation efficiency of copper at pH 10 and MIBC dosage of 35 g/t
3.3 Optimization
The maximum copper separation efficiency in a multiple-factor study occurs where all parameters simultaneously meet the desirable criteria. This could be determined and visualised graphically by superimposing the contours of the response surfaces in an overlay plot. Figure 12 presents the graphical optimizations which display the area of feasible response values in the factor. According to Figs. 12 and 13, in the multi-factor study, the maximum copper separation efficiency (88.7%) was found to be at SIPX dosage of 8.6 g/t, IPETC dosage of 7 g/t, MIBC dosage of 20 g/t and the solution pH of 11. Under this condition, the desirability and copper grade reached 0.655 and 3.4%, respectively. On the other hand, in one-factor experiments, a higher copper recovery (96%) and grade (4.2%) were achieved at the pH of 11 (with lime) and the MIBC and SIPX dosages of 5 g/t and 10 g/t, respectively. The main reason for this higher efficiency could be related to the lower dosage of the frother (5 g/t in one-factor experiments and 20-50 g/t for multiple- factor experiments).
In order to verify the predicting of the model for the output variable (separation efficiency), an additional flotation experiment was carried out under the optimum condition. Results showed an acceptable repeatability, in which the separation efficiency was 91.1%. The error between the predicted and experimental values was less than 3%. It can be concluded that the model can well predict the output variable.
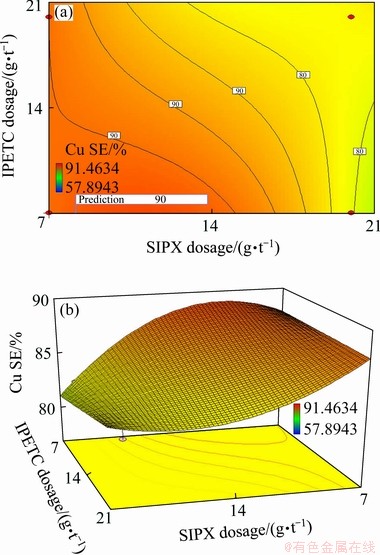
Fig. 12 Contour (a) and 3D (b) graphical optimization for separation efficiency in MIBC dosage of 20 g/t at pH 11
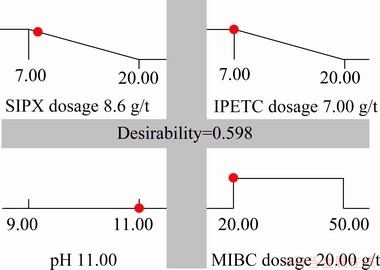
Fig. 13 Desirability ramp for numerical optimization of four independent parameters and responses
4 Conclusions
(1) Results of one-factor experiments showed that IPETC compared with PAX and SIPX collectors presented the highest kinetics rate (0.75 min-1), whereas SIPX demonstrated the highest copper recovery (94.2%). Thus, the mixture of SIPX and IPETC as collectors and lime as pH regulator were utilised to evaluate the interaction of the effective variables on the copper separation efficiency using a central composite design.
(2) The results of ANOVA indicated that SIPX dosage, MIBC dosage and pH were the most effective parameters with a positive effect on the copper separation efficiency.
(3) The interaction effect between the collectors and the frother indicated that at the low dosage of SIPX (7 g/t), increasing MIBC dosage from 20 to 50 g/t enhanced the separation efficiency from 80% to 83%; while, at the high dosage of IPETC dosage (21 g/t) increasing the MIBC dosage decreased separation efficiency from 84% to 78%.
(4) The interaction between collectors and pH level showed that at the low level of IPETC dosage, increasing pH from 9 to 11 led to enhancing the copper separation efficiency from 81% to 86%. Furthermore, at the low dosage of SIPX, separation efficiency was enhanced from 77% to 86% with increasing the pH level from 9 to 11.
(5) The optimal conditions of flotation for copper efficiency, in the multi-factor experiments, were determined to be at SIPX dosage of 8.6 g/t, the IPETC dosage of 7 g/t, the MIBC dosage of 20 g/t and the pH of 11.
Acknowledgments
The authors would like to thank the assistance of Bavanat Copper Concentrator Company in providing the ore and analyzing the samples.
References
[1] WILLS B A, NAPIER-MUNN T. WILLS’ Mineral Processing Technology: An introduction to the practical aspects of ore and mineral recovery [M]. 7th Ed. Butterworth-Heinemann, 2006.
[2] FUERSTENAU D W, FUERSTENAU M C. The flotation of oxide and silicate minerals, Principles of flotation [M]. Johannesburg: South African Institute of Mining and Metallurgy, 1982, 109-158.
[3] ACKERMAN P K, HARRIS G H, KLIMPEL R R, APLAN F F. Evaluation of flotation collectors for copper sulfides and pyrite. I: Common sulfhydryl collectors [J]. International Journal of Mineral Processing, 1987, 21: 105-127.
[4] VAZIFEH Y, JORJANI E, BAGHERIAN A. Optimization of reagent dosages for copper flotation using statistical technique [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(12): 2371-2378.
[5] LIU S, LIU G Y, HUANG Y G, ZHONG H. Hydrophobic intensification flotation: Comparison of collector containing two minerophilic groups with conventional collectors [J]. Transactions of Nonferrous Metals Society of China, 2020, 30(9): 2536-2546.
[6] MAREE W, KLOPPERS L, HANGONE G, OYEKOLA O. The effects of mixtures of potassium amyl xanthate (PAX) and isopropyl ethyl thionocarbamate (IPETC) collectors on grade and recovery in the froth flotation of a nickel sulfide ore [J]. South African Journal of Chemical Engineering, 2017, 24: 116-121.
[7] BRADSHAW D J, O'CONNOR C T. The flotation of pyrite using mixtures of dithiocarbamates and other thiol collectors [J]. Minerals Engineering, 1994, 7: 681-690.
[8] QUINN J J, KRACHT W, GOMEZ C O, GAGNON C, FINCH J A. Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties [J]. Minerals Engineering, 2007, 20: 1296-1302.
[9] PEREZ-GARIBAY R, RAMIREZ-AGUILERA N, BOUCHARD J, RUBIO J. Froth flotation of sphalerite: Collector concentration, gas dispersion and particle size effects [J]. Minerals Engineering, 2014, 57: 72-78.
[10] FINCH J A, SMITH G W. Dynamic surface tension of alkaline dodecylamine acetate solutions in oxide flotation [J]. Transactions of the Institution of Mining and Metallurgy, 1972, 81: 213-215.
[11] SHEN W Z, FORNASIERO D, RALSTON J. Effect of collectors, conditioning pH and gases in the separation of sphalerite from pyrite [J]. Minerals Engineering, 1998, 11: 145-158.
[12] HUANG H J, ZHU H F, HU Y H. Hydrophobic-surface of copper from converter slag in the flotation system [J]. International Journal of Mining Science and Technology, 2013, 23: 613-617.
[13] FAIRTHORNE G, FORNASIERO D, RALSTON J. Interaction of thionocarbamate and thiourea collectors with sulphide minerals: A flotation and adsorption study [J]. International Journal of Mineral Processing, 1997, 50: 227-242.
[14] HELBIG C, BRADSHAW D T, HARRIS P J, O′CONNOR C T, BALDAUF H. Synergistic interactions between reagents in sulphide flotation [J]. Journal of the Southern African Institute of Mining and Metallurgy, 1998, 98: 189-193.
[15] EL-SHALL H, ABDEL-KHALEK N A, SVORONOS S. Collector–frother interaction in column flotation of Florida phosphate [J]. International Journal of Mineral Processing, 2000, 58: 187-199.
[16] SOMASUNDARAN P, WANG D Z. Solution chemistry: Minerals and reagents [M]. Elsevier, 2006.
[17] GOKTEPE F. Effect of pH on pulp potential and sulphide mineral flotation [J]. Turkish Journal of Engineering and Environmental Sciences, 2002, 26: 309-318.
[18] LEJA J, SCHULMAN J H. Flotation theory: Molecular interactions between frothers and collectors at solid–liquid– air interfaces [J]. Trans AIME, 1954, 199: 221-228.
[19] HOPE G A, WOODS R, BOYD S E, WATLING K. A SERS spectroelectrochemical investigation of the interaction of butylethoxycarbonylthiourea with copper surfaces [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004, 234: 129-137.
[20] ZHENG Z M, HU Q L, HAO J F, GUO N N, SUN Y, LIU D H. Statistical optimization of culture conditions for 1, 3-propanediol by Klebsiella pneumoniae AC 15 via central composite design [J]. Bioresource Technology, 2008, 99: 1052-1056.
[21] CELIK A G, CAKAL G D. Characterization of espey colemanite and variation of its physical properties with temperature [J]. Physicochemical Problems of Mineral Processing, 2016, 52: 182-192.
[22] BECHER P. Surface chemistry of froth flotation [M]. New York: Plenum Press, 1982.
[23] DARLING P. SME mining engineering handbook [M]. Society for Mining, Metallurgy, and Exploration (SME), 2011: 1255-1270.
[24] HARRIS G H. Xanthates [M]. Kirk-Othmer Encyclopedia of Chemical Technology, 2000.
[25] SIMATE G S, NDLOVU S, GERICKE M. Bacterial leaching of nickel laterites using chemolithotrophic microorganisms: Process optimisation using response surface methodology and central composite rotatable design [J]. Hydrometallurgy, 2009, 98: 241-246.
[26] ASLAN N. Application of response surface methodology and central composite rotatable design for modeling and optimization of a multi-gravity separator for chromite concentration [J]. Powder Technology, 2008, 185: 80-86.
[27] PREETHA B, VIRUTHAGIRI T. Application of response surface methodology for the biosorption of copper using Rhizopus arrhizus [J]. Journal of Hazardous Materials, 2007, 143: 506-510.
[28] HAN C Y, PU H P, LI H Y, DENG L, HUANG S, HE S F, LUO Y M. The optimization of As(V) removal over mesoporous alumina by using response surface methodology and adsorption mechanism [J]. Journal of Hazardous Materials, 2013, 254-255: 301-309.
[29] AHMED N, JAMESON G J. Flotation kinetics [J]. Mineral Processing and Extractive Metallurgy Review, 1989, 5: 77-99.
[30] POLAT M, CHANDER S. First-order flotation kinetics models and methods for estimation of the true distribution of flotation rate constants [J]. International Journal of Mineral Processing, 2000, 58: 145-166.
[31] GRAY P M J, BOWYER G J, CASTLE J F, VAUGHAN D J, WARNER N A. Sulphide deposits—Their origin and processing [M]. Springer, 2012.
[32] WOODS R, HOPE G A. A SERS spectroelectrochemical investigation of the interaction of O-isopropyl-N- ethylthionocarbamate with copper surfaces [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 146: 63-74.
[33] RAO K H, FORSSBERG K. Mixed collector systems in flotation [J]. International Journal of Mineral Processing, 1997, 51: 67-79.
[34] HELBIG C, BRADSHAW D, HARRIS P, O'CONNOR C, BALDAUF H. The synergistic interactions of mixtures of thiol collectors in the flotation of sulphide minerals [C]//11th International Mineral Processing Congress. Elsevier, 2000: B8b1-B8b8.
[35] LIU H L, CHIOU Y R. Optimal decolorization efficiency of reactive red 239 by UV/TiO2 photocatalytic process coupled with response surface methodology [J]. Chemical Engineering Journal, 2005, 112: 173-179.
[36] IMANDI S B, BANDARU V V R, SOMALANKA S R, BANDARU S R, GARAPATI H R. Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste [J]. Bioresource Technology, 2008, 99: 4445-4450.
[37] DEMIM S, DROUICHE N, AOUABED A, BENAYAD T, COUDERCHET M, SEMSARI S. Study of heavy metal removal from heavy metal mixture using the CCD method [J]. Journal of Industrial and Engineering Chemistry, 2014, 20: 512-520.
[38] HAMZAOUI A H, JAMOUSSI B, MNIF A. Lithium recovery from highly concentrated solutions: Response surface methodology (RSM) process parameters optimization [J]. Hydrometallurgy, 2008, 90: 1-7.
[39] SMAR V D, KLIMPEL R R, APLAN F F. Evaluation of chemical and operational variables for the flotation of a copper ore. Part I—Collector concentration, frother concentration, and air flow rate [J]. International Journal of Mineral Processing, 1994, 42: 225-240.
[40] FARROKHPAY S. The significance of froth stability in mineral flotation—A review [J]. Advances in Colloid and Interface Science, 2011, 166: 1-7.
[41] WIESE J, HARRIS P. The effect of frother type and dosage on flotation performance in the presence of high depressant concentrations [J]. Minerals Engineering, 2012, 36: 204-210.
[42] MA X, BRUCKARD W J, HOLMES R. Effect of collector, pH and ionic strength on the cationic flotation of kaolinite [J]. International Journal of Mineral Processing, 2009, 93: 54-58.
[43] LIU G Y, ZHONG H, DAI T G. The separation of Cu/Fe sulfide minerals at slightly alkaline conditions by using ethoxycarbonyl thionocarbamates as collectors: Theory and practice [J]. Minerals Engineering, 2006, 19:1380-1384.
[44] EJTEMAEI M, IRANNAJAD M, GHARABAGHI M. Influence of important factors on flotation of zinc oxide mineral using cationic, anionic and mixed (cationic/anionic) collectors [J]. Minerals Engineering, 2011, 24: 1402-1408.
[45] LOTTER N O, BRADSHAW D J. The formulation and use of mixed collectors in sulphide flotation [J]. Minerals Engineering, 2010, 23: 945-951.
[46] BASILIO C I. Fundamental studies of thionocarbamate interactions with sulfide minerals [D]. Virginia Polytechnic Institute and State University, 1989.
[47] ZHAO G, PENG J, ZHONG H, WANG S, LIU G Y. Synthesis of novel ether thionocarbamates and study on their flotation performance for chalcopyrite [J]. Minerals, 2016, 6: 97.
[48] DAVIDSON M S. An investigation of copper recovery from a sulphide-oxide ore with a mixed collector system [D]. Kingston, Ontario, Canada: Queen’s University, 2009.
[49] RAO K H, DWARI R K, LU S, VILINSKA A, SOMASUNDARAN P. Mixed anionic/non-ionic collectors in phosphate gangue flotation from magnetite fines [J]. The Open Mineral Processing Journal, 2011, 4: 14-24.
Ali AHMADI, Mojtaba REZAEI, Seyed Mohammad SADEGHIEH
Department of Mining Engineering, Isfahan University of Technology, Isfahan 84156-83111, Iran
摘 要:浮选药剂在黏土矿中基础矿物的选矿中表现出复杂的行为。利用中心组合设计法研究浮选药剂对低品位高黏质硫化物矿中铜浮选效率的交互作用。初步结果表明,在石灰作pH调节剂的情况下,异丙基黄原酸钠(SIPX)和O-异丙基-N-乙基硫基氨基甲酸酯(IPETC)是最有效的捕收剂。在不同的pH值下,评估捕收剂(SIPX和IPETC)用量和起泡剂甲基异丁基甲醇(MIBC)用量对分离效率的影响。基于方差分析(ANOVA)结果,对于分离效率而言,捕收剂用量与pH值以及捕收剂用量与起泡剂用量之间的交互作用显著。在低捕收剂用量时,pH值从9提高到11可使IPETC的铜分离效率从81%提高到86%,SIPX的铜分离效率从77%提高到86%。方差分析结果表明,在pH值11、8.6 g/t SIPX、7 g/t IPETC和20 g/t MIBC的条件下,铜分离效率最高(88.7%)。最终,可得出结论,捕收剂SIPX和IPETC更适合于处理高黏质硫化矿。
关键词:浮选;硫化铜;试剂;交互作用;废矿
(Edited by Bing YANG)
Corresponding author: Ali AHMADI, Tel: +98-311-3915113; Fax: +98-311-3915176; E-mail: a.ahmadi@iut.ac.ir
DOI: 10.1016/S1003-6326(21)65539-5
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press

