Solvent extraction separate of zinc and cadmium from magnesium and calcium in sulfuric acid medium by mixing extractants
来源期刊:中南大学学报(英文版)2017年第10期
论文作者:李晓晖 艾仙斌 何力 计少石 胡淼 丁建南 李凤
文章页码:2253 - 2259
Key words:synergistic extraction; cadmium; zinc; Mextral V10; aromatic hydroxamic
Abstract: Synergistic extraction has been proven to enhance extractability and selectivity for the separation of cadmium and zinc from magnesium and calcium in the sulfuric acid medium with Mextral V10 and Mextral 622H (aromatic hydroxamic) mixtures diluted in DT100. Mixtures of Mextral V10 and 622H are highly selective for Zn and Cd over Mg and Ca compared with the single Mextral V10, resulting in larger synergistic shifts for zinc and cadmium with the ΔpH50 (Zn–Ca) values increasing substantially from 0.83 to 1.73 pH units and ΔpH50 (Cd–Ca) values increasing from 0.63 to 2.13 pH units. The aqueous to organic ratio (A/O ratio), the saponification ratio of Mextral V10 and extracting agent concentration were studied on the effect of metal ions extraction, which helped to increase the organic capacity of metal extraction. The McCabe-Thiele plot for Cd and Zn extraction with 5% (volume fraction) Mextral V10 and 5% Mextral 622H extractants mixture indicate the necessity of only one theoretical stages at an A/O ratio of 1.5:1. One stage extraction simulation test conducted at pH 6.50 shows that Cd and Zn reach the extraction of 99.6% and 97.9%, respectively, and only low levels of magnesium and calcium are extracted in the organic phase. The extracted Zn2+ or Cd2+-organic species are Zn(A1)(A2) or Cd(A1)(A2) with the mixture system by slope analysis.
Cite this article as: LI Xiao-hui, AI Xian-bin, HE Li, JI Shao-shi, HU Miao, DING Jian-nan, LI Feng. Solvent extraction separate of zinc and cadmium from magnesium and calcium in sulfuric acid medium by mixing extractants [J]. Journal of Central South University, 2017, 24(10): 2253–2259. DOI:https://doi.org/10.1007/s11771-017-3635-1.
J. Cent. South Univ. (2017) 24: 2253-2259
DOI: https://doi.org/10.1007/s11771-017-3635-1
LI Xiao-hui(李晓晖)1, AI Xian-bin(艾仙斌)2, HE Li(何力)1, JI Shao-shi(计少石)1,
HU Miao(胡淼)1, DING Jian-nan(丁建南)1, LI Feng(李凤)3
1. Institute of Biological Resources, Jiangxi Academy of Science, Nanchang 330096, China;
2. Institute of Energy, Jiangxi Academy of Science, Nanchang 330096, China;
3. Kopper Chemical Industrial Corp., Ltd., Chongqing 401221, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract: Synergistic extraction has been proven to enhance extractability and selectivity for the separation of cadmium and zinc from magnesium and calcium in the sulfuric acid medium with Mextral V10 and Mextral 622H (aromatic hydroxamic) mixtures diluted in DT100. Mixtures of Mextral V10 and 622H are highly selective for Zn and Cd over Mg and Ca compared with the single Mextral V10, resulting in larger synergistic shifts for zinc and cadmium with the △pH50 (Zn–Ca) values increasing substantially from 0.83 to 1.73 pH units and △pH50 (Cd–Ca) values increasing from 0.63 to 2.13 pH units. The aqueous to organic ratio (A/O ratio), the saponification ratio of Mextral V10 and extracting agent concentration were studied on the effect of metal ions extraction, which helped to increase the organic capacity of metal extraction. The McCabe-Thiele plot for Cd and Zn extraction with 5% (volume fraction) Mextral V10 and 5% Mextral 622H extractants mixture indicate the necessity of only one theoretical stages at an A/O ratio of 1.5:1. One stage extraction simulation test conducted at pH 6.50 shows that Cd and Zn reach the extraction of 99.6% and 97.9%, respectively, and only low levels of magnesium and calcium are extracted in the organic phase. The extracted Zn2+ or Cd2+-organic species are Zn(A1)(A2) or Cd(A1)(A2) with the mixture system by slope analysis.
Key words: synergistic extraction; cadmium; zinc; Mextral V10; aromatic hydroxamic
1 Introduction
The rapid development of industrialization brought huge economic benefits, at the same time heavy metal (e.g., Cu, Pb, Cd and Zn) contamination has become one of the major environmental problems all over the world [1, 2]. Recently, great efforts have been focused on the treatment of mine wastewater due to its potential danger to ecosystems and human health [3, 4]. It is therefore necessary to remove heavy metals from mine wastewater [5, 6].
At present, chemical precipitation has become less and less attractive to remove heavy metals from mine wastewater because of high energy requirements, high cost and unstable efficiencies. Microbial and phytoremediation methods are difficult to thoroughly eliminate heavy metals and to recycle heavy metals because of low selective extraction of microorganisms and plants. But solvent extraction, as a mature stage in the hydrometallurgical process, has the advantages of simple equipment, easy operation, and small secondary pollution and so on. At present, heavy metal ions are difficult to separate directly from mining wastewater with the single extraction system. However, the synergistic extraction has been proven to enhance extractability and selectivity [7, 8]. The synergistic solvent extraction systems have been developed by the solvent extraction technology team of the Parker Centre to separate nickel and cobalt from manganese, magnesium and calcium in sulphuric acid medium using direct solvent extraction processes without intermediate precipitation and releach steps [9–12]. The reagent LIX63 (5, 8-diethyl-7-hydroxy-6-dodecanone oxime) is versatile synergistic reagent [13, 14]. CHENG et al [11, 12] reported that the separation of nickel and cobalt from impurities such as manganese, magnesium and calcium using solvent extraction with Versatic 10 was largely improved by the addition of a synergistic reagent LIX63. The synergistic system consisting of LIX63, Versatic 10 and TBP in Shellsol D70 can be used to separate copper, nickel, cobalt and zinc from chloride solutions [15]. In addition, LIX 984N, LIX
984N, LIX 84-IC and other hydroxamic reagents are studied as synergistic reagents at present [16, 17]. But much work in solvent extraction with aliphatic hydroxamic was conducted, and less attention was paid to aromatic hydroxamic. The reagent of Mextral 622H is aromatic hydroxamic in the mixture with high flash point hydrocarbon diluent.
84-IC and other hydroxamic reagents are studied as synergistic reagents at present [16, 17]. But much work in solvent extraction with aliphatic hydroxamic was conducted, and less attention was paid to aromatic hydroxamic. The reagent of Mextral 622H is aromatic hydroxamic in the mixture with high flash point hydrocarbon diluent.
In the present work, a direct solvent extraction process using the mixture of Mextral V10 and Mextral 622H to separate zinc and cadmium from magnesium and calcium has been proposed. The mixture of Mextral V10 and 622H system was investigated to identify any synergistic or antagonistic effects with extraction pH isotherms. The effect of experimental conditions on the removal efficiency of the metals was also evaluated, including the A/O ratio, extracting agent concentration and the saponification rate of Mextral V10.
2 Experimental
2.1 Aqueous and organic phase
The simulation acid mining water was prepared by dissolving AR grade sulphates of zinc and magnesium, nitrate of cadmium and calcium in distilled water. The composition of simulation solution is shown in Table 1.
Table1 Concentration of simulation acid mining water (Unit: mg/L)

The reagent Mextral V10 (2-methul-2- ethylheptanoic acid), Mextral 622H and the diluent DT100 were purchased from Kopper Chemical Industral Corp., Ltd. The composition of the Mextral 622H was reported by active oxime component in a diluent. All reagents were used as received.
2.2 Extraction test
2.2.1 Extraction pH isotherms
The solvent extraction tests of extraction pH isotherms were carried out in a 125 mL separating funnel and placed on oscillator at the speed of 150 r/min. The pH of aqueous phase was monitored with a Mettler Toledo pH meter (model FE 20). To determine metal extraction pH isotherms, the organic phase was mixed with the simulation solution at an A/O ratio of 1:1 and 25 °C. The solution mixture (20 mL) was sampled over a pH range of 4.0–8.0. The system was allowed to equilibrate sufficiently at each pH point before sampling. The organic system containing 7% Mextral V10 and 3% Mextral 622H diluted in DT100 was selected as the composition and the saponification rate of Mextral V10 was 70% with 10 mol/L sodium hydroxide solution.
2.2.2 Extraction distribution isotherms
The organic systems were loaded by mixing with the simulation solution in a range of A/O ratios of 1:2, 1:1.5, 1:1.25, 1:1 and 1.5:1 at 25 °C with optimum pH values as determined in the above pH isotherm tests based on the separation of cadmium and zinc from magnesium and calcium. The extraction distribution isotherms were obtained at pH 6.50 for the Mextral V10/Mextral 622H system.
2.2.3 Effect of aqueous and organic ratio (A/O), saponification rate of Mextral V10 and extracting agent concentration on extraction of metal ions
The organic system containing 5% Mextral V10 and 5% Mextral 622H were selected as the composition. Composites with varying A/O ratios (1:2, 1:1, 1.5:1, 2:1 and 2.5:1) were added to 125 mL separating funnel and the reaction mixtures were placed on oscillator at 150 r/min and 25 °C. The saponification rate of Mextral V10 was 70% with 10 mol/L sodium hydroxide solution. The metal ion concentration in the aqueous phase was determined after 10 min of extraction. The effect of extracting agent concentration on the extraction of metal ion was studied at 5%, 10%, 15% and 20%. The volume ratio of Mextral V10 and Mextral 622H was 1:1 and the saponification rate of Mextral V10 was 70% at an A/O ratio of 1:1 and 25 °C. The effect of Mextral V10 saponification rate on the extraction of metal ion was studied at 50%, 60%, 70% and 80%. The organic systems containing 5% Mextral V10 and 5% Mextral 622H were selected as the composition at an A/O ratio of 1:1 and 25 °C.
2.2.4 Saponification of Mextral V10
Mextral V10 can be saponified by sodium hydroxide solution to a certain degree, the saponification rate of Mextral V10 were adjusted to 50%, 60%, 70% and 80%. The saponification of Mextral V10 was performed in a flask with a stirrer (300 r/min), at reaction temperature of 25 °C, adding 10 mol/L sodium hydroxide solution rapidly while the stirrer was in operation.
2.3 Chemical analysis
The distribution ratio was calculated as the ratio between the concentrations of metal ion in the organic phase and the aqueous phase. The metal ion concentration in the aqueous phase was determined by using an atomic absorption spectrometry (AA6880, Shimadzu, Japan). The metal ion concentration in the organic phase was calculated from mass balance.
3 Results and discussion
3.1 Metal extraction pH isotherms
Based on the extraction of zinc and cadmium, and the rejection of magnesium and calcium, the organic system containing 7% Mextral V10 and 3% Mextral 622H was selected as the composition at an O/A ratio of 1:1 and 25 °C, the saponification rate of Mextral V10 was 70%. The results show that the mixture system is highly selective for Zn and Cd over Mg and Ca, compared to the organic system containing 10% Mextral V10 alone (Fig. 1), the pH isotherms of calcium and magnesium are shifted to a higher pH range, and the pH isotherms of zinc and cadmium are shifted to a lower pH range. This mixture system results in larger synergistic shifts for zinc and cadmium with the △pH50 (Zn–Ca) values (the difference in the pH50 values of zinc and calcium) increasing substantially from 0.83 to 1.73 pH units and △pH50 (Cd–Ca) values from 0.63 to 2.13 pH units (Fig. 2).
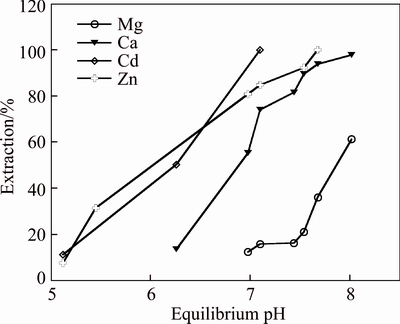
Fig. 1 Metal extraction pH isotherms with 10% Versatic10 in DT100
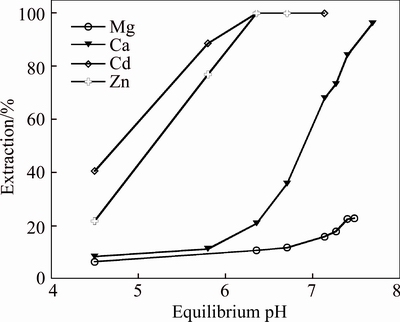
Fig. 2 Metal extraction pH isotherms with mixtures of 7% Mextral V10 and 3% Mextral 622H in DT100
It is shown in Fig. 3 that the highest separation factors (β=DA/DB, where A represents Cd or Zn, and B represents Ca or Mg) value of cadmium and magnesium achieved is 566.25 at an equilibrium pH 7.44 where Cd extraction is 99.1% and Mg co-extraction is 16.28%. The highest β value of zinc and magnesium achieved is 218.54 at an equilibrium pH 7.68 where Zn extraction is 99.2% and Mg co-extraction is 36.2%. The separation factors of cadmium and zinc over calcium are low, indicating impossible separation of cadmium and zinc from calcium with a number of extraction stages. However, the highest β of cadmium and magnesium achieved is 912 at an equilibrium pH 7.27 and the highest β of zinc and magnesium achieved is 543 at an equilibrium pH 6.36 in Fig. 4. And the separation factors of cadmium and zinc over calcium are significantly increased where Zn and Cd extractions are 98.5% and 99.1%, respectively, and Mg co-extraction is 10.8% at an equilibrium pH 6.36. The results show that the mixture system has highly selective for Zn and Cd over Mg and Ca compared with the organic system containing 10% Mextral V10 alone. The addition of Mextral 622H may suppress magnesium and calcium extraction, then increase the selectivity for the separation of cadmium and zinc over magnesium and calcium.
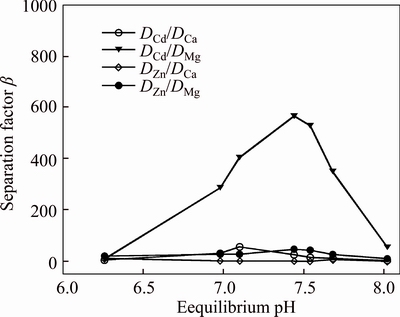
Fig. 3 Separation factor with 10% Mextral V10 in DT100
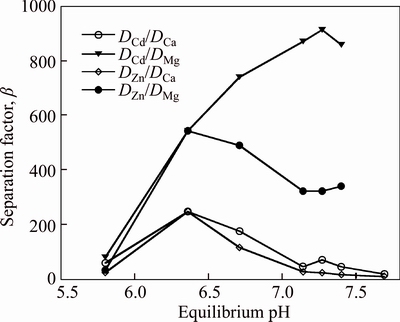
Fig. 4 Separation factor with mixtures of 7% Mextral V10 and 3% Mextral 622H in DT100
3.2 Effect of Mextral V10 and Mextral 622H volume ratio on extraction pH isotherms of zinc and cadmium
A series of tests were conducted with Mextral V10 concentrations in the range of 0–10%, Mextral 622H concentrations in the range of 0–10% in DT100 at an O/A ratio of 1:1 and 25 °C, the saponification rate of Mextral V10 was 70% with 10 mol/L sodium hydroxide solution (Figs. 5 and 6). Compared to the organic system containing Mextral V10 or Mextral 622H alone, the pH isotherms of zinc and cadmium are shifted to a lower pH range, this mixture organic system results in larger synergistic shifts for zinc with their pH50 values (the pH value at 50% extraction) reduced to 0.9, 0.93 and 1.45 pH units and the pH50 values of cadmium were reduced to 0.9, 0.84 and 1.57 pH units with the decreasing Mextral V10 and Mextral 622H volume ratio, respectively.

Fig. 5 Effect of Mextral V10 and Mextral 622H volume ratio on extraction pH isotherms of cadmium
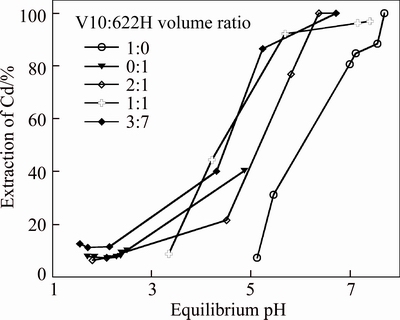
Fig. 6 Effect of Mextral V10 and Mextral 622H volume ratio on extraction pH isotherms of zinc
3.3 Effect of A/O ratio on extraction of metal ions
In this set of tests, the A/O ratios of 1:2, 1:1, 1.5:1, 2:1 and 2.5:1 were tested using the extraction of metal ion to investigate the effect of A/O ratio on the metal extraction behavior at the initial solution pH of 2.0 (Fig. 7). It is indicated that the calcium and magnesium concentration in the loaded organic solutions decreases with the increasing A/O ratio. The concentration of calcium and magnesium in the loaded organic phase are the highest approximately 243.46 and 152.45 mg/L at the A/O ratio of 1:2, respectively. The cadmium and zinc extractions for the different A/O ratios tested are more than 99% in the range of 0.5–1.5. The cadmium and zinc concentrations in the raffinate are found to significantly increase with increasing A/O ratio from 1.5 to 2.5, which indicates that the A/O ratio at 1.5:1 works very well in terms of cadmium and zinc extraction and the concentration of calcium and magnesium in the loaded organic are only 38.57 and 16.35 mg/L under this condition and the separation factors of cadmium and zinc over calcium are 1835 and 1668, respectively.

Fig. 7 Effect of A/O ratio on extraction of metal ions
3.4 Effect of extracting agent concentration on extraction of metal ions
The extracting agent concentrations of 5%, 10%, 15% and 20% were investigated on the effect of metal ion at the initial solution pH of 1.83 with the A/O ratio of 1:1 and the saponification rate of Mextral V10 was 70% (Fig. 8). It is clear that higher extracting agent concentration would result in significant loading of cadmium and zinc and the concentration of magnesium and calcium in the loaded organic increases from 45.9 to 110.49 mg/L and 39.94 to 223.0 mg/L, respectively. While lower extracting agent concentration would cause considerable cadmium and zinc remained in the raffinate when the extracting agent concentration is less than 10%, which is unable to complete the separation of zinc and cadmium from simulation acid mining water. Effect of extracting agent concentration on the extraction of metal ion is attributed to increasing the organic capacity of metal extraction.
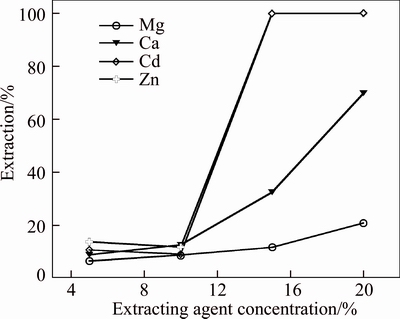
Fig. 8 Effect of extracting agent concentration on extraction of metal ions
3.5 Effect of saponification rate of Mextral V10 on extraction of metal ions
The Mextral V10 saponification rates of 50%, 60%, 70% and 80% were investigated on the effect of metal ions extraction at the initial solution pH of 1.83 with the O/A ratio of 1:1 (Fig. 9). It is shown that higher saponification rate of Mextral V10 would result in significant cadmium and zinc loading, but the magnesium and calcium concentrations in the loaded organic solution gradually increase with the increasing saponification rate. While lower saponification rate of Mextral V10 would cause considerable cadmium and zinc remained in the raffinate. With all tested, little magnesium and calcium are loaded in the organic solution and almost no zinc and cadmium are lost in the raffinate at the saponification rate of Mextral V10 of 70%.
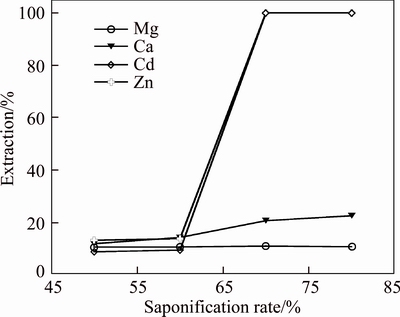
Fig. 9 Effect of Mextral V10 saponification rate on extraction of metal ions
3.6 Cadmium and zinc extraction distribution isotherm
McCabe-Thiele plot on cadmium and zinc extraction with mixture of 5% Mextral V10 and 5% Mextral 622H in DT100 at Mextral V10 saponification rate of 70% are shown in Figs. 10 and 11, respectively. Only one theoretical extraction stage was needed to extract almost all the cadmium and zinc. After extraction simulation test at A/O ratio of 1.5:1 was carried out at equilibrium pH 6.50 and 25 °C, the extractions of cadmium and zinc are 99.6% and 97.9%, respectively. Only low levels of magnesium and calcium were extracted, with 38.56 mg/L Mg and 5.43 mg/L Ca in the organic phase. These results show that at pH 6.50, much higher metal extraction is obtained while the concentrations of magnesium and calcium in the loaded organic solution are still relatively low.
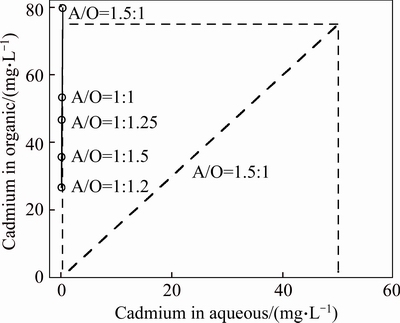
Fig. 10 Cadmium extraction distribution isotherm with 5% Mextral V10 and 5% Mextral 622H in DT100 and its McCabe- Thiele diagram
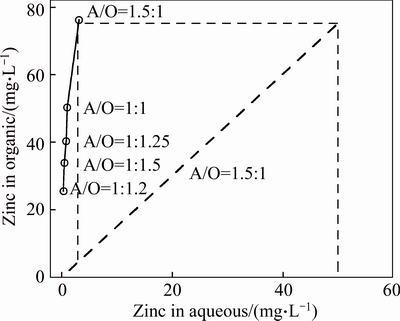
Fig. 11 Zinc extraction distribution isotherm with 5% Mextral V10 and 5% Mextral 622H in DT100 and its McCabe-Thiele diagram
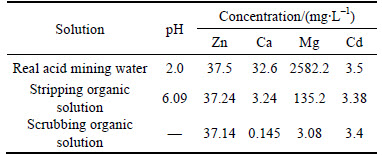
The practical efficiency of the synergistic system was tested based on the data obtained from the McCabe- Thiele plot and the extraction distribution isotherm on cadmium and zinc extraction at one theoretical extraction stage and one scrubbing stage. The result is shown in Table 2. With extraction tested, little magnesium and calcium were loaded in the organic solution and almost no zinc and cadmium were lost in the raffinate. The co-extracted magnesium and calcium could be removed by scrubbing.
Table 2 Test of real acid mining water for synergistic system
3.7 Synergistic extraction mechanism
The possible reaction of cadmium and zinc extraction with mixtures of Mextral V10 and Mextral 622H system in simulation solution can be expressed as
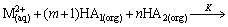
 (1)
(1)
The extraction equilibrium constant K can be defined as
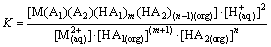 (2)
(2)
Hence, the following logarithmic expression can be derived:
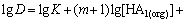
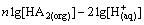 (3)
(3)
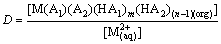 (4)
(4)
where M represents metal ions; D denotes the distribution coefficient of cadmium and zinc between organic and aqueous phases as shown in Eq. (4) and D is calculated by the extraction of the metal ions; K is a constant under a certain temperature; HA1(org) and HA2(org) are Mextral V10 and Mextral 622H, respectively, and they are reasonably constant owing to very small amounts of the extractants consumed in the formation of metal-organic complexes. Therefore, the plot of lgD against pH should be a straight line as shown in Eq. (3).
Extraction tests were conducted with the mixture system consisting of 5% Mextral 622H and various concentrations of Mextral V10 in DT100 with the aqueous phase containing 50 mg/L Zn2+ or Cd2+ at an A/O ratio of 1:1 and 25 °C. It is found that the trend line slopes of the lgD against pH curve are 0.238 and 0.294 for Zn2+ and Cd2+, respectively (Fig. 12), which are reasonably close to 0. Therefore, the m value in Eq. (3) should be 0, indicating that one Mextral V10 molecule is involved in the extraction of one Zn2+ or Cd2+.
The plot of lgD against lg[Mextral 622H] is shown in Fig. 13 with the mixture system consisting of 5% Mextral V10 and various concentrations of Mextral 622H in DT100 with the aqueous phase containing 50 mg/L Zn2+ or Cd2+ at an A/O ratio of 1:1 and 25 °C. It is found that the trend line slopes of the lgD against lg[Mextral 622H] curve are 0.90587 and 1.0822 for Zn2+ and Cd2+, respectively, which are also reasonably close to 1. Therefore, the n value in Eq. (3) should be 1, indicating that one Mextral 622H molecule is involved in the extraction of one Zn2+ or Cd2+. Based on the above analysis, the reaction of zinc and cadmium extraction with mixtures system can be represented by Eqs. (5) and (6).
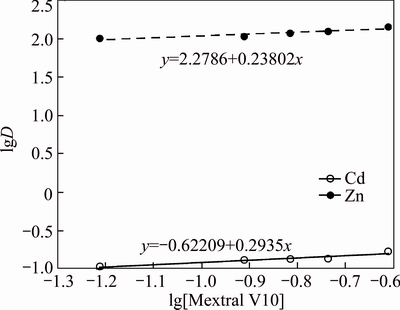
Fig. 12 lgD against lg[Mextral V10] for mixture system of Mextral V10 and Mextral 622H
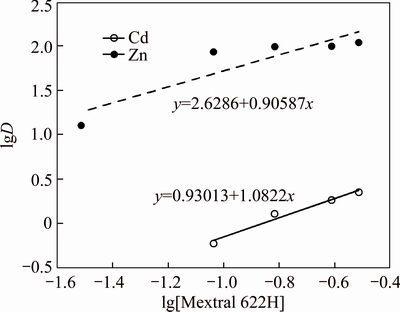
Fig. 13 lgD against lg[Mextral 622H] for mixture system of Mextral V10 and Mextral 622H
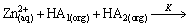
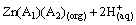 (5)
(5)
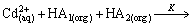
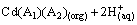 (6)
(6)
4 Conclusions
The mixture system of Mextral V10 and Mextral 622H was tested to separate zinc and cadmium from magnesium and calcium solution. This mixture system results in larger synergistic shifts for zinc and cadmium with the △pH50 (Zn-Ca) values increasing substantially from 0.83 to 1.73 pH units and △pH50 (Cd-Ca) values from 0.63 to 2.13 pH units at an O/A ratio of 1:1 and 25℃, the saponification rate of Mextral V10 is 70%. And the highest β of cadmium and magnesium achieved is 912 at an equilibrium pH 7.27 and the highest β of zinc and magnesium achieved was 543 at an equilibrium pH 6.36. The A/O ratio, the saponification rate of Mextral V10 and extracting agent concentration has a significant effect on the metal ions extraction, which is attributed to increasing the organic capacity of metal ions extraction. Only one theoretical extraction stage is needed to extract almost all the cadmium and zinc. The practical efficiency of the syngistic system is tested at A/O ratio of 1.5:1 that little magnesium and calcium are loaded in the organic solution and almost no zinc and cadmium are lost in the raffinate. The extracted Zn2+ or Cd2+-organic species is Zn(A1)(A2) or Cd(A1)(A2) with the mixture system of Mextral V10 and Mextral 622H.
References
[1] PEJMAN A, BIDHENDI G N, ARDESTANI M, SAEEDI M, BAGHVAND A. A new index for assessing heavy metals contamination in sediments: A case study [J]. Ecological Indicators, 2015, 58: 365–373.
[2] DUAN Xing-wu, ZHANG Guang-li, LI Rong, FANG Hai-yan, HE Da-ming, FENG De-tai. Spatial distribution and environmental factors of catchment-scale soil heavy metal contamination in the dry-hot valley of Upper Red River in southwestern China [J]. Catena, 2015, 135: 59–69.
[3] MCNINCH R M, ROSE J B, DREELIN E A. Aquatic ecosystems and human health [J]. Earth Systems and Environmental Sciences, Encyclopedia of Inland Waters, 2009, 3: 13–20.
[4] BEOLCHINI F, FONTI V, ROCCHETTI L, SARACENI G, PIETRANGELI B, DELL’ ANNO A. Chemical and biological strategies for the mobilization of metals/semi-metals in contaminated dredged sediments: Experimental analysis and environmental impact assessment [J]. Chem Ecol, 2013, 29(5): 415–426.
[5] MAYES W M, JARVIS A P. Remediation of aquatic post-industrial inorganic pollutants [J]. Earth Systems and Environmental Sciences. Encyclopedia of Environmental Health, 2011, 4: 789–800.
[6] CHEN Chiu-wen, KAO Chih-ming, CHEN Chih-feng, DONG Cheng-di. Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan [J]. Chemosphere, 2007, 66(8): 1431–1440.
[7] YANG Tian-zu, SHUI Chen-jing, BIN Wan-da. The synergistic mechanism of solvent extraction of gold in HCl media with TOA and TOPO [J]. Journal of Central South University of Technology, 1999, 6(1): 28–31.
[8] HU Jiu-gang, CHEN Qi-yuan, HU Hui-ping, YIN Zhou-lan. Synergistic extraction of zinc from ammoniacal solutions using β-diketone mixed with CYANEX923 or LIX84I [J]. Trans Nonferrous Met Soc China, 2012, 22(5): 1217–1223.
[9] CHENG C Y, URBANI M D. Solvent extraction process for separation cobalt and or nickel from impurities in leach solutions: US, 7935322B2 [P]. 20110503.
[10] CHENG C Y. Solvent extraction of nickel and cobalt with synergistic systems consisting of carboxylic acid and aliphatic hydroxyoxime [J]. Hydrometallurgy, 2006, 84(1, 2): 109–117.
[11] CHENG C Y, BODDY G, ZHANG W, GODFREY M, ROBINSON D J, PRANOLO Y, ZHU Z, WANG W. Recovery of nickel and cobalt from laterite leach solutions using direct solvent extraction: Part 1-selection of a synergistic SX system [J]. Hydrometallurgy, 2010, 104(1): 45–52.
[12] CHENG C Y, BODDY G, ZHANG W, GODFREY M, ROBINSON D J, PRANOLO Y, ZHU Z, ZENG L, WANG W. Recovery of nickel and cobalt from laterite leach solutions using direct solvent extraction. Part 2: Semi- and fully-continuous tests [J]. Hydrometallurgy, 2010, 104(1): 53–60.
[13] FLETT D S, TITMUSS S. Synergistic effect of LIX63 on the extraction of copper and cobalt by naphthenic acid [J]. J Inorg Nucl Chem, 1969, 31(8): 2612–2613.
[14] PREATON J S. Solvent extraction of nickel and cobalt by mixtures of carboxylic acids and non-chelating oximes [J]. Hydrometallurgy, 1983, 11(1): 105–124.
[15] ZHU Z, ZHANG W, PRANOLO Y, CHENG C Y. Separation and recovery of copper, nickel, cobalt and zinc in chloride solutions by synergistic solvent extraction [J]. Hydrometallurgy, 2012, 127–128: 1–7.
[16] FOUAD E A. Separation of copper from aqueous sulfate solutions by mixtures of Cyanex301 and LIX 984N [J]. J Hazard Mater, 2009, 166(2, 3): 720–727.
984N [J]. J Hazard Mater, 2009, 166(2, 3): 720–727.
[17] NDLOVU B, MAHLANGU T. Calcium and magnesium rejection from sulphate solutions in lateritic nickel solvent extraction using Versatic 10 acid-LIX 84-IC system [J]. J S Afr Inst Min Metall, 2008, 108(4): 223–228.
84-IC system [J]. J S Afr Inst Min Metall, 2008, 108(4): 223–228.
(Edited by FANG Jing-hua)
Cite this article as: LI Xiao-hui, AI Xian-bin, HE Li, JI Shao-shi, HU Miao, DING Jian-nan, LI Feng. Solvent extraction separate of zinc and cadmium from magnesium and calcium in sulfuric acid medium by mixing extractants [J]. Journal of Central South University, 2017, 24(10): 2253–2259. DOI:https://doi.org/10.1007/s11771-017-3635-1.
Foundation item: Projects(20151BBG70009, 20151BBE50115) supported by Jiangxi Science and Technology Projects, China; Projects(2014-YYB-06, 2014-YYB-11) supported by Scientific Research and Development Project of Jiangxi Academy of Sciences, China
Received date: 2016-04-13; Accepted date: 2016-10-06
Corresponding author: LI Xiao-hui, PhD; Tel/Fax: +86–791–88177324; E-mail: lixiaohui211121@163.com

