Temperature dependence on reaction of CaCO3 andSO2 in O2/CO2 coal combustion
来源期刊:中南大学学报(英文版)2009年第5期
论文作者:王宏 徐辉碧 郑楚光 邱建荣
文章页码:845 - 850
Key words:CaCO3; SO2; O2/CO2 coal combustion; temperature dependence
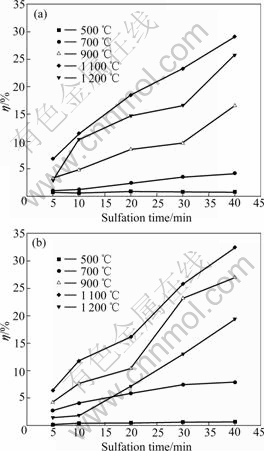
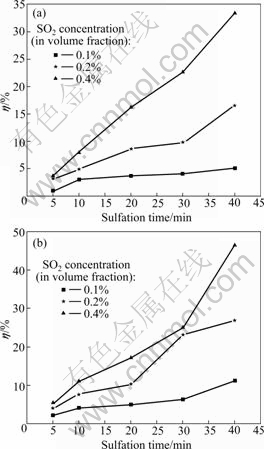
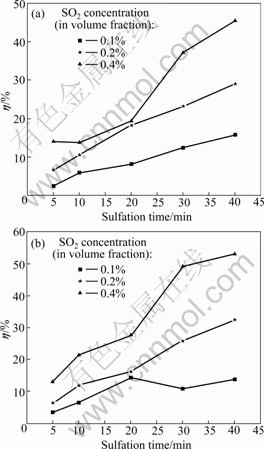
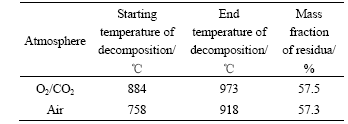
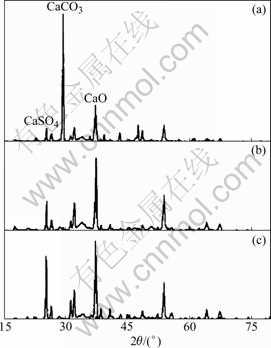
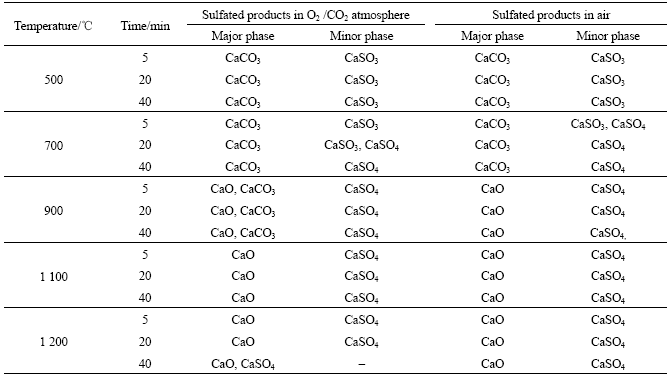
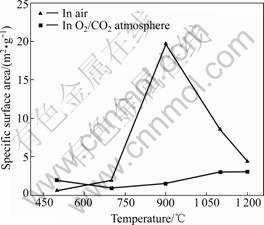
Abstract: The temperature dependence on the reaction of desulfurization reagent CaCO3 and SO2 in O2/CO2 coal combustion was investigated by thermogravimetric analysis, X-ray diffraction measurement and pore structure analysis. The results show that the conversion of the reaction of CaCO3 and SO2 in air is higher at 500-1 100 ℃ and lower at 1 200 ℃ compared with that in O2/CO2 atmosphere. The conversion can be increased by increasing the concentration of SO2, which causes the inhibition of CaSO4 decomposition and shifting of the reaction equilibrium toward the products. XRD analysis of the product shows that the reaction mechanism of CaCO3 and SO2 differs with temperature in O2/CO2 atmosphere, i.e. CaCO3 directly reacts with SO2 at 500 ℃ and CaO from CaCO3 decomposition reacts with SO2 at 1 000 ℃. The pore analysis of the products indicates that the maximum specific surface area of the products accounts for the highest conversion at 1 100 ℃ in O2/CO2 atmosphere. The results reveal that the effect of the atmosphere on the conversion is temperature dependence.
基金信息:the National Natural Science Foundation of China
the Key Foundation of Ministry of Education of China
J. Cent. South Univ. Technol. (2009) 16: 0845-0850
DOI: 10.1007/s11771-009-0140-1