
J. Cent. South Univ. (2018) 25: 21-28
DOI: https://doi.org/10.1007/s11771-018-3713-z
Improving of copper extraction from chalcopyrite by using NaCl
M. Deniz Turan1, Mustafa Boyrazl 1, H. Soner Altundo
1, H. Soner Altundo an2
an2
1. Department of Metallurgical and Materials Engineering, F rat University, Elaz
rat University, Elaz
 23119, Turkey;
23119, Turkey;
2. Department of Bioengineering, F rat University, Elaz
rat University, Elaz
 23119, Turkey
23119, Turkey
 Central South University Press and Springer-Verlag GmbH Germany,part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany,part of Springer Nature 2018
Abstract: The addition of NaCl in the ammonium persulfate-APS (as an oxidant) leaching was investigated. APS has some advantages compared with conventional oxidants and its standard redox potential (E°) is 2.0 V. Effect of six parameters such as NaCl concentration, APS concentration, temperature, time, liquid–solid ration (L/S), and stirring speed on the leaching behavior was studied. Results showed that metals extraction increased with increasing of NaCl concentration, APS concentration, leaching temperature (up to 333 K), and L/S ratio. During oxidative leaching of sulfide minerals, the occurrence of elemental sulfur layer on particle surface is known as primary problem that causes low metal extraction. According to the results, the passivation effect of sulfur layer and low dissolution problems can be eliminated in the presence of chloride ions. Copper and iron extraction yields were obtained as 75% and 80%, respectively under leaching conditions as follows: APS concentration 250 g/L; NaCl concentration 150 g/L; time 180 min; temperature 333 K; stirring speed 400 r/min; and L/S 250 mL/g.
Key words: copper; chalcopyrite; oxidation; leaching; dissolution
Cite this article as: M. Deniz Turan, Mustafa Boyrazl , H. Soner Altundo
, H. Soner Altundo an. Improving of copper extraction from chalcopyrite by using NaCl [J]. Journal of Central South University, 2018, 25(1): 21–28. DOI: https://doi.org/10.1007/s11771-018-3713-z.
an. Improving of copper extraction from chalcopyrite by using NaCl [J]. Journal of Central South University, 2018, 25(1): 21–28. DOI: https://doi.org/10.1007/s11771-018-3713-z.
1 Introduction
Copper is generally produced from its sulfide concentrate by using pyrometallurgical route. Pyrometallurgical production route mainly consists of the conventional smelting and converting processes. However, these high temperature processes have always been plagued with environmental issues concerning pollution. Because of increasing sensitivity of environmental issue and various economical factors, alternative copper extraction processes from sulfide ores have been researched for many years. Researched alternative copper production processes have been focused on the hydrometallurgical techniques. Hydrometallurgical processes mainly consist of leaching of materials by using suitable lixiviate, afterwards to produce metals from pregnant solutions. There are some patented and commercial hydrometallurgical processes for leaching of copper from its sulfide concentrates. These processes need some segments having high cost such as mechanical activation, effective oxidative leaching stage, and treatment of pregnant solution obtained after leaching [1–6]. However, leaching of copper sulfides such as chalcopyrite in the presence of sulfuric acid [7–13], nitric acid [14], and ammonia medium [15, 16] has been investigated. The most important problems encountered are low extraction yield and high iron content of pregnant solutions during the atmospheric leaching conditions. The reason of low extraction yield is passivity effect on the particle surface owing to oxidation of sulfide sulfur to metallic sulfur during the oxidative leach process.
To overcome these problems, researchers have focused on some combined methods. One of the methods is mechanical activation/extended milling as known pre-treatment of the concentrate before leaching process [17–21]. Another way is pressure leaching of concentrate or/and ore under the condition of high oxygen potential and acidic solution [22–26]. Although all of these methods are supplying high metal extraction, they are known to having high cost processes. Because of the above reasons, using of complexing agents such as chloride ions in the leaching solutions has a rising popularity. The reasons for using of chlorine ions can be listed as follows: to provide high redox potential, catalytic effect and, to generate soluble copper compounds. The related processes intend to increase the dissolution rate of metals from sulfide ores in the chloride media [27–39]. Although many researchers have reported relevant works, there is not any research about using the persulfate compounds together with chloride ions in literature and consideration of iron dissolution in the leaching solution. Iron is an important polluting for leaching solutions due to the resistance for the further purification stages. Therefore, the soluble iron should not be ignored in this kind of leaching studies.
Persulfate compounds have strong oxidizing effect, and reduced to sulfates after performing the process of oxidation in solution. Because of these properties of persulfate, its compound is thought to be a significant advantage in terms of leaching of sulfide materials. Cathode reaction of persulfate salts may be represented by following reaction:
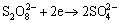 (1)
(1)
and its standard redox potential (E°) is 2.0 V [40]. The anodic reaction in the course of oxidizing leach of metal sulfides and total reaction may be represented by following equations:
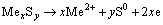 (2)
(2)
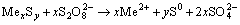 (3)
(3)
where Me represents metals.
On the other hand, ammonium persulfate (APS) has active oxygen of 7% and, when it decomposes in solution, it can produce active oxygen represented by following reaction [41]:
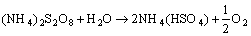 (4)
(4)
Under the condition of chlorine ions with persulfate used, oxidative agent may cause the formation of chlorate or perchlorate which in turn acted as oxidant. Nevertheless, in the course of acidic leaching to complex sulfides such as chalcopyrite, some invisible reactions may occur so that bornite (Cu5FeS4), chalcocite (Cu2S), covellite (CuS) may form depending on the pH and oxidation potential of leaching medium [40]. Moreover, it seems to be possible converting of Fe2+ ions to Fe3+ and to elemental sulfur of SO42- respectively in the leaching solution that are represented by following reactions:
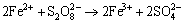 (5)
(5)
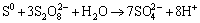 (6)
(6)
In some earlier studies, persulfate leaching of chalcopyrite concentrates has been studied under the atmospheric conditions and closed systems. Under the atmospheric pressure, it has been determined that the copper extraction limited, and it could be improved by a further milling to obtain better extraction yields [42, 43]. And also, some studies showed that using of persulfate compounds provided several benefits because of owning high potential [44, 45].
In the present work, the leaching of chalcopyrite concentrate by using of ammonium persulfate in the presence of sodium chloride was investigated under atmospheric pressure. In the study, effects of some parameters such as temperature, APS concentration, NaCl concentration, leaching time, liquid/solid ratio, stirring speed were studied.
2 Experimental
2.1 Materials and characterization
Chalcopyrite concentrate was obtained from Ber-Oner Flotation Plant, Elazig, Turkey. This concentrate was sieved through 75 μm mesh. The fraction passed through this sieve was used in all experiments. This fraction was dried at 353 K for 12 h and sample was stored in a closed vessel for later use.
Metal analyses of the chalcopyrite concentrate were made by using ICP-OES (Perkin-Elmer, Optima 2000DV) following dissolution by using microwave-assisted digestion route. Sulfur content of the concentrate was determined gravimetrically [46]. Results of chemical analyses are shown in Table 1.
Table 1 Chemical analysis of chalcopyrite concentrate sample (mass fraction, %)
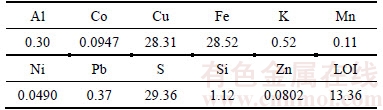
Mineralogical analyses of the concentrate were made by using X-ray diffraction system (Shimadzu XRD-6000) and powder diffraction technique. Mineralogical characteristics showed that the chalcopyrite concentrate was mainly composed of chalcopyrite (CuFeS2) and pyrite (FeS2).
Particle size distribution and surface area measurements of the concentrate were carried out by using laser scattering technique (Malvern Instruments MasterSizer X) and N2-BET method (Micromeritics ASAP 2020), respectively. The results of particle size and surface area measurements are shown in Table 2.
Table 2 Particle size distribution and N2-BET surface area of chalcopyrite concentrate

The chemicals used were 98% ammonium persulfate salt (Merck. 101200) and technical sodium chloride that procured, and all these chemicals were used without any further purification. Distilled water was used in all experiments.
2.2 Method
All solutions used in experiments were prepared freshly by dissolving appropriate amount of APS and NaCl with distilled water in a flask.
All leaching experiments were performed under the atmospheric conditions by using a magnetic multi stirrer (Velp Scientifica MultiStirrer 15). Experiments were carried out in 0.2 L flasks by stirring magnetically under reflux. APS-NaCl solution-chalcopyrite concentrate mixtures were stirred in a flask under various conditions to determine the effect of parameters on the metals extraction. At the end of the preferred leaching time, stirring was stopped and, the slurry filtrated.
Obtained leaching solutions were diluted with distilled water and analyzed by AAS (Perkin-Elmer, AAnalyst 400) for Cu and Fe metals. Pipetted leaching solution was mixed with distilled water in 100 mL flask. While all analyses were performed by using single hallow cathode lamp, wave length were used in 324 nm and 254 nm for Cu and Fe, respectively.
SEM analyses of original chalcopyrite concentrate and leaching residue were performed in 20 kV voltages after a thin coating with gold. Appropriate images of coated powders were determined and mapped.
3 Results and discussion
In our earlier studies, it has been determined that the APS is suitable oxidizing agent for extraction of major constituents such as copper and iron in chalcopyrite concentrate. Copper and iron dissolution yields from original concentrate could be obtained as about 20% and 22% respectively under the conditions of 0.25 kg/L of APS concentration, 333 K of leaching temperature,240 min of leaching time, 0.25 mol/L of H2SO4 concentration, liquid/solid ratio of 10 L/kg and, atmospheric pressure. Also, in the same study, copper and iron dissolution yields could be increased to 69% and 77% by further milling of the concentrate (d50=3.00 μm) under the similar conditions [42]. According to the detailed mineralogical analysis of leaching residues in relevant studies, it was indicated that the copper extraction yields might be limited due to formed elemental sulfur layer on the particle surface [7, 47, 48].
RU Z et al [39] reported that chloride ions significantly accelerated the copper dissolution from chalcopyrite in the H2SO4–NaCl–O2 system. This result supports the idea that copper extraction yields can be increased by using chloride ions together with APS for leaching of chalcopyrite.
Z et al [39] reported that chloride ions significantly accelerated the copper dissolution from chalcopyrite in the H2SO4–NaCl–O2 system. This result supports the idea that copper extraction yields can be increased by using chloride ions together with APS for leaching of chalcopyrite.
Firstly, effect of the NaCl concentration in the range of 0–0.25 kg/L was investigated. The results of these experiments are shown in Figure 1. As seen in Figure 1, the copper and iron dissolution yields were about 14% and 21%, respectively, under following conditions: 0 kg/L of NaCl concentration, 0.25 kg/L of APS concentration, 200 min of leaching time, 333 K of leaching temperature,10 L/kg of liquid/solid ratio and 400 r/min of stirring speed. Metal yields increased significantly with increasing the NaCl concentration up to 0.15 kg/L. The copper extraction yield reached about 52% at this point. After this point, metal extraction yields were not affected with increasing NaCl concentration. It can be clearly observed that the presence of sodium chloride has positive effect on the metal extraction. This tendency may be related to catalytic effect of chloride ions on the oxidation process. Some researchers have interpreted this case by the formation of intermediate copper chloride complexes [33, 39]. When chloride ions are present in the leaching solution, a second redox couple, the Cu2+/Cu+ can also be operative in the system [39, 49]. Also, it can be said that chloride ions may have some positive effects on the low metal dissolution that relates with formed elemental sulfur layer during oxidation process. The mechanism of this behavior is attributed that leaching agent passes easily through cracked sulfur layer on particle surface.
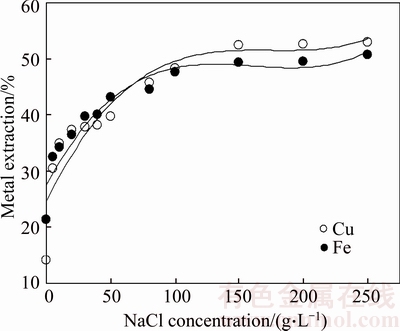
Figure 1 Effect of NaCl concentration on metal extraction from chalcopyrite concentrate by leaching APS (APS concentration: 250 g/L; leaching time:200 min; leaching temperature: 333 K (60°C); liquid/solid ratio: 10 mL/g; stirring speed: 400 r/min)
Figure 2 shows the effect of APS concentration on the metal extraction. As expected, metal extraction yields increased with increasing of APS concentration. Copper extraction yield reached to a plateau beyond APS concentration of 0.25 kg/L. For this concentration value, Cu and Fe extractions were about 52% and 49%, respectively. With increasing APS concentration, active oxygen occurring increases in leaching solution and it provides further oxidation in the metal dissolution mechanism. Nevertheless, as known, solubility of salts as APS is limited in the leaching solution.
Effect of the leaching time on the metal extraction is given in Figure 3. According this figure, it can be said that copper extraction rate in the first 15 min interval is higher than the subsequent period. This situation gives the impression as existence of two different leaching mechanisms for initial and subsequent periods. Probably, exchanging in the leaching mechanism may be occurred by formation of porous elemental sulfur layer on the surface. It has been reported that similar results occur for APS leaching of chalcopyrite in absence of NaCl.
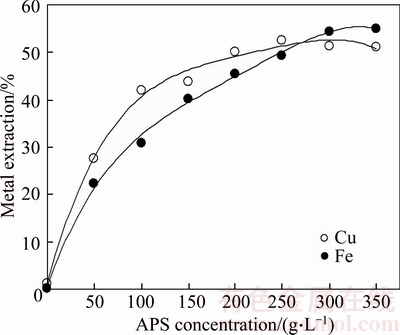
Figure 2 Effect of APS concentration on metal extraction from chalcopyrite concentrate (NaCl concentration:150 g/L; leaching time: 200 min; leaching temperature: 333 K (60°C); liquid/solid ratio: 10 mL/g; stirring speed: 400 r/min)
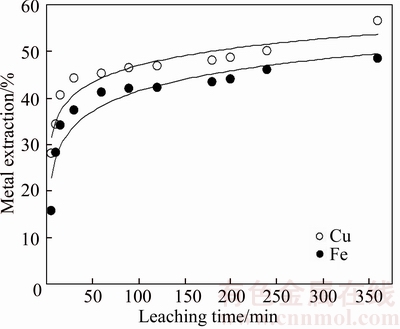
Figure 3 Effect of leaching time on metal extraction from chalcopyrite concentrate (NaCl concentration:150 g/L; APS concentration: 250 g/L; leaching temperature: 333 K (60°C); liquid-solid ratio: 10 ml/g; stirring speed: 400 r/min)
As known, leaching temperature is an important factor on the dissolution rate. On the other hand, decomposition rate of ammonium persulfate increases with increasing the temperature according to Eq. (4). This decomposition phenomenon depending on the temperature can be considered as useful for the oxidation process. However, this active oxygen can not be used effectively in an open system because the decomposition rate may be higher than that of oxidation process. For that reason, leaching temperature is most critical factor for APS leaching of chalcopyrite since the decomposition accelerate by heating.
Metal extraction yields increased with increasing temperature up to 333 K afterwards decreased with increasing above this temperature (Figure 4). It could be concluded that decomposition rate was faster than the oxidation over 333 K. Unlike similar studies using oxygen, metal extraction yields do not increase with increasing leaching temperature because of escaped active oxygen. However, metal extraction yields achieved may be accepted as respectable although using low leaching temperature.
Effects of the liquid/solid ratio and stirring speed are shown in Figures 5 and 6, respectively. As shown in Figure 5, extraction yield increased with increasing liquid/solid ratio. Although the copper extraction yield was obtained about 75%, copper concentration of the leaching solution decreased with increasing the ratio. For that reason, lower liquid/solid ratio may be preferred to obtain concentrated pregnant solution. Under these leaching conditions, iron passing amount into solution was more over 80%. This is also important point, so that the iron is known as contaminate for leaching process. Some studies have not referred to information about iron contamination, but iron ions are also generated complex with chloride ions.
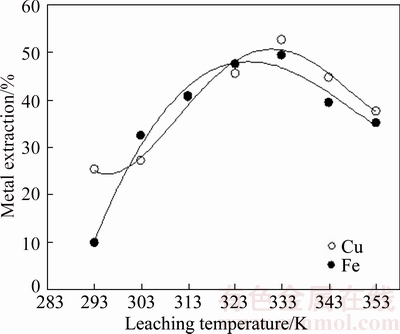
Figure 4 Effect of leaching temperature on metal extraction from chalcopyrite concentrate (NaCl concentration:150 g/L; APS concentration: 250 g/L; leaching time: 180 min; liquid/solid ratio: 10 ml/g; stirring speed: 400 r/min)
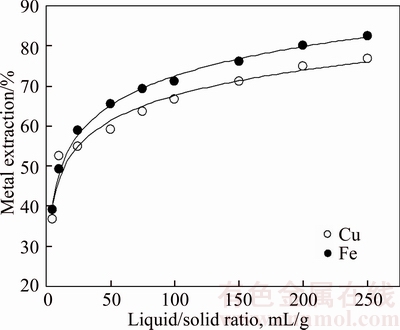
Figure 5 Effect of liquid/solid ratio on metal extraction from chalcopyrite concentrate (NaCl concentration:150 g/L; APS concentration: 250 g/L; leaching time:180 min; leaching temperature: 333 K (60°C); stirring speed: 400 r/min)
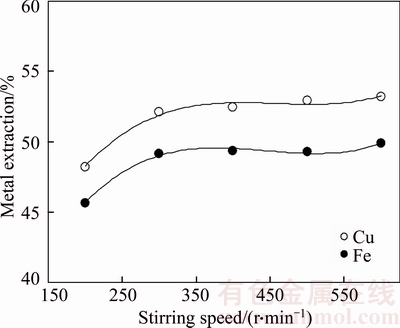
Figure 6 Effect stirring speed on metal extraction from chalcopyrite concentrate (NaCl concentration:150 g/L; APS concentration: 250 g/L; leaching time: 180 min; leaching temperature: 333 K (60°C); liquid/solid ratio: 10 ml/g)
Metal extraction was not influenced over 300 r/min of stirring speed as shown in Figure 6. Liquid/solid ratio and stirring speed can be evaluated as economical factors which have effect to production costs. Leaching process is propelled solid/liquid heterogeneous reactions so that mass transfer between phases is important for metal extraction. In the leaching process, stirring speed is the driving force because it improves the mass transfer. On the other hand, stirring requests a mechanical devices and process cost increases with increasing stirring time. According to obtained experimental results, magnetic stirring has limited effectives after 300 r/min for this study.
Figure 7 shows the X-ray diffraction pattern of leaching residue. As expected, chalcopyrite structure was partially converted to other mineral phases after oxidative leaching with APS in the presence of sodium chloride. Also, there are SEM images of original chalcopyrite concentrate and leaching residue in Figure 8. According to mapped SEM image of chalcopyrite concentrate, basic chalcopyrite metals such as copper, iron, and sulfur were determined besides silicon. On the other hand, metal oxide forms and compounds of leaching reactant were determined on the particle of leaching residue.
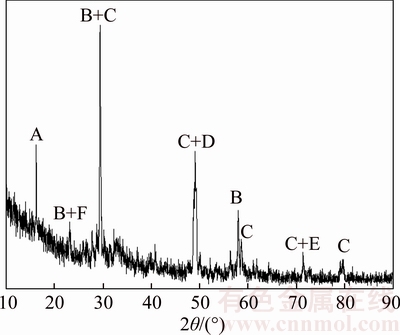
Figure 7 XRD pattern of leached chalcopyrite concentrate [A: Sodium copper oxide (Na7Cu3O8); B: Sodium iron oxide (Na2FeO3); C: Chalcopyrite (CuFeS2); D: Cubanite (CuFe2S3); E: Copper chlorate (Cu (ClO4)2); F: Sulfur (S)]
Based on the results, it is possible to say that using of sodium chloride in APS leaching of chalcopyrite concentrate can be important to solve a scientific problem for hydrometallurgical metal production.
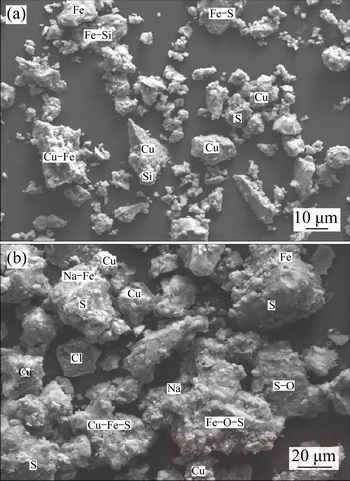
Figure 8 SEM images of chalcopyrite concentrate (a) and leaching residue (b)
4 Conclusions
1) Due to oxygen and acidity provided by ammonium persulfate, leaching process does not require a further oxidizing agent.
2) This study has some advantages. Particle size of chalcopyrite concentrate is original size, and it does not require mechanical activation.
3) NaCl concentration was a significant parameter on the metal extraction. While copper and iron extraction yields reached 52% and 50% by using 0.15 kg/L of NaCl, respectively; however, the metal extraction values did not exceed 14% for Cu and 21% for Fe in the absence of NaCl.
4) Copper and iron extraction increased with increasing of the APS concentration. While there was not significant metal extraction in the absence of APS. Cu and Fe extractions were about 52% and 49%, respectively, under the leaching conditions of 0.15 kg/L of NaCl concentration, 200 min of leaching time, 333 K of leaching temperature, 10 L/kg of liquid/solid ratio, 400 r/min of stirring speed.
5) Metal extraction yields increased with increasing temperature up to 333 K, afterwards extraction values decreased with increasing above 333 K temperature. Also, it was determined that metal extraction rate in first 15 min was higher than the subsequent period.
6) It is possible to obtain metal extraction yield of 75% for Cu and 80% for Fe by increasing liquid/solid ratio. Nevertheless, it is difficult to attain concentrated metal solution at high liquid/solid ratio conditions. Additionally, it was noticed that metal extractions were not influenced over 300 r/min of stirring speed.
Acknowledgments
This study was supported by the FUBAP (F rat University scientific research projects) under the project No: MF. 12. 32. The authors wish to express their thanks to metallurgical engineers O
rat University scientific research projects) under the project No: MF. 12. 32. The authors wish to express their thanks to metallurgical engineers O uz K
uz K z
z lkaya and Yasin Mutlu for their help in conducting the experiments.
lkaya and Yasin Mutlu for their help in conducting the experiments.
References
[1] HIROYOSHI N, HIROTA M, HIRAJIMA T, TSUNEKAWA M. A case of ferrous sulfate addition enhancing chalcopyrite leaching [J]. Hydrometallurgy, 1997, 47: 37–45.
[2] ARBITER N, MCNULTY T. Ammonia leaching of copper sulfide concentrates [C]// Copper 99-Proceedings of the Fourth International Conference Phoenix Arizona, USA: CIM, TMS, 1999: 197–212.
[3] BALAZ P. Mechanical activation in hydrometallurgy [J]. International Journal of Mineral Processing, 2003, 72: 341–354.
[4] AGNEW C J, WELHAM N J. Oxidation of chalcopyrite by extended milling [J]. International Journal of Mineral Processing, 2005, 77: 208–216.
[5] HARAHSHEH M A, KINGMAN S, HARAHSHEH A A. Ferric chloride leaching of chalcopyrite: Synergetic effect of CuCl2 [J]. Hydrometallurgy, 2008, 91: 89–97.
[6] PAD LLA R, RODR
LLA R, RODR GUEZ G, RU
GUEZ G, RU Z M C. Copper and arsenic dissolution from chalcopyrite–enargite concentrate by sulfidation and pressure leaching in H2SO4–O2 [J]. Hydrometallurgy, 2010, 100: 152–156.
Z M C. Copper and arsenic dissolution from chalcopyrite–enargite concentrate by sulfidation and pressure leaching in H2SO4–O2 [J]. Hydrometallurgy, 2010, 100: 152–156.
[7] BAFGH M S H, EMAM
M S H, EMAM A H, ZAKER
A H, ZAKER A. Effect of specific surface area of a mechanically activated chalcopyrite on its rate of leaching in sulfuric acid-ferric sulfate media [J]. Metallurgical and Materials Transaction B, 2013, 44(5): 1166–1172. DOI: 10.1007/s11663-013-9890-0.
A. Effect of specific surface area of a mechanically activated chalcopyrite on its rate of leaching in sulfuric acid-ferric sulfate media [J]. Metallurgical and Materials Transaction B, 2013, 44(5): 1166–1172. DOI: 10.1007/s11663-013-9890-0.
[8] LU Z Y, JEFFREY M I, LAWSON F. The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions [J]. Hydrometallurgy, 2000, 56: 189–202.
[9] HIROYOSHI N, MIKI H, HIRAJIMA T, TSUNEKAWA M. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions [J]. Hydrometallurgy, 2001, 60: 185–197.
[10] DREISINGER D, ABED N. A fundamental study of the reductive leaching of chalcopyrite using metallic iron part: kinetic analysis [J]. Hydrometallurgy, 2002, 66: 37–57.
[11] HAN K N, MENG X. Recovery of copper from its sulphides and other sources using halogen reagents and oxidants [J]. Minerals & Metallurgical Processing, 2003, 20: 160–164.
[12] H ROYOSH
ROYOSH N, KURO
N, KURO WA S, M
WA S, M K
K H, TSUNEKAWA M, H
H, TSUNEKAWA M, H RAJ
RAJ MA T. Effects of coexisting metal ions on the redox potential dependence of chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2007, 87: 1–10.
MA T. Effects of coexisting metal ions on the redox potential dependence of chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2007, 87: 1–10.
[13] HIROYOSHI N, KITAGAWA H, TSUNEKAWA M. Effect of solution composition on the optimum redox potential for chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2008, 91: 144–149.
[14] SOK
 M D, MARKOV
M D, MARKOV
 B,
B, 
 VKOV
VKOV
 D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid [J]. Hydrometallurgy, 2009, 95: 273–279.
D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid [J]. Hydrometallurgy, 2009, 95: 273–279.
[15] BJORLING G, FALDT I, LINDGREN E, TOROMANOV I. A nitric acid route in combination with solvent extraction for hydrometallurgical treatment of chalcopyrite [C]// Extractive Metallurgy of Copper. USA, New York: AIME, 1976, 2: 725–737.
[16] SARVESWARE RAO K, RAY H.S. A new look at characterisation and oxidative ammonia leaching behaviour of multimetal sulphides [J]. Minerals Engineering, 1998, 11: 1011–1024.
[17] FENG D, VAN DEVERTER J S J. Leaching behavior of sulphides in ammoniacal thiosulfate systems [J]. Hydrometallurgy, 2002, 63: 189–200.
[18] TKACOVA K, BALAZ P. Reactivity of mechanically activated chalcopyrite [J]. International Journal of Mineral Processing, 1996, 44: 197–208.
[19] MAURICE D, HAWK J A. Ferric chloride leaching of mechanically activated chalcopyrite [J]. Hydrometallurgy, 1998, 49: 103–123.
[20] ACH MOV
MOV COV M, BALAZ P, BR
COV M, BALAZ P, BR ANC
ANC N J. The influence of mechanical activation of chalcopyrite on the selective leaching of copper by sulphuric acid [J]. Metalurgija, 2006, 45: 9–12.
N J. The influence of mechanical activation of chalcopyrite on the selective leaching of copper by sulphuric acid [J]. Metalurgija, 2006, 45: 9–12.
[21] BAFGH M SH, EMAM
M SH, EMAM A H, ZAKER
A H, ZAKER L A, KHAK
L A, KHAK J V. Effect of mechanical activation on the kinetics of leaching of chalcopyrite in the ferric sulfate media [J]. Iranian Journal of Materials Science & Engineering, 2010, 7: 30–35.
J V. Effect of mechanical activation on the kinetics of leaching of chalcopyrite in the ferric sulfate media [J]. Iranian Journal of Materials Science & Engineering, 2010, 7: 30–35.
[22] AK IL A. A preliminary research on acid pressure leaching of pyritic copper ore in Kure copper mine Turkey [J]. Minerals Engineering, 2002, 15: 1193–1197.
IL A. A preliminary research on acid pressure leaching of pyritic copper ore in Kure copper mine Turkey [J]. Minerals Engineering, 2002, 15: 1193–1197.
[23] BREWER R E. Copper concentrate pressure leaching-plant scale-up from continuous laboratory testing [C]// SME Annual Meeting. USA, Denver, 2004: 1–6.
[24] DRE S
S NGER D. Copper leaching from primary sulfides: Options for biological and chemical extraction of copper [J]. Hydrometallurgy, 2006, 83: 10–20.
NGER D. Copper leaching from primary sulfides: Options for biological and chemical extraction of copper [J]. Hydrometallurgy, 2006, 83: 10–20.
[25] WODKA J, CHM ELEWSK
ELEWSK T, Z
T, Z OLKOWSK
OLKOWSK B. Pressure leaching of shale ore in oxygenated sulphuric acid [J]. Physicochemical Problems of Mineral Processing, 2007, 41: 349–364.
B. Pressure leaching of shale ore in oxygenated sulphuric acid [J]. Physicochemical Problems of Mineral Processing, 2007, 41: 349–364.
[26] TSH LOMBO K G, BAFUB
LOMBO K G, BAFUB AND
AND A F M. Ammonia/nitric acid leaching of copper-cobalt oxidized ore [C] // International Conference on Mining, Mineral Processing and Metallurgical Engineering. South Africa: ICMMME'2013, 2013: 120–122
A F M. Ammonia/nitric acid leaching of copper-cobalt oxidized ore [C] // International Conference on Mining, Mineral Processing and Metallurgical Engineering. South Africa: ICMMME'2013, 2013: 120–122
[27] DALTON R F, D AZ G, PR
AZ G, PR CE R, ZUNKEL A D. The cuprex metal extraction process recovering copper from sulfide ores: Recovering copper from sulphide ores [J]. JOM, 1991, 43(8): 51–56.
CE R, ZUNKEL A D. The cuprex metal extraction process recovering copper from sulfide ores: Recovering copper from sulphide ores [J]. JOM, 1991, 43(8): 51–56.
[28] BARR G, DEFREYNE J, JONES D, MOORE R. The CESL process- successful refinery of a low grade copper concentrates [M]. Proceedings ALTA Copper, Perth Australia, 2000.
[29] HYVAR NEN O, HAMALA
NEN O, HAMALA NEN M, LE
NEN M, LE MALA R. Outokumpu hydrocopper process—A novel concept in copper production [C]// Chloride Metallurgy 2002: Practice and Theory of Chloride/metal Interaction, 32nd Annual Hydrometallurgy Meeting.Montreal, Quebec, Canada, Metallurgical Society, CIM, 2002: 712–713.
MALA R. Outokumpu hydrocopper process—A novel concept in copper production [C]// Chloride Metallurgy 2002: Practice and Theory of Chloride/metal Interaction, 32nd Annual Hydrometallurgy Meeting.Montreal, Quebec, Canada, Metallurgical Society, CIM, 2002: 712–713.
[30] MOYES J HOULL S F. Intec base metal processes—releasing the potential of chloride metallurgy technical update and commercialization status [C]// Chloride Metallurgy 2002: Practice and Theory of Chloride/metal Interaction, 32nd Annual Hydrometallurgy Meeting. Montreal, Quebec, Canada, Metallurgical Society, CIM. 2002.
S F. Intec base metal processes—releasing the potential of chloride metallurgy technical update and commercialization status [C]// Chloride Metallurgy 2002: Practice and Theory of Chloride/metal Interaction, 32nd Annual Hydrometallurgy Meeting. Montreal, Quebec, Canada, Metallurgical Society, CIM. 2002.
[31] LUNDSTR M M, AROMA J, FORSEN O, HYVAR
M M, AROMA J, FORSEN O, HYVAR NEN O, BARKER M H. Leaching of chalcopyrite in cupric chloride solution [J]. Hydrometallurgy, 2005, 7: 89–95.
NEN O, BARKER M H. Leaching of chalcopyrite in cupric chloride solution [J]. Hydrometallurgy, 2005, 7: 89–95.
[32] SKROB AN M, HAVL
AN M, HAVL K T, UKAS
K T, UKAS K M. . Effect of NaCl concentration and particle size on chalcopyrite leaching in cupric chloride solution [J]. Hydrometallurgy, 2005, 77: 109–114.
K M. . Effect of NaCl concentration and particle size on chalcopyrite leaching in cupric chloride solution [J]. Hydrometallurgy, 2005, 77: 109–114.
[33] HERREROS O, V NALS J. Leaching of sulfide copper ore in a NaCl-H2SO4-O2 media with acid pre-treatment [J]. Hydrometallurgy, 2007, 89: 260–268.
NALS J. Leaching of sulfide copper ore in a NaCl-H2SO4-O2 media with acid pre-treatment [J]. Hydrometallurgy, 2007, 89: 260–268.
[34] N COL M, M
COL M, M K
K H, Y
H, Y VENES L V. The dissolution of chalcopyrite in chloride solutions Part 3. Mechanisms [J]. Hydrometallurgy, 2010, 103: 86–95.
VENES L V. The dissolution of chalcopyrite in chloride solutions Part 3. Mechanisms [J]. Hydrometallurgy, 2010, 103: 86–95.
[35] Y VENES L V, N
VENES L V, N COL M, M
COL M, M K
K H. The dissolution of chalcopyrite in chloride solutions Part 1. The effect of solution potential [J]. Hydrometallurgy, 2010a, 103: 108–113.
H. The dissolution of chalcopyrite in chloride solutions Part 1. The effect of solution potential [J]. Hydrometallurgy, 2010a, 103: 108–113.
[36] Y VENES L V, M
VENES L V, M K
K H, N
H, N COL M. The dissolution of chalcopyrite in chloride solutions Part 2: Effect of various parameters on the rate [J]. Hydrometallurgy, 2010b, 103: 80–85.
COL M. The dissolution of chalcopyrite in chloride solutions Part 2: Effect of various parameters on the rate [J]. Hydrometallurgy, 2010b, 103: 80–85.
[37] GUO Zhao-hui, PAN Feng-kai, XIAO Xi-yuan, ZHANG Long, JIANG Kai-qi. Optimization of brine leaching of metals from hydrometallurgical residue [J]. Transaction of Nonferrous Metals Society of China, 2010, 20: 2000–2005.
[38] M K
K H N
H N COL M. The dissolution of chalcopyrite in chloride solutions. IV. The kinetics of the auto-oxidation of copper(I) [J]. Hydrometallurgy, 2011, 105: 246–250.
COL M. The dissolution of chalcopyrite in chloride solutions. IV. The kinetics of the auto-oxidation of copper(I) [J]. Hydrometallurgy, 2011, 105: 246–250.
[39] RU Z M C, MONTES K S, PAD
Z M C, MONTES K S, PAD LLA R. Chalcopyrite leaching in sulfate–chloride media at ambient pressure [J]. Hydrometallurgy, 2011, 109: 37–42.
LLA R. Chalcopyrite leaching in sulfate–chloride media at ambient pressure [J]. Hydrometallurgy, 2011, 109: 37–42.
[40] JACKSON E. Hydrometallurgicall extraction and reclamation [M]. New York: Ellis Harwood Ltd, USA, 1986.
[41] MELLOR J W. A Comprehensive treatise on inorganic and theoretical chemistry [M]. London: Lowe and Brydone Printers Ltd, Great Britain, 10: 1960.
[42] BAHAR N. An investigation on persulphate leaching of chalcopyrite concentrate [D]. Elaz
 :F
:F rat University, 2004.
rat University, 2004.
[43] TURAN M D. Investigation of the pressure leaching of chalcopyrite in the presence of active oxidizing [D]. Elaz
 :F
:F rat University, 2010.
rat University, 2010.
[44] BABU M N, SAHU K K, PANDEY B D. Zinc recovery from sphalerite concentrate by direct oxidative leaching with ammonium, sodium and potassium persulfates [J]. Hydrometallurgy, 2002, 64: 119–129.
[45] DAKUBO F, BAYGENTS J C, FARRELL J. Peroxidisulfate assisted leaching of chalcopyrite [J]. Hydrometallurgy, 2012, 121: 68–73
[46] VOGEL A I. Vogel’s textbook of quantitative chemical analysis [M]. 5th edition, London: Great Britain, John Wiley & Sons Inc. 1989.
[47] HACKL R P, DREISINGER D B, PETERS E, KING J A. Passivation of chalcopyrite during oxidative leaching in sulfate media [J]. Hydrometallurgy, 1995, 39: 25–48.
[48] KOLEINI S M J, AGHAZADEH V, SANDSTR M A. Acidic sulphate leaching of chalcopyrite concentrates in presence of pyrite [J]. Minerals Engineering, 2011, 24: 381–386.
M A. Acidic sulphate leaching of chalcopyrite concentrates in presence of pyrite [J]. Minerals Engineering, 2011, 24: 381–386.
[49] BONAN M, DEMARTHE J M, RENON H, BARAT N F. Chalcopyrite leaching by CuCl2 in strong NaCl solutions [J]. Metall. Trans. 12B, 1981: 269–274.
N F. Chalcopyrite leaching by CuCl2 in strong NaCl solutions [J]. Metall. Trans. 12B, 1981: 269–274.
(Edited by HE Yun-bin)
中文导读
添加NaCl 提高黄铜矿的铜提取率
摘要:本文研究了在过硫酸铵-APS作为氧化剂中添加NaCl对黄铜矿浸出行为的影响。 APS与传统氧化剂相比有许多优势,其标准氧化还原电位为2.0 V。研究了6个参数,包括NaCl浓度,APS浓度,温度,时间,液固比,搅拌速率对浸出行为的影响,结果表明金属的提取率随NaCl浓度,APS浓度,浸出温度和液固比的增加而增加。在硫化物矿物的氧化浸出过程中,在颗粒表面出现的硫元素层会降低金属的提取率。本研究结果表明,由于Cl离子的存在,硫元素层的钝化和低溶解度问题都得到消除。在APS浓度为250 g/L,NaCl浓度为150 g/L,时间为180 min,温度为333 K,搅拌速率为400 r/min及液固比为 250 mL/g的条件下,铜和铁的提取率分别为75%和80%。
关键词:铜;黄铜矿;氧化;浸出;溶解
Received date: 2016-08-29; Accepted date: 2017-12-01
Corresponding author: M. Deniz Turan, PhD, Associate Professor; Tel: +90–0424–2370000–6379; Fax: +90–0424–2415526; E-mail: mdturan@firat.edu.tr; ORCID: 0000-0002-2136-1435
 , H. Soner Altundo
, H. Soner Altundo an. Improving of copper extraction from chalcopyrite by using NaCl [J]. Journal of Central South University, 2018, 25(1): 21–28. DOI: https://doi.org/10.1007/s11771-018-3713-z.
an. Improving of copper extraction from chalcopyrite by using NaCl [J]. Journal of Central South University, 2018, 25(1): 21–28. DOI: https://doi.org/10.1007/s11771-018-3713-z.