J. Cent. South Univ. Technol. (2010) 17: 720-725
DOI: 10.1007/s11771-010-0546-9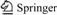
Citrate-stabilized CdSe/CdS quantum dots as fluorescence probe for protein determination
FU Xin(傅昕), HUANG Ke-long(黄可龙), LIU Su-qin(刘素琴)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: A rapid, ultrasensitive and convenient fluorescence measurement technology based on the enhancement of the fluorescence intensity resulting from the interaction of functionalized CdSe/CdS quantum dots (QDs) with bovine serum albumin (BSA) was proposed. The citrate-stabilized CdSe/CdS (QDs) were synthesized by using Se powder and Na2S as precursors instead of any pyrophoric organometallic precursors. The modified CdSe/CdS QDs are brighter and more stable against photobleaching in comparison with organic fluorophores. At pH 7.0, the fluorescence signal of CdSe/CdS is enhanced by increasing the concentration of BSA in the range of 0.1-10 μg/mL, and the low detection limit is 0.06 μg/mL. A linear relationship between the enhanced fluorescence peak intensity (?F) and BSA concentration (c) is established using equation ?F=50.7c+16.4 (R=0.996 36). Results of determination for BSA in three synthetic samples are identical with the true values, and the recovery (98.9%-102.4%) and relative standard deviation (RSD, 1.8%-2.5%) are satisfactory.
Key words: CdSe/CdS quantum dots; bovine serum albumin; protein; fluorescence measurement
1 Introduction
The quantitative analysis of protein continues to be an important area because it can be used as a reference for measurements in biochemistry, food tests and clinical diagnoses. There were many conventional detection methods to detect protein, such as Lowry method [1], bromcresol green (BCG) method [2], Bradford method [3], Kjeldahl method [4] and capillary electrophoresis [5]. But the simplicity and sensitivity of these methods were not dissatisfied. Nowadays, fluorescence analysis is more widely used in peptide and protein chemistry [6-9]. Many fluorescence methods are developed for the fluorimetric quantitative detection of protein using fluorescent dye, such as erythrosine sodium (ES) [10], rose bengal (RB) [11], albumin blue (AB) [12] and methyl blue [13]. However, due to narrow excitation spectra, broad emission bands, spectral overlap and low photobleaching, these methods based on fluorescent dye still comprise some limitations in long-term imaging, multiplexing and sensitivity. For example, the detection limit of erythrosine sodium-based luminescence measurement was 0.5 μg/mL [10] and that of albumin blue 580-based method was 0.4 μg/mL [12].
Quantum dots (QDs) are a kind of ideal fluorophore for biological imaging and have been widely used in the fields of chemistry and biomedical science as a fluorescence marker [14-20]. Compared to conventional fluorescent dye, QDs have a broad absorption with narrow, symmetric photoluminescene (PL) spectra and the emission wavelength can be continuously tuned by changing the particle size [21-22]. QDs offer advantages of considerably greater resistance to quenching, photobleaching and chemical degradation. Moreover, after overcoating the core (CdSe) with one shell, the core/shell QDs will have more favorable optical properties than “bare” CdSe QDs because of reducing nonradiative recombination by confining the wave function of electron–hole pairs to the interior of nanoparticles. On the other hand, the aqueous synthesized approach, which often uses thiols as stabilizers [23-25], is a simple, green, non-toxic, highly reproducible method compared to the traditional organometallic routes. These methods have attracted considerable attention in the preparation and design of the surface properties of hydrophilic quantum dots. Recently, several groups have paid efforts to the design of thiol-functionalized QDs for protein determination. However, the detection limits were relatively high [23-24]. In order to achieve the sensitive detection of protein, citrate-stabilized QDs have been developed by our group. Citrate-stabilized QDs were widely reported because of more favorable optical properties than thiol-stabilized QDs [26-27].
In this work, citrate-stabilized water-soluble CdSe/CdS nanoparticles were synthesized at lower temperature using Se powder as a selenium source and Na2S as a sulfur source instead of any pyrophoric organometallic precursors, respectively. The shell (CdS) can greatly passivate the core surface to protect CdSe from oxidation, prevent CdSe leaching into the surrounding solution and improve the PL yield as well as the photostability by coating with CdS. Based on the enhanced fluorescence of CdSe/CdS nanoparticles by protein, a rapid, ultrasensitive and convenient determining technology was demonstrated for quantitative detection of the protein by using water- soluble CdSe/CdS nanoparticles as fluorescence marker and bovine serum albumin (BSA) as the target protein. It has higher sensitivity and wider linear range. The obtained results demonstrate that the application of CdSe/CdS nanoparticles as fluorescent probes in quantitative detection of protein is feasible.
2 Experimental
2.1 Reagents and chemicals
Selenium (Se) powder (>99.5%, mass fraction), sodium borohydride (99%), Na2S·9H2O (>98%), CdCl2·2.5H2O (>99%), trisodium citrate dihydrate (99%), tris(hydroxymethyl)-aminomethane (Tris) (99%), hydro- chloric acid, sulfuric acid, ethanol and sodium hydroxide (96%) were of analytical grade without further purification. Stock standard solution of BSA (Sigma–Aldrich Chemical Co., America) was prepared by dissolving BSA in water to the desired concentration and stored at 4 ℃.
2.2 Apparatus
Fluorescence intensity and emission spectra were recorded by a HITACHI F-2500 spectrofluoro- photometer. All pH values were measured with a PHS-25 pH meter (Shanghai, China). SHZ-82 water thermostat was used to keep the reaction temperature. All optical measurements were carried out at room temperature under ambient conditions.
2.3 Synthesis of citrate-stabilized CdSe/CdS quantum dots
Water-soluble citrate-stabilized CdSe/CdS composite nanoparticles were synthesized using the previous reported method [28-29] with minor revision: 232.26 mg trisodium citrate dihydrate and 68.4 mg CdCl2·2.5H2O were added to 200 mL Tris–HCl buffer solution (pH 9.2) in a three-necked flask with magnetic stirring. Then, 1 mL of freshly prepared Se precursor solution was injected drop by drop under continually stirring. The initial molar ratio of Cd to Se to citrate was approximately 1:0.25:3. The Se precursor solution (NaHSe) was prepared according to the method in Ref.[30]. In the presence of a molar ratio of NaBH4 to Se 1:1 in ethanol, NaHSe was formed according to the reaction equation as follows:
NaBH4+Se+3C2H5OH=NaHSe+B(OC2H5)3+3H2(g) (1)
Briefly, under N2 atmosphere, ethanol (1 mL) was added dropwise to selenium (6 mg) and excessive sodium borohydride (7.5 mg) under magnetic stirring, kept at 45 ℃ for 20 min. The mixture was refluxed at 50 ℃ under N2 atmosphere for 1 h. Finally, the solution blown was by H2S gas generated by the reaction of Na2S solution with diluted H2SO4 with a slow nitrogen flow at 30 ℃ for 30 min. The purified CdSe/CdS/citrate powder was obtained through the ethanol precipitation procedure.
2.4 Detection of BSA
The functionalized CdSe/CdS composite nanoparticles were used to detect the protein based on the change of the relative fluorescence intensity (?F) when protein was added to CdSe/CdS solution. The possible explanation for the increased fluorescence intensity might be (1) BSA can eliminate part of the non-radiation relaxation. (2) BSA can neutralize the charge on the surface of the nanoparticles and form core/shell analogues to enhance the fluorescence intensity [31]. The details of the procedure are as follows [23-24]: 1.5 mL CdSe/CdS QDs solution (0.3 mmol/L), 1.0 mL Clark–Lubs buffer (pH 7.0) and different concentrations of BSA standard solution were sequentially added into 10 mL volumetric bottle, diluted to the mark with water and mixed thoroughly, and incubated in the water bath at 25 ℃ for 20 min before the measurement of fluorescence intensity. The relative fluorescence enhancing intensities ?F (?F=F0-F, where F and F0 were the relative fluorescence intensities of the nano-CdSe/CdS/ citrate solution with and without BSA, respectively) was measured at λem=565 nm by means of spectrofluorophotometer.
3 Results and discussion
3.1 Fluorescence spectroscopy and TEM image
The fluorescence spectra of citrate-stabilized CdSe/CdS and CdSe/CdS-BSA nanoparticles are shown in Fig.1(a). The fluorescence emission spectrum of the CdSe/CdS–BSA system is similar to that of CdSe/CdS nanoparticles, but the intensities are obviously enhanced. The TEM image of CdSe/CdS nanoparticles is shown in Fig.1(b). This indicates that the nanocrystals are irregularly spherical and monodispersive. The results indicate that functionalized nano-CdSe/CdS is a potential better fluorescence probe for the sensitive determination of BSA.

Fig.1 Fluorescence spectra of citrate-stabilized CdSe/CdS and CdSe/CdS-BSA nanoparticles (a) and TEM image of CdSe/ CdS nanoparticles (b)
3.2 Stability of citrate-stabilized CdSe/CdS core/shell nanoparticles
Fig.2 shows the compared photostability of CdSe/CdS nanocrystals against traditional fluorescence dye-FITC. Under respective optimal excitation wavelength fluorescence intensity is measured every 10 min by the spectrafluorometer. As shown in Fig.2, the total fluorescence intensity of FITC is reduced by about 75% within 15 min and evenly disappeared at 30 min, whereas there is no obvious change in that of CdSe/CdS nanocrystals in 60 min. These data illustrate that CdSe/CdS nanocrystals have higher luminescence and photostability. It is better than conventional organic fluorophores as a fluorescence labeling.
3.3 Optimization of determination conditions
In this work, a series of factors were investigated to find the better determination condition. It is found that pH has great effect on luminescence intensity of the CdSe/CdS solution (Fig.3(a)). The maximum fluorescence intensity occurs when pH is 7.0. This phenomenon may be attributed to reasons as follows: at low pH, the decreased fluorescence intensity of CdSe/ CdS QDs may result from protonation of surface-binding citrate in acid medium [31], which induced loss of ligand protection and direct exposure of the QDs surface to the solution. With the increase of pH environment, the fluorescence intensity decreases because of the electrostatic repulsion between electronegative BSA and QDs. Hence, pH 7.0 is selected for all of the experiments.
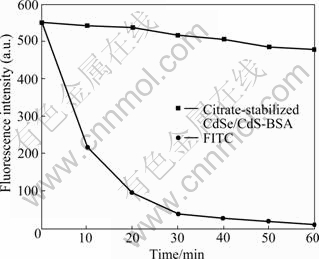
Fig.2 Fluorescence intensities and photostability of citrate- stabilized CdSe/CdS-BSA nanoparticles and FITC
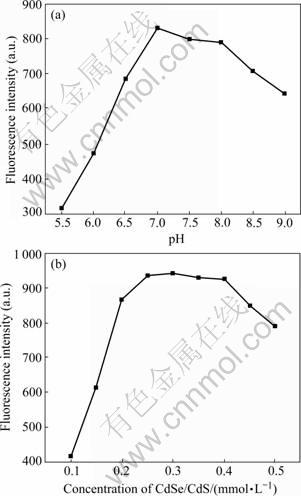
Fig.3 Effect of pH (a) and concentration of CdSe/CdS (b) on fluorescence intensity
The effect of the concentration of the functionalized colloidal solution was also investigated from 0.1 to 0.5 mmol/L (Fig.3(b)) as an important factor. It can be seen that the maximum fluorescence intensity can be obtained when the concentration of nano-CdSe/CdS/ citrate is 0.3 mmol/L. This is because QDs do not completely occupy all nonspecific binding sites of BSA in low concentration. On the contrary, high concentration of QDs will cause the self-quenching [24]. So, the concentration of 0.3 mmol/L is used in subsequent experiments.
The effect of the reaction time and temperature on the fluorescence intensity is shown in Fig.4. The fluorescence intensity increases with the reaction time from 0 to 20 min, and then reaches the equilibrium after 20 min (Fig.4(a)). It can be seen that the reaction is completed in 20 min and the excess time does not contribute to the enhancement of the PL intensity. Moreover, higher temperature will speed up the reaction between the functionalized nano-CdSe/CdS and BSA. The solution was incubated in 25 ℃, and then the fluorescence intensity reaches the maximum (Fig.4(b)). It is explained that (1) if the temperature is too low, QDs will not react with BSA; (2) too high temperature can lead to the aggregation of the system. So, 25 ℃ and 20 min are recommended to use.
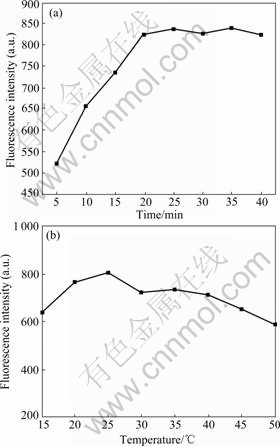
Fig.4 Effect of reaction time (a) and temperature (b) on fluorescence intensity
3.4 Influence of coexisting substances
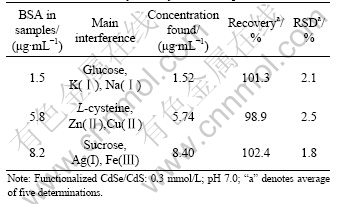
The influence of coexisting substances on the relative fluorescence intensity of the system was studied (Table 1). From the results, it can be seen that high concentrations of Na(Ⅰ), K(Ⅰ), Zn(Ⅱ), Cl- and SO42- do not cause significant interference to detect BSA, but Cu(Ⅱ), Ag(Ⅰ) and Fe(Ⅲ) can be allowed only at a relatively low concentration. Also, high concentration of common saccharine and amino acids such as glucose, sucrose, citric acid, L-cysteine and histidine do not interfere with the determination of BSA. It demonstrates that this method has high selectivity.
Table 1 Test results for interference of coexisting substances

3.5 Calibration curve and detection limit
The fluorescence intensity of 0.1-10 μg/mL BSA is presented in Fig.5. The fluorescence signal increases rapidly with the BSA concentration from 0.1 to 10 μg/mL. Because the excessive BSA does not contribute to the enhancement of the PL intensity, the fluorescence intensity is not obviously increased when the concentration of BSA is above 10 μg/mL. The detection limit is estimated to be 0.06 μg/mL (according to 3×SD, where SD is the standard deviation of five measurements of a blank solution). This method has a wider linear range and a higher sensitivity, which is superior to the recently reported methods for detecting BSA that use classical dyes like erythrosine sodium (ES) (detection limit of 0.5 μg/mL) [10], and albumin blue 580 (detection limit of 0.4 μg/mL) [12]. In addition, it is simpler than the fluorescent dye-based methods. Furthermore, a linear relationship between the enhancement of fluorescence intensity (?F) and the concentration of BSA (c) over the range of 0.1-10 μg/mL can be established using equation ?F=50.7c+16.4 (Fig.5, insert). The correlation coefficient is 0.996 36.
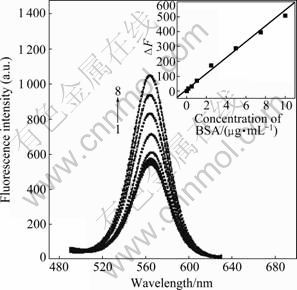
Fig.5 Variation in differential fluorescence signal intensity with BSA concentration (Relationship followed equation of ?F= 50.7c+16.4 (R=0.996 36) (insert)): 1—0 μg/mL; 2—0.1 μg/mL; 3—0.5 μg/mL; 4—1.0 μg/mL; 5—2.5 μg/mL; 6—5.0μg/mL; 7—7.5 μg/mL; 8—10.0 μg/mL
3.6 Sample determination
To demonstrate the applicability of proposed method, three synthetic samples were quantified. The results are shown in Table 2. The concentration of each coexisting substance is 2.0 μg/mL. From Table 2, it can be seen that the values found for the three samples are identical with the true values, and the recovery and relative standard deviation are satisfactory. Therefore, the fluorescence enhancing method of detecting BSA is applicable.
Table 2 Results of analysis of synthetic samples
4 Conclusions
(1) Citrate-stabilized water-soluble CdSe/CdS composite nanoparticles are synthesized at lower temperature using Se powder as a selenium source and Na2S as a sulfur source. The fluorescence of CdSe/CdS composite nanoparticles can be enhanced by protein.
(2) A rapid, ultrasensitive and convenient determining technology is demonstrated for quantitative detection of the protein by using water-soluble CdSe/CdS nanoparticles as fluorescence marker and bovine serum albumin (BSA) as target protein. The extent of the fluorescence intensity enhancement (?F) is proportional to the concentration of BSA (c) in the range of 0.1-10.0 μg/mL.
(3) The effect of parameters such as pH, concentration of CdSe/CdS, time and temperature of the reaction are discussed. At pH 7.0, concentration of CdSe/CdS (0.3 mmol/L), room temperature (25 ℃) and reaction time (20 min) are chosen as the optimal condition for the experiment. The results of determination for BSA in three synthetic samples are identical with the true values, the recovery (98.9%- 102.4%) and relative standard deviation (RSD, 1.8%- 2.5%) are satisfactory.
References
[1] LOWRY O H, ROSEBROUGH N J, FARR A L. Protein measurement with the folin phenol reagent [J]. The Journal of Biological Chemistry, 1951, 193(1): 265-275.
[2] DOUMAS B T, WATSON W A, BIGGS H G. Albumin standards and the measurement of serum albumin with bromcresol green [J]. Clinica Chimica Acta, 1971, 31(1): 87-96.
[3] BRADFORD M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding [J]. Analytical Biochemistry, 1976, 72(7): 248-254.
[4] LYNCH J M, BARBANO D M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products [J]. Journal of AOAC International, 1999, 82(6): 1389-1398.
[5] LEE I H, PINTO D, ARRIAGA E A, ZHANG Z, DOVICHI N J. Picomolar analysis of proteins using electrophoretically mediated microanalysis and capillary electrophoresis with laser-induced fluorescence detection [J]. Analytical Chemistry, 1998, 70(21): 4546-4548.
[6] GUO Chang-ying, WU Xia, YANG Jing-he, WANG Fei, JIA Zhen, RAN De-huan, ZHENG Jin-hua. Determination of proteins using fluorescence enhancement of Tb3+-benzoyl-acetone-sodium dodecyl benzene sulfonate-protein system [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2006, 181(1): 50-55.
[7] POSOKHOV Y O, LADOKHIN A S. Lifetime fluorescence method for determining membrane topology of proteins [J]. Analytical Biochemistry, 2006, 348(1): 87-93.
[8] WANG Fei, YANG Jing-he, WU Xia, SUN Chang-xia, LIU Shu-fang, GUO Chang-ying, WANG Feng. Fluorescence enhancement effect for the determination of proteins with morin-Al3+-cetyltrimethylammonium bromide [J]. Talanta, 2005, 67(4): 836-842.
[9] SANTOS M, NADI S, GOICOECHEA H C, HALDAR M K, CAMPIGLIA A D, MALLIK S. Artificial neural networks for qualitative and quantitative analysis of target proteins with polymerized liposome vesicles [J]. Analytical Biochemistry, 2007, 361(1): 109-119.
[10] ZHU Xia-shi, SUN Jing, HU Yan-yan. Determination of protein by hydroxypropyl-β-cyclodextrin sensitized fluorescence quenching method with erythrosine sodium as a fluorescence probe [J]. Analytica Chimica Acta, 2007, 596(2): 298-302.
[11] PEREZ-RUIZ T, MARTINEZ-LOZANO C, TOMAS V, FENOLL J. Determination of proteins in serum by fluorescence quenching of Rose Bengal using the stopped-flow mixing technique [J]. Analyst, 2000, 125(3): 507-510.
[12] WARD K M. Renal function (microalbuminuria) [J]. Analytical Chemistry, 1995, 67(12): 383R-391R.
[13] HOU Xiao-li, TONG Xiao-fei, DONG Wen-juan, DONG Chuan, SHUANG Shao-min. Synchronous fluorescence determination of human serum albumin with methyl blue as a fluorescence probe [J]. Spectrochimica Acta Part A, 2007, 66 (3): 552-556.
[14] LIANG Jian-gong, HUANG Shan , ZENG Dan-yun, HE Zhi-ke, JI Xing-hu, AI Xin-ping, YANG Han-xi. CdSe quantum dots as luminescent probes for spironolactone determination [J]. Talanta, 2006, 69(1): 126-130.
[15] MA Ying, YANG Cheng, LI Nan, YANG Xiu-rong. A sensitive method for the detection of catecholamine based on fluorescence quenching of CdSe nanocrystals [J]. Talanta, 2005, 67(5): 979-983.
[16] XIE Min, LIU Hui-hui, CHEN Ping, ZHANG Zhi-ling, WANG Xiao-hui, XIE Zhi-xiong, DU Yu-min, PAN Bo-qun, PANG Dai-wen. CdSe/ZnS-labeled carboxymethyl chitosan as a bioprobe for live cell imaging [J]. Chemical Communications, 2005, 44: 5518-5520.
[17] DUBERTRET B, SKOURIDES P, NORRIS D J, NOIREAUX V, BRIVANLOU A H, LIVCHABER A. Invivo imaging of quantum dots encapsulated in phospholipid micelles [J]. Science, 2002, 298(5599): 1759-1762.
[18] YANG Dong-zhi, XUA Shu-kun, CHEN Qi-fan, WANG Yan. One system with two fluorescence resonance energy transfer (FRET) assembles among quantum dots, gold nanoparticles and enzyme [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 329(1): 38-43.
[19] LIAO Ping, YAN Zheng-yu, XUA Zhi-ji, SUN Xiao. A novel fluorescent assay for edaravone with aqueous functional CdSe quantum dots [J]. Spectrochimica Acta Part A, 2009, 72(5): 1066-1070.
[20] HUANG Chin-ping, LIU Shu-wei, CHEN Teng-ming, LI Yaw-kuen. A new approach for quantitative determination of glucose by using CdSe/ZnS quantum dots [J]. Sensors and Actuators B, 2008, 130(1): 338-342.
[21] SUKHANOVA A, DEVY J, VENTEO L, KAPLAN H, ARTEMYEV M, OLEINIKOV V, KLINOV D, PLUOT M, COHEN J H M, NABIEVA I. Biocompatible fluorescent nanocrystels for immunolabeling of membrane proteins and cells [J]. Analytical Biochemistry, 2004, 324(1): 60-67.
[22] WILLARD D M, CARILLO L L, JUNG J, ORDEN A V. CdSe-ZnS quantum dots as resonance energy transfer donors in a model protein-protein binding assay [J]. Nano Letters, 2001, 1(9): 469-474.
[23] YU Ying, LAI Yan, ZHENG Xiu-li, WU Jian-zhong, LONG Zhao-yang, LIANG Chun-sui. Synthesis of functionalized CdTe/CdS QDs for spectrofluorimetric detection of BSA [J]. Spectrochimica Acta Part A, 2007, 68(5): 1356-1361.
[24] WANG Lun, WANG Le-yu, ZHU Chang-qing, WEI Xian-wen, KAN Xian-wen. Preparation and application of functionalized nanoparticles of CdS as a fluorescence probe [J]. Analytica Chimica Acta, 2002, 468(1): 35-41.
[25] GU Li, LI Fei, ZHANG Yang, GU Dan-qing. Preparation of CdSe quantum dots and coating with polylactide [J]. Journal of Central South University: Science and Technology, 2009, 40(4): 904-908. (in Chinese).
[26] ROGACH A L, NAGESHA D, OSTRANDER J W, GIERSIG M, KOTOV N A. “Raisin bun”—Type composite spheres of silica and semiconductor nanocrystals [J]. Chemistry of Material , 2000, 12(9): 2676-2685.
[27] WANG Y, TANG Z, CORREA-DUARTE M A, LIZ-MARZAN L M, KOTOV N A. Multicolor luminescence patterning by photoactivation of semiconductor nanoparticle films [J]. Journal of the American Chemical Society, 2003, 125(10): 2830-2831.
[28] DENGDa-wei, YUJun-sheng, YI Pan. Water-soluble CdSe and CdSe/CdS nanocrystals: A greener synthetic route [J]. Journal of Colloid and Interface Science, 2006, 299(1): 225-232.
[29] HUANG Feng-hua, CHEN Guo-nan. Preparation and application of L-cysteine-modified CdSe/CdS core/shell nanocrystals as a novel fluorescence probe for detection of nucleic acid [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2008, 70(2): 318-323.
[30] KLAYMAN D L, GRIFFIN T S. A facile method for the introduction of selenium into organic molecules [J]. Journal of the American Chemical Society, 1973, 95(1): 197-199.
[31] CHANG Wen-gui, SHEN Yu-hua, XIA An-jian, ZHANG Hui, WANG Juan, LU Wen-sheng. Controlled synthesis of CdSe and CdSe/CdS core/shell nanoparticles using Gemini surfactant Py-16-10-16 and their bioconjugates with BSA [J]. Journal of Colloid and Interface Science, 2009, 335(2): 257-263.
Foundation item: Project(50772133) supported by the National Natural Science Foundation of China
Received date: 2009-10-19; Accepted date: 2009-12-21
Corresponding author: HUANG Ke-long, PhD, Professor; Tel: +86-731-88879850; E-mail: klhuang@mail.csu.edu.cn
(Edited by YANG You-ping)