
Preparation of YVO4:RE (RE=Yb3+/Er3+, Yb3+/Tm3+) nanoparticles via microemulsion-mediated hydrothermal method
ZHANG Jun(张 军)1, Wulantuya(乌兰图雅)1, DI Xiao-wei(狄晓威)1, LIU Zhi-liang(刘志亮)1,
XU Gang(徐 刚)2, XU Sheng-ming(徐盛明)2
1. College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot 010021, China;
2. Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
Received 6 July 2009; accepted 30 December 2009
_____________________________________________________________________________________________________
Abstract: A microemulsion-mediated hydrothermal method for synthesis of YVO4?RE (RE=Yb3+/Er3+, Yb3+/Tm3+) nanoparticles by hydrothermal treatment of quaternary microemulsion medium consisting of Na3VO4/NaOH and RE(NO3)3 aqueous solution,surfactant cetyltrimethylammonium bromide (CTAB), cosurfactant n-hexanol and oil phase n-heptane was report. The confinement of microemulsion droplets acting as microreactors during the reaction process allows the formation of small size YVO4?RE nanoparticles with relatively narrow size distribution and less aggregation. The structure, size and shape of YVO4?RE nanoparticles were investigated by means of X-ray diffractometry (XRD) and transmission electron microscopy (TEM). Compared with the conventional solid annealing diffusion method, the microemulsion-mediated hydrothermal method shows superiority in obtaining YVO4?RE nanoparticles with controllable size, narrow size distribution and less aggregation. The microemulsion-mediated hydrothermal method may be potentially applicable for synthesis of other rare earth doped up-converting luminescence nanomaterials.
Key words: yttrium orthovanadate; doping; up-conversion luminescence; microemulsion; hydrothermal method
_____________________________________________________________________________________________________
1 Introduction
Since the first discovery of up-conversion luminescence phenomenon, up-converting luminescent materials have been widely investigated due to their unique up-conversion luminescent properties that may find potential applications in several new technologies, such as solid lighting, displaying, biological assays and imaging[1-2]. Many series of materials systems showing up-converting luminescent properties have been developed, and it was recently found that novel and efficient up-conversion luminescence could be realized in nanoscaled up-converting materials[3-6].
Among the widely investigated up-converting nanomaterials systems, up-conversion luminescent yttrium orthovanadate (YVO4) nanoparticles doped with rare-earth ions have attracted much research attention in recent years[7-8]. Rare earth ions of Yb3+/Er3+ and Yb3+/Tm3+ are believed to be the most effective ions for doping into YVO4 host lattice to result in efficient up-conversion luminescence. However, most of these reports so far are mainly focused on the study of YVO4 nanomaterials doped with one kind of rare earth ion, such as Eu3+, Tm3+, Dy3+, Sm3+ or Er3+[9-11]. The reports on co-doping of Yb3+/Er3+ and Yb3+/Tm3+ in YVO4 nanomaterials are still far more adequate, and the effects of co-doping Yb3+/Er3+ and Yb3+/Tm3+ into YVO4 nanomaterials resulting in the enhanced up-converting luminescence still need to be further studied.
We have developed a microemulsion-mediated hydrothermal method available for preparation of luminescent nanoparticles with controllable sizes and narrow distribution[12-13]. The method can also be used for preparation of undoped and rare earth doped YVO4 nanoparticles[13]. The sizes of the nanoparticles could be adjusted by modulating the microemulsion compositions and reaction conditions. In this work, we reported the synthesis of YVO4?RE (RE=Yb3+/Er3+, Yb3+/Tm3+) nanoparticles via this microemulsion- mediated hydrothermal method. A quaternary microemulsion medium consists of Na3VO4/NaOH and RE(NO3)3 aqueous solution, surfactant cetyltrimethylammonium bromide (CTAB), cosurfactant n-hexanol and oil phase n-heptane were developed, in which the microemulsion droplets acting as microreactors during the reaction process could show confinement effects allowing the formation of small size YVO4?RE nanoparticles with relatively narrow size distribution and less aggregation. The microemulsion- mediated hydrothermal method shows superiority in obtaining YVO4?RE nanoparticles with controllable size, narrow size distribution and less aggregation, and may be potentially effective for synthesis of a wide series of rare earth doped luminescent nanomaterials.
2 Experimental
2.1 Preparation of YVO4?RE (RE=Yb3+/Er3+, Yb3+/Tm3+) nanoparticles
RE(NO3)3 (RE=Yb3+, Er3+, Tm3+), Na3VO4, hexanol, heptane, and cetyltrimethylammoniumbromide (CTAB) were purchased from J&K Chemical Company, and were used as received.
The synthesis of undoped YVO4 and YVO4?RE (RE=Yb3+/Er3+, Yb3+/Tm3+) nanoparticles via microemulsion-mediated hydrothermal method follows the same procedures. Taking the preparation of YVO4?Yb3+/Er3+ as an example, two microemulsions were separately prepared. One contained CTAB, hexanol, heptane and aqueous solution of Na3VO4, and NaOH, and the other consisted of CTAB, hexanol, heptane and aqueous solution of Y(NO)3, Yb(NO)3 and Er(NO)3 (Table 1). Then one microemulsion was dropwise added into the other at room temperature with continuous stirring. Subsequently, the mixture was transferred into a 50 mL autoclave for hydrothermal treatment at 150 ℃ for 2 h to make the nanoparticles well crystallized. By naturally cooled down to room temperature, the YVO4?Yb3+/Er3+ nanoparticles were separated from the microemulsion media by centrifugation and were washed with absolute ethanol and distilled water several times. Finally, the obtained YVO4?Yb3+/Er3+ nanoparticles were dried at room temperature. Similar procedures were followed for the preparation of undoped YVO4 and YVO4?Yb3+/Tm3+ nanoparticles. For synthesis of YVO4?Yb3+/Tm3+ nanoparticles, the same reaction conditions were kept except using Tm(NO)3 to substitute Er(NO)3.
Table 1 Compositions of microemulsion for preparation of YVO4, YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles
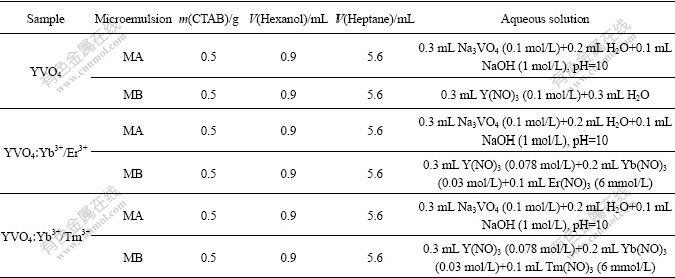
For comparison, a solid annealing diffusion method was carried out to synthesize YVO4?RE (RE=Yb3+/Er3+, Yb3+/Tm3+) nanoparticles. Typically, a certain amount of YVO4 nanoparticles obtained via microemulsion- mediated hydrothermal method were weighted, and a certain amount of Yb(NO3)3 (20%, mole fraction) and Er(NO3)3 or (Tm(NO3)3 (2%, mole fraction) were weighted and mixed with YVO4 nanoparticles. After grinding,the samples were calcined at 600 ℃ for 4 h to obtain the final YVO4?RE (RE=Yb3+/Er3+, Yb3+/Tm3+) nanoparticles.
2.2 Characterization
Powder X-ray diffractometry (XRD) was used to characterize the phase structures of the nanoparticles. Measurements were performed using a Bruker AXS-D8 diffractometer (German) operated at 40 kV and 40 mA with a slit of 1/2 at a scanning rate of 3 (?)/min in a scanning range 2θ of 20–80?, using Cu Ka radiation (l=0.154 06 nm). Samples for XRD analysis were prepared by gently crushing the obtained products with a mortar and pestle, and were placed in a quartz glass holder for characterization. Transmission electron microscopy (TEM) characterization was performed on a JEM-2010 system operated at an acceleration voltage of 200 kV to evaluate the structure, size and shape of the nanoparticles.
Samples for TEM analysis were prepared by drying a drop of nanoparticles dispersied on an amorphous carbon-coated copper grid for the observation. Energy dispersive X-ray spectroscopy (EDX) was performed using an EDAX system attached to TEM.
3 Results and discussion
3.1 X-ray powder diffractometry
The crystalline phases of YVO4, YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles were determined by X-ray powder diffractometry, and XRD patterns are shown in Fig.1. The XRD patterns suggest that the good crystallinity of the nanoparticles with phase structures indexed to tetragonal structured YVO4 (JCPDS No.17-0341). No impurity phase except tetragonal structured YVO4 was evidenced from the XRD characterization. Compared with bulk YVO4, the diffraction peaks were broadened, indicating the formation of nanosized YVO4. The average particles size of the nanoparticles can be roughly deduced to about 30 nm by fitting the full width at half maximum of (200) peaks according to Scherrer equation [14], which was consistent with the results determined by TEM observations.
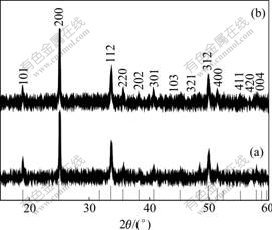
Fig.1 XRD patterns of YVO4?Yb3+/Er3+(a) and YVO4?Yb3+/ Tm3+(b) nanoparticles
3.2 TEM characterization
The sizes and shapes of the as-prepared YVO4, YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles prepared via microemulsion-mediated hydrothermal method were examined by TEM, and the images are shown in Fig.2. Fig.2(a) displays the TEM image of YVO4 nanoparticles without doping. It can be seen from Fig.2(a) that the nanoparticles show the spherical shape with particle sizes of about 30 nm. Fig.2(b) presents the high resolution TEM image of YVO4 nanoparticles. The lattice fringe observed from the HRTEM image suggests the formation of well crystalline YVO4 nanoparticles with tetragonal phase structure in consistence with XRD results. Fig.2(c) and (d) show the TEM images of YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles, respectively. It is found that after co-doping the Yb3+/Er3+ and Yb3+/Tm3+ ions entered into YVO4, no changes of phase structure of YVO4 nanoparticles were observed, and the particle shape and size keep almost the same, indicating the effectiveness of microemulsion method for homogenous doping of Yb3+/Er3+ and Yb3+/Tm3+ ions into YVO4.
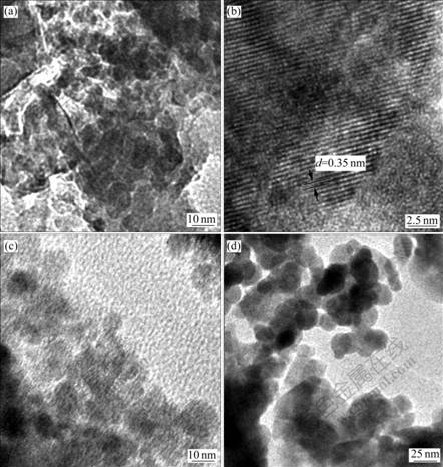
Fig.2 TEM images of YVO4(a) and (b), VO4?Yb3+/Er3+(c), and YVO4?Yb3+/Tm3+(d) nanoparticles prepared via microemulsion-mediated hydrothermal method
3.3 Energy dispersive X-ray analysis
To confirm the contents of Yb, Er and Tm elements existing in the doped YVO4 nanoparticles, energy dispersive X-ray (EDX) analysis was carried out, as shown in Fig.3. Beside V, O and Y elements, the representative peaks of Yb/Er and Yb/Tm elements appear in the spectra of YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles, respectively, which confirms the existence of Yb/Er and Yb/Tm elements in YVO4 nanoparticles and suggests the realization of the co-doping of Yb3+/Er3+ and Yb3+/Tm3+ ions into YVO4 nanoparticles via microemulsion mediated hydrothermal method.
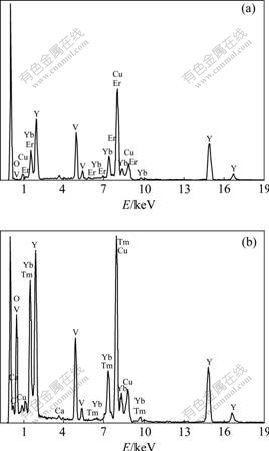
Fig.3 EDX patterns of as-prepared YVO4?Yb3+/Er3+(a) and YVO4?Yb3+/Tm3+(b) nanoparticles
3.4 Comparison of different preparation methods on effect of size and shape of YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles
To compare the co-doping effectiveness, the particle sizes and shapes of nanoparticles prepared via microemulsion mediated hydrothermal method were compared with those by other methods, a solid annealing diffusion approach, as described in experimental section. The size and shape of the nanoparticles obtained by solid annealing diffusion method are shown in Fig.4. It is found that solid annealing diffusion method may result in the formation of YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles with larger particle size and severe aggregation. Meanwhile, the XRD characterization of the obtained YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles indicates the formation of Y2O3 phase except the tetragonal structured YVO4. This suggests that the microemulsion-mediated hydrothermal method shows superiority in obtaining YVO4?Yb3+/Er3+ and YVO4?Yb3+/ Tm3+ nanoparticles with controllable size, narrow size distribution and less aggregation.
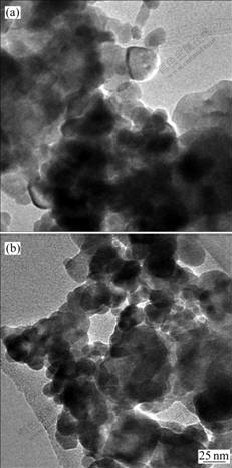
Fig.4 TEM images of YVO4?Yb3+/Er3+(a) and YVO4?Yb3+/ Tm3+(b) nanoparticles prepared via solid annealing diffusion method
4 Conclusions
1) Microemulsion-mediated hydrothermal method was successfully developed for preparation of YVO4, YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles.
2) The microemulsion-mediated hydrothermal method shows effectiveness in obtaining YVO4, YVO4?Yb3+/Er3+ and YVO4?Yb3+/Tm3+ nanoparticles with small size and narrow size distribution and less aggregation.
3) Compared with the conventional solid annealing diffusion method, homogenous co-doping of Yb3+/Er3+ and Yb3+/Tm3+ into YVO4 lattice can be easily realized by the microemulsion-mediated hydrothermal method.
4) The microemulsion-mediated hydrothermal method may be potentially applicable for synthesis of other rare earth doped up-converting luminescence nanomaterials.
References
[1] LEVINE A K, PALILLA F C. A new, highly efficient red-emitting cathodoluminescent phosphor (YVO4?Eu) for color television [J]. Appl Phy Lett, 1964, 5: 118-124.
[2] ZHANG M, SHI S, MENG J, WANG X, FAN H, ZHU Y, WANG X, QIAN Y. Preparation and characterization of near-infrared luminescent bifunctional core/shell nanocomposites [J]. J Phys Chem C, 2008, 112: 2825-2830.
[3] KR?MER K W, BINER D, FREI G, G?DEL H U, HEHLEN M P, L?THI S R. Hexagonal sodium yttrium fluoride based green and blue emitting upconversion phosphors [J]. Chem Mater, 2004, 16: 1244-1251.
[4] BOYER J C, CUCCIA L A, CAPOBIANCO J A. Synthesis of colloidal upconverting NaYF4?Er3+/Yb3+ and Tm3+/Yb3+ monodisperse nanocrystals [J]. Nano Letters, 2007, 7: 847-852.
[5] SUYER J F, GRIMM J, KR?MER K W, G?DEL H U. Highly efficient near-infrared to visible up-conversion process in NaYF4?Er3+, Yb3+ [J]. J Lumin, 2005, 114: 53-59.
[6] CHEN Z G, CHEN H L, HU H, YU M X, LI F Y, ZHANG Q, ZHOU Z G, YI T, HUANG C H. Versatile synthesis strategy for carboxylic acid- functionalized upconverting nanophosphors as biological labels [J]. J Am Chem Soc, 2008, 130: 3023-3029.
[7] YANG K, ZHENG F, WU R, LI H, ZHANG X. Upconversion luminescent properties of YVO4?Yb3+, Er3+ nano-powder by sol-gol method [J]. J Rare Earths, 2006, 24: 162-166.
[8] CHEN X, LIU K, ZHUANG J, WANG G, CHEN C. The upconversion luminescent research of HoYb?YVO4 [J]. Acta Physica Sinica, 2002, 51: 690-695.
[9] ZHANG H, FU X, NIU S, SUN G, XIN Q. Photoluminescence of nanocrystalline YVO4?TmxDy1-x prepared by a modified pechini method [J]. Materials Letters, 2007, 61: 308-311.
[10] SUN Y, LIU H, WANG X, KONG X, ZHANG H. Optical spectroscopy and visible upconversion studies of YVO4?Er3+ nanocrystals synthesized by a hydrothermal process [J]. Chem Mater, 2006, 18: 2726-2732.
[11] PENG H, HUANG S, SUN L, YAN C. Analysis of surface effect on luminescent properties of Eu3+ in YVO4 nanocrystals [J]. Physics Letters A, 2007, 367: 211-214.
[12] ZHANG J, SUN L, LIAO C, YAN C. Size control and photoluminescence enhancement of CdS nanoparticles prepared via reverse micelle method [J]. Solid State Commun, 2002, 124: 45-48.
[13] SUN L, ZHANG Y, ZHANG J, YAN C, LIAO C, LU Y. Fabrication of size controllable YVO4 nanoparticles via microemulsion-mediated synthetic process [J]. Solid State Commun, 2002, 124: 35-38.
[14] WARREN B E. X-ray diffraction[M]. New York: Dover Publications Inc, 1990.
_________________________
Foundation item: Projects(20601012, 20601016, 20961005) supported by the National Natural Science Foundation of China; Project(209024) supported by the Ministry of Education of China; Projects(206077, 206043, 10013-121008) supported by Inner Mongolia University, China
Corresponding author: ZHANG Jun; Tel: +86-471-4992175; E-mail: cejzhang@imu.edu.cn
(Edited by CHEN Ai-hua)